Abstract
Small-cell lung carcinoma (SCLC) is an aggressive, rapidly growing and metastasizing, and highly fatal neoplasm. We report that vasoactive intestinal peptide inhibits the proliferation of SCLC cells in culture and dramatically suppresses the growth of SCLC tumor-cell implants in athymic nude mice. In both cases, the inhibition was mediated apparently by a cAMP-dependent mechanism, because the inhibition was enhanced by the adenylate cyclase activator forskolin and the phosphodiesterase inhibitor 3-isobutyl-1-methylxanthine in proportion to increases in intracellular cAMP levels, and the inhibition was abolished by selective inhibition of cAMP-dependent protein kinase. If confirmed in clinical trials, this antiproliferative action of vasoactive intestinal peptide may offer a new and promising means of suppressing SCLC in human subjects, without the toxic side effects of chemotherapeutic agents.
Keywords: neuropeptides/cAMP/cell culture/athymic nude mice
Small-cell lung carcinoma (SCLC) constitutes 20–25% of cases of lung cancer, currently the leading cause of death from malignant disease among men and women in the U.S. (1). SCLC is also a highly fatal cancer; it metastasizes early and rapidly, and it is rarely curable (1). Therefore, means of controlling the growth and multiplication of SCLC are needed urgently.
The neuroendocrine nature of SCLC is well known; the tumor cells produce a variety of hormones and neurotransmitters and are in turn influenced by their secretory products, some of which (e.g., bombesin-like peptides such as gastrin-releasing peptide) act as autocrine growth factors (2). We recently reported that vasoactive intestinal peptide (VIP), a naturally occurring 28-aa residue neuropeptide (3), binds to specific adenylate cyclase-linked receptors on SCLC cell lines, NCI-H345 and NCI-H69 (as designated by the National Cancer Institute; ref. 4), and inhibits the growth and multiplication of these cell lines in vitro (5), especially if the growth-promoting action of endogenously produced gastrin-releasing peptide (6) is blocked with an anti-bombesin monoclonal antibody (5). In this paper we further document the antiproliferative activity of VIP against SCLC cells in vitro, establish its mediation by cAMP and cAMP-dependent protein kinase (protein kinase A), and report that VIP also suppresses the growth of SCLC tumor implants in athymic nude mice in vivo.
MATERIALS AND METHODS
Cell Culture.
SCLC cell lines NCI-H345 and NCI-H69, belonging to the classic subclass of SCLC, were obtained from Adi F. Gazdar and colleagues (National Cancer Institute, Bethesda), who originally established these and other SCLC cell lines (7, 8). The cells were maintained in RPMI medium 1640 containing 10 nM hydrocortisone, 5.0 μg/ml insulin, 10 μg/ml transferrin, 10 nM 17β-estradiol, 30 nM sodium selenite, 100 units/ml penicillin, and 100 μg/ml streptomycin (HITES medium; ref. 9). As a control, the SCLC cell line NCI-H128 (American Type Culture Collection), which lacks VIP receptors (10), was also tested. The cells were incubated in tissue-culture flasks in a humidified atmosphere of 5% CO2 in air at 37°C and grown as floating cell aggregates. All chemicals needed for cell culture were purchased from Sigma.
Cell Counts.
Cells (2.0 × 104 per ml) in the logarithmic growth phase were harvested and plated into 24-well cluster plates in 2.0 ml of HITES medium. VIP was added to four final concentrations from 1.0 nM to 1.0 μM. In other experiments, the structurally related peptide glucagon was added to the same final concentrations, and, in a third group of experiments, only diluent was added. Both VIP and glucagon were stored in 0.01 N acetic acid at −80°C before use to minimize proteolytic degradation. Different concentrations of the peptides were prepared in the culture medium and then added to the plates.
To assess the role of cAMP in inhibiting the proliferation of SCLC cells, NCI-H345 cells were incubated with forskolin (100 nM to 100 μM), isobutyl methylxanthine (IBMX; 1.0 μM to 1.0 mM), or either of these agents together with 1.0 μM VIP. To examine the role of protein kinase A in mediating the action of cAMP, we tested the effect of four concentrations (1.0 nM to 1.0 μM) of KT5720, a selective inhibitor of this enzyme, on the inhibition of cell proliferation by VIP (1.0 μM). The culture medium was partially (1.0 ml) replaced on the second day with fresh medium containing freshly prepared peptides, forskolin, IBMX, or KT5720. The viable cells (those not stained by trypan blue) were counted in triplicate on the fourth day with a hemocytometer.
[3H]Thymidine Incorporation.
Cultured NCI-H345 cells (5.0 × 104 in 180 μl of HITES medium) were seeded into a 96-well plate. VIP was added to the wells in 25 μl of HITES medium to achieve final concentrations in the wells of 1.0 nM to 1.0 μM. Forskolin or IBMX was added in 25 μl of HITES medium in four concentrations of 100 nM to 100 μM or 1.0 μM to 1.0 mM, respectively, with or without 1.0 μM VIP. After a 20-h incubation at 37°C, 25 μl of [methyl-3H]thymidine solution [DuPont/NEN; 5.0 Ci/mmol, originally in ethanol/water (1:1, vol/vol), 0.1 μCi per well] was added to each well. The cells were harvested 4 h later on a filter paper by exhaustive elution with PBS and exposed to ice-cold 10% trichloracetic acid in a semiautomatic cell harvester. The dried filter was counted in triplicate in a liquid scintillation counter.
Intracellular cAMP Levels.
NCI-H345 cells (1.0 × 106 per ml) were incubated in 1.0 ml of DMEM, pH 7.3, containing 2% BSA and four concentrations of forskolin or IBMX in the same concentration range as described above, with or without 1.0 μM VIP (4). After incubation for 10 min at 25°C, 1.0 ml of methanol was added to stop the reaction and extract cAMP. Cell samples were then sonicated and centrifuged for 5 min at 3,000 × g. The pellet was washed with 1.0 ml of methanol, and the washings were added to the supernatant of each sample. The combined solution was evaporated to dryness, and the residue was dissolved in 0.5 ml of cAMP assay buffer. After centrifugation, supernatants were assayed for cAMP in triplicate by radioimmunoassay, with the use of a RIANEN kit from DuPont/NEN.
In Vivo Experiments.
Female athymic BALB/c nude mice, 4–5 weeks old, were housed in filter-top cages in a pathogen-free, temperature-controlled, laminar-flow, filtered-air, isolated room and were exposed to light from 7:00 a.m. to 7:00 p.m. NCI-H69 cells (1.0 × 107) were injected subcutaneously into the right flank of each mouse. There were four experimental groups, of four mice each, three of which received VIP (1.0, 5.0, or 10 μg/day) in PBS; as a control, the fourth received only PBS. All solutions were infused for 8 weeks, beginning 1 week after injection of the cells, and delivered by Alzet osmotic pumps (model 2002; Palo Alto, CA) placed aseptically under the skin of the back of the mice. The pump released its contents at a rate of 0.5 μl/h for a duration of 2 weeks. The spent pumps were removed every 2 weeks, and new pumps, containing fresh solutions, were implanted (11); this procedure was repeated three times. The tumors were measured with calipers, and the mice were weighed weekly for 8 weeks. Tumor volume was calculated for an ellipsoid as maximal length × maximal height × maximal width × π/6. On the last day of the experiment, blood was sampled from the retroorbital plexus into chilled heparin-containing tubes rinsed with 0.05% NaEDTA and containing three protease inhibitors, 10 μg/ml soybean trypsin inhibitor, 100 TIU/ml aprotinin, and 10 μg/ml phosphamidon; all from Sigma), as well as 0.1 mM IBMX for measurement of plasma VIP and cAMP levels. The mice were then euthanized. The tumors were excised, weighed, and frozen in liquid nitrogen for subsequent extraction (in methanol) and for measurement of protein content (12); a portion of the tumor was fixed in 10% neutral buffered formalin for morphologic examination.
In another experiment, six groups of mice received, via two pumps in each mouse, 123 μg/day of forskolin in dimethyl sulfoxide (DMSO) and PBS (n = 6), 0.5 μg/day of VIP in PBS and DMSO (n = 5), 1.0 μg/day of VIP in PBS and DMSO (n = 6), 0.5 μg/day of VIP in PBS and forskolin in DMSO (n = 5), 1.0 μg/day of VIP in PBS and forskolin in DMSO (n = 7), or only PBS and DMSO daily (n = 6) as a control group. The pumps were replaced every 2 weeks, as described above. The tumors were measured with calipers, and the mice were weighed weekly for 8 weeks. On the last day of the experiment, blood was sampled, and the tumors were weighed and then frozen in liquid nitrogen.
Statistical Analysis.
Data are expressed as mean ± SD. Statistical differences were examined by ANOVA and Tukey’s protected t test. In vivo data were transferred to the logarithmic scale before statistical analysis.
RESULTS
In Vitro Experiments.
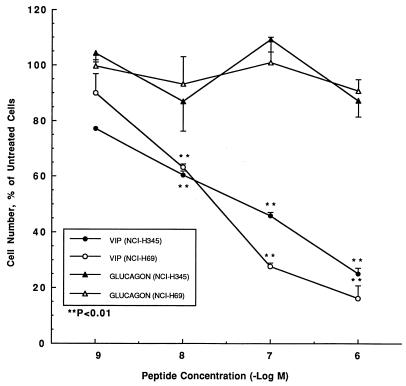
Selective, dose-dependent inhibition of cell proliferation by VIP. In concentration-response experiments limited to 4 days, VIP dose-dependently inhibited the basal increase of NCI-H69 and NCI-H345 cell counts, respectively, by up to 84% and 75%, over a concentration range of from 1.0 nM to 1.0 μM (Fig. 1). Equimolar concentrations of glucagon did not affect the cell count of either cell line. In addition to inhibiting cell counts, VIP also dose-dependently reduced [3H]thymidine incorporation into NCI-H345 cells by 23% at 1.0 nM, 31% at 10 nM, 44% at 100 nM, and 45% at 1.0 μM (data not shown in figure).
Figure 1.
VIP dose-dependently inhibits NCI-H345 and NCI-H69 cell proliferation in culture, whereas glucagon does not. The cells (2.0 × 104 per ml) were cultured for 4 days in the presence of between 1.0 × 10−9 and 1.0 × 10−6 M VIP (NCI-H345, closed circles, and NCI-H69, open circles) or glucagon (NCI-H345, closed triangles, and NCI-H69, open triangles). ∗∗, P < 0.01 vs. untreated cells.
Enhancement of inhibition by other cAMP-promoting agents.
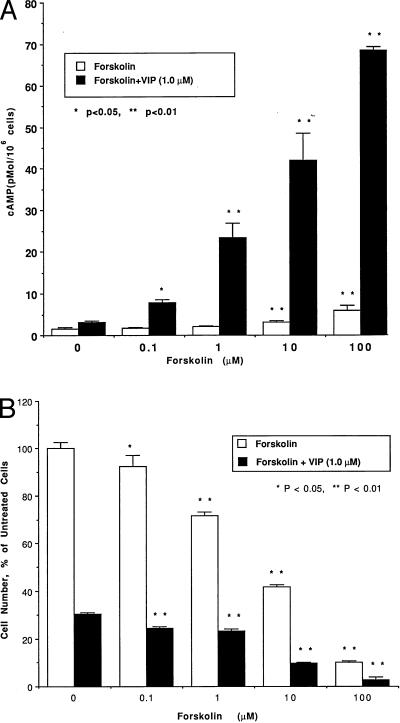
In experiments designed to clarify the relationship between the inhibition of cell proliferation and intracellular cAMP levels, NCI-H345 cells were incubated with VIP (1.0 nM to 1.0 μM), with forskolin (100 nM to 100 μM), with IBMX (1.0 μM to 1.0 mM), or with forskolin or IBMX together with 1.0 μM VIP. In these experiments, we measured cAMP content, cell counts, and [3H]thymidine incorporation. VIP dose-dependently elevated intracellular cAMP levels by 103% at 1.0 nM, 149% at 10 nM, 188% at 100 nM, and 200% at 1.0 μM. The cAMP elevation caused by VIP alone was small and transient, probably because cAMP is metabolized quickly by phosphodiesterase. Alone, forskolin dose-dependently elevated intracellular cAMP levels by up to 290% (Fig. 2A); combined with VIP, it stimulated cAMP production by up to 2120% (Fig. 2A). Forskolin dose-dependently reduced the increase in NCI-H345 cell counts by up to 90% (Fig. 2B); combined with 1.0 μM VIP, it reduced the cell counts by up to 97% (Fig. 2B). Forskolin dose-dependently reduced [3H]thymidine incorporation into NCI-H345 cells by up to 36% (Fig. 3); combined with 1.0 μM VIP, the inhibition of [3H]thymidine incorporation reached 58% (Fig. 3). IBMX alone increased cAMP levels by 75%. In combination with VIP, the effects closely paralleled those of VIP and forskolin on cAMP, cell counts, and [3H]thymidine incorporation into NCI-H345 cells. The inhibition of cell counts and [3H]thymidine incorporation correlated highly with the increase in intracellular cAMP levels (r = 0.97 and 0.98 for cell counts and [3H]thymidine incorporation with forskolin and VIP, respectively, and r = 0.995 and 0.96 with IBMX and VIP, respectively).
Figure 2.
(A) Dose-related stimulation of cAMP production in NCI-H345 cells by forskolin alone (0.1–100 μM) or combined with 1.0 μM VIP. The cells (1.0 × 106 per ml) were incubated for 10 min in the presence of forskolin alone (open bars) or with VIP (solid bars). ∗, P < 0.05 and ∗∗, P < 0.01 vs. values in absence of forskolin. (B) Dose-related inhibition of NCI-H345 cell proliferation by forskolin alone (0.1–100 μM), or combined with 1.0 μM VIP. The cells (2.0 × 104 per ml) were cultured for 4 days in the presence of forskolin alone (open bars) or with forskolin and VIP (closed bars). ∗, P < 0.05 and ∗∗, P < 0.01 vs. values without forskolin.
Figure 3.
Dose-related inhibition of [3H]thymidine incorporation into NCI-H345 cells by forskolin alone (0.1–100 μM) or combined with 1.0 μM VIP. The cells (5.0 × 104 per well) were incubated for 24 h in the presence of forskolin alone (open bars) or of forskolin and VIP (solid bars). ∗, P < 0.05 and ∗∗, P < 0.01 vs. values without forskolin.
Role of protein kinase A.
To examine the role of protein kinase A in inhibiting the proliferation of SCLC cells, NCI-H345 cells were incubated for 4 days with KT5720, a selective inhibitor of this enzyme, with or without VIP. Combined with 1.0 μM VIP, KT5720 (1.0 nM to 1.0 μM) dose-dependently reversed the inhibition of NCI-H345 cell counts by VIP, abolishing it at a concentration of 1.0 μM (Fig. 4). The same concentrations of KT5720 alone did not alter the cell counts.
Figure 4.
Dose-related attenuation by KT5720 (0.001–1.0 μM) of the inhibition of NCI-H345 cell proliferation by 1.0 μM VIP. The cells (2.0 × 104 per ml) were cultured for 4 days in the presence of KT5720 alone (open bars) or of KT5720 and VIP (solid bars). ∗∗, P < 0.01 vs. values without KT5720.
In Vivo Experiments.
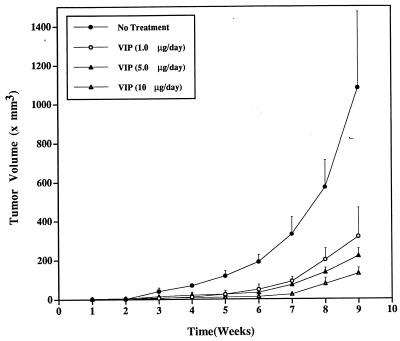
Growth of tumor-cell implants and suppression by VIP. The injected SCLC cells grew into palpable tumors in the nude mice within 1 week; see Figs. 5 and 6. In mice treated with buffer only, the tumors grew exponentially, increasing in volume during weeks 1 to 9 from 1.5 mm3 to 1100 mm3. In mice treated with VIP (1.0, 5.0, or 10 μg/day), the rate of tumor growth was dose-dependently slowed by up to 80% at week 5 in the 1.0 μg/day group, by up to 84% at week 6 in the 5.0 μg/day group, and by up to 95% at week 6 in the 10 μg/day group. The maximal tumor suppression rate at week 9 was 88%, at 10 μg/day. Tumor volume was significantly smaller in all VIP-treated mice than in PBS-treated mice after week 4. After week 6, tumor volume was significantly smaller in mice treated with 10 μg/day of VIP than in those treated with 1.0 μg or 5.0 μg/day of VIP but was not different between mice treated with 1.0 μg/day of VIP and those treated with 5.0 μg/day of VIP. Dramatic, dose-dependent suppression of SCLC tumor growth was observed after treatment with any of the doses of VIP, whereas PBS-treated mice experienced rapid tumor growth. Histological examination of the tumors showed no increased necrosis with VIP treatment, suggesting that VIP slowed cell proliferation, rather than causing cell death.
Figure 5.
SCLC tumor growth in nude mice and its dose-dependent suppression by VIP. A week after injection of NCI-H69 cells into mice, four groups of four mice each were treated with PBS (closed circles), 1.0 μg/day of VIP (open circles), 5.0 μg/day of VIP (closed triangles), or 10 μg/day of VIP (open triangles), via Alzet osmotic pumps. Tumor volume was measured at weekly intervals, as described in Materials and Methods. For analysis of results, please see text.
Figure 6.
Representative nude mice 9 weeks after implantation of NCI-H69 cells and treatment for 8 weeks with PBS (mouse A), 1.0 μg/day of VIP (mouse B), 5.0 μg/day of VIP (mouse C), and 10 μg/day of VIP (mouse D). The largest tumor is seen in mouse A, with progressively smaller tumor masses in the mice treated with increasingly larger doses of VIP (mice B–D). The cylindrical object under the skin of the back is the infusion pump (Alzet model 2002). (Bar = 10 mm.)
Potentiation of VIP effect by forskolin.
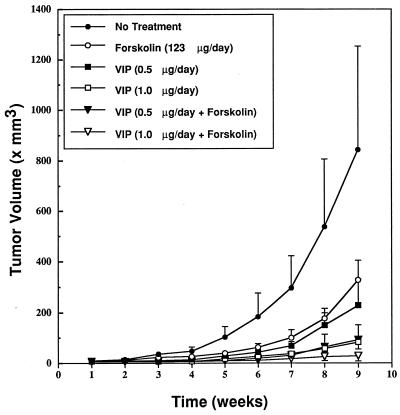
As in the cultured cells in vitro, the inhibitory effect of VIP on the growth of SCLC in nude mice was enhanced by forskolin; see Fig. 7. In PBS and DMSO-treated mice, the tumor volume increased exponentially during weeks 1–9, from 11 mm3 to 840 mm3. In mice treated with 123 μg/day of forskolin, the rate of tumor growth was slowed by up to 70% at week 6 and 62% at week 9 (P < 0.01). In mice treated with either 0.5 or 1.0 μg/day of VIP, the rate of tumor growth was dose-dependently slowed by up to 81% at week 6 in the 0.5 μg/day group and by up to 91% at week 9 in the 1.0 μg/day group (P < 0.01). Maximal tumor-suppression rate at week 9 was 91% at the 1.0 μg/day dose level. In mice treated with the combination of VIP (0.5 or 1.0 μg/day) and 123 μg/day of forskolin, the rate of tumor growth was attenuated by up to 96% at week 5 and 97% at week 9. Tumor volume was significantly smaller in all VIP- or forskolin-treated mice than in PBS and DMSO-treated mice after week 4 (P < 0.01); after week 4 (except for week 6) tumor volume in mice treated with 1.0 μg/day of VIP was significantly smaller than in mice treated with 0.5 μg/day of VIP (P < 0.05). After week 5, tumor volume was significantly smaller in mice treated with VIP (0.5 or 1.0 μg/day) and forskolin than in mice treated with VIP alone (P < 0.01); after week 7, tumor volume was smaller in mice treated with 1.0 μg/day of VIP and forskolin than in those treated with 0.5 μg/day of VIP and forskolin (P < 0.05).
Figure 7.
SCLC tumor growth and its potentiated suppression by combined treatment with VIP and forskolin. The experiment was designed as described for Fig. 5, except that six treatment groups were examined: buffer only (closed circles), forskolin (123 μg/day, open circles), VIP (0.5 μg/day, closed squares), VIP 1.0 μg/day, open squares), VIP (0.5 μg/day) and forskolin (closed triangles), and VIP (1.0 μg/day) and forskolin (open triangles). For analysis of results, please see text.
Treatment with 1.0, 5.0, and 10 μg/day of VIP, dose-dependently decreased tumor weights from 617 ± 233 g (buffer only), respectively to 229 ± 91.8, 155 ± 20.1, and 91.5 ± 15.7 g (P < 0.05 or < 0.01 among groups). At the same time, tumor protein content decreased dose-dependently from 49.3 ± 15.6 mg (buffer only), respectively to 24.0 ± 8.16, 16.0 ± 1.16, and 9.53 ± 1.77 mg (P < 0.05 or < 0.01 among groups). In another group of experiments, tumor weights decreased from 484 ± 227 (buffer only) to 231 ± 52.0 g with 123 μg/day of forskolin, 157 ± 67.6 g with 0.5 μg/day of VIP, 58.5 ± 16.2 g with 1.0 μg/day of VIP, 64.8 ± 38.6 g with 0.5 μg/day of VIP and forskolin, and 19.6 ± 12.8 g with 1.0 μg/day of VIP and forskolin (P < 0.05 or <0.01 among groups). In these experiments too, tumor protein content decreased in parallel with tumor weight.
Plasma VIP levels.
In the first group of in vivo experiments, plasma VIP levels, determined in blood collected 14 days after the last pump replacement (n = 4), were 23.9 ± 7.73 pM in mice receiving buffer, and 29.9 ± 3.37 pM, 58.0 ± 4.24 pM (P < 0.01), and 63.6 ± 21.0 pM (P < 0.01) in those treated, respectively, with 1.0, 5.0, and 10 μg/day of VIP. In the second group of experiments, conducted in the same way, plasma VIP levels (n = 5–7) were 27.1 ± 7.61 pM in mice treated with buffer and DMSO, and 27.2 ± 3.07 pM and 58.7 ± 29.8 pM (P < 0.01) in those treated with 0.5 and 1.0 μg/day of VIP, respectively. In the two groups of mice treated with 1.0 μg/day of VIP, tumor growth at week 9 was suppressed by 71% and 91%. The greater inhibition of tumor growth correlated with a higher mean plasma VIP level: 58.7 ± 29.8 pM vs. 29.9 ± 3.37 pM (P < 0.05). Plasma cAMP levels did not increase in mice treated with VIP or with VIP and forskolin.
No change in body weight.
Total body weight was the same in solvent-treated mice as in mice treated with VIP or VIP and forskolin, suggesting that no systemic toxicity was associated with even the highest dose regimen used.
DISCUSSION
Our results show that VIP inhibits the growth and multiplication of human SCLC cells NCI-H345 and NCI-H69 in culture, and markedly suppresses the growth of SCLC tumor-cell implants in vivo. The specificity of this effect is supported by the lack of any inhibition by glucagon, a peptide that is related structurally to VIP. In an earlier study, we found that glucagon failed to compete with VIP binding to SCLC cells (4). However, helodermin, another VIP-like peptide, which binds to helodermin-preferring receptors on NCI-H345 cells (4), inhibited their proliferation(5). By contrast, the proliferation of NCI-H128 cells, which lack VIP receptors (10), was not inhibited by VIP (data not shown).
The proliferation of NCI-H69 cells was inhibited more than that of NCI-H345 cells by VIP alone (Fig. 1). Because the inhibition of proliferation of NCI-H345 cells by VIP was comparable to that by helodermin (5), we focused on these cells in our studies of inhibition in vitro, including cell counting, [3H]thymidine incorporation, and the stimulation of cAMP production. Consistent with an earlier report (13), we were unable to implant NCI-H345 cells in nude mice; therefore, our in vivo experiments were limited to NCI-H69 cells. Both cell lines belong to the classic subclass of SCLC and retain characteristic SCLC morphology as well as amine-precursor-uptake and decarboxylation (APUD) cell characteristics (7, 8).
Apparently, the inhibition of SCLC cell proliferation by VIP and other agents was mediated by increased intracellular cAMP levels. (i) Forskolin and IBMX, which elevated intracellular cAMP content in tumor cells, inhibited SCLC growth and potentiated the inhibitory effect of VIP in vitro and in vivo. (ii) In all instances, the inhibition of SCLC cell counts and of [3H]thymidine incorporation into SCLC cells was highly correlated with augmentation of cAMP levels. (iii) The VIP-induced inhibition of SCLC cell counts was reversed totally by KT5720, a selective inhibitor of protein kinase A. The results further suggest that the activation of protein kinase A is required and may completely account for the antimitogenic effect of VIP on SCLC cell proliferation.
The in vivo inhibition by VIP was evident at picomolar concentrations of plasma VIP, whereas in vitro inhibition required at least 100-fold higher concentrations. Several factors may account for this large difference in effective concentrations. (i) A continuous supply of VIP, as provided in the in vivo experiments, may be essential for delivering inhibitory concentrations of the peptide to the SCLC cells. (ii) The concentration of VIP within a solid tumor may be variable and impossible to simulate in vitro. (iii) The environment of the cells in vitro may lower their sensitivity to VIP, relative to in vivo conditions. (iv) VIP, given in vivo, may release other, more potent peptides with similar activity, such as the recently proposed activity-dependent neurotrophic peptide, found to possess highly potent neuroprotective properties (14).
For systemic delivery of peptides in vivo, constant infusion by osmotic pumps increases efficacy and reduces side effects relative to bolus injection. Daily intraperitoneal injections over 3 weeks of the d-Phe5-substituted analogue of the neuropeptide substance P had no effect on the growth of human SCLC tumor in nude mice (15). Administered by constant infusion via osmotic pump, however, the analogue inhibited tumor growth (15). Similarly, continuous infusions of several antagonists of substance P were tolerated better in 5-fold higher doses by tumor-bearing nude mice than were bolus injections (16). Further, the constant, low plasma levels obtained with continuous infusion may not be cytotoxic to normal cells (17). The greater therapeutic effect of continuously infused VIP may be caused by a sustained plasma level that suppresses tumor-cell growth but spares normal cells. The absence of any weight loss by the animals in these experiments supports a relatively low systemic toxicity of VIP as administered.
Our results seem to be at variance with recent reports that VIP stimulated colony formation by NCI-H345 SCLC cells (18) and that a VIP antagonist/VIP hybrid inhibited colony formation by NCI-H209 and NCI-H345 SCLC cells (19) and suppressed non-SCLC tumor growth in athymic nude mice (20). In clonogenic assays, however, only a small number of the cells originally seeded into the layer of agarose form colonies in the assay. Plating efficiencies are low in clonogenic assays (21, 22), and proliferation is evaluated from colony-forming cells only, rather than the entire cell population (23). Clonogenic assays may also be affected by a clumping artifact and cell migration (21, 23, 24). Further, no one has described an inhibitory effect of these analogues on the stimulatory action of VIP on SCLC colony formation or a direct stimulation of SCLC xenograft growth by VIP in vivo.
There is a great need for developing new and more successful strategies for the treatment of SCLC. Although it is premature to predict whether VIP will pass the test of clinical trials, our findings suggest that VIP, or a related analogue or peptide mimetic, holds promise as an effective and relatively nontoxic anti-SCLC agent. The SCLC-inhibitory effect of VIP reported here may be similar to that recently reported for cytotoxic analogs of somatostatin (25). Our data have additional implications in light of recent observations on the expression and localization of the human VIP type I receptor (26). This receptor, expressed with highest prevalence in the lung (26, 27), has been localized to the short arm of chromosome 3 (3p22) where allele loss has been linked to SCLC and other cancers (28–30). The marked inhibition of SCLC by VIP is consistent with the view that this chromosomal region contains a functional tumor-suppressor gene and suggests that VIP and its receptor may have a role in regulating the genesis of SCLC malignancy (29, 30).
Acknowledgments
We thank Rosalind Antoniazzi for preparation of the manuscript. This work was supported by National Institutes of Health Grant HL-30450 and by research funds from the Department of Veterans Affairs, for which S.I.S. was a Medical Investigator.
ABBREVIATIONS
- protein kinase A
cAMP-dependent protein kinase
- DMSO
dimethyl sulfoxide
- IBMX
3-isobutyl-1-methylxanthine
- NCI
designator of cell lines developed by the National Cancer Institute
- SCLC
small-cell lung cancer
- VIP
vasoactive intestinal peptide
References
- 1.Chia M M, Gazdar A F, Carbone D P, Minna J P. In: Textbook of Respiratory Medicine. 2nd Ed. Murray J, Nadel J, editors. Philadelphia: Saunders; 1994. pp. 1485–1503. [Google Scholar]
- 2.Cuttitta F, Carney D N, Mulshine J, Moody T W, Fedorko J, Tischler A, Minna J D. Nature (London) 1985;316:823–826. doi: 10.1038/316823a0. [DOI] [PubMed] [Google Scholar]
- 3.Said S I, Mutt V. Science. 1970;169:1217–1218. doi: 10.1126/science.169.3951.1217. [DOI] [PubMed] [Google Scholar]
- 4.Luis J, Said S I. Peptides (Tarrytown, NY) 1990;11:1239–1244. doi: 10.1016/0196-9781(90)90158-2. [DOI] [PubMed] [Google Scholar]
- 5.Maruno K, Said S I. Life Sci. 1993;52:PL267–PL271. doi: 10.1016/0024-3205(93)90640-o. [DOI] [PubMed] [Google Scholar]
- 6.Korman L Y, Carney D N, Citron M L, Moody T W. Cancer Res. 1986;46:1214–1218. [PubMed] [Google Scholar]
- 7.Gazdar A F, Carney D N, Russell E K, Sims H L, Baylin S B, Bunn P A, Jr, Guccion J G, Minna J D. Cancer Res. 1980;40:3502–3507. [PubMed] [Google Scholar]
- 8.Carney D N, Gazdar A F, Bepler G, Guccion J G, Marangos P J, Moody T W, Zweig M H, Minna J D. Cancer Res. 1985;45:2913–2923. [PubMed] [Google Scholar]
- 9.Simms E, Gazdar A F, Abrams P G, Minna J D. Cancer Res. 1980;40:4356–4363. [PubMed] [Google Scholar]
- 10.Shaffer M M, Carney D N, Korman L Y, Lebovic G S, Moody T W. Peptides (Tarrytown, NY) 1987;8:1101–1106. doi: 10.1016/0196-9781(87)90143-4. [DOI] [PubMed] [Google Scholar]
- 11.Szende B, Redding T W, Schally A V. Proc Natl Acad Sci USA. 1990;87:901–903. doi: 10.1073/pnas.87.3.901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lowry O H, Rosebrough N J, Farr A L, Randall R J. J Biol Chem. 1951;192:265–275. [PubMed] [Google Scholar]
- 13.Mahmoud S, Staley J, Teylor J, Bogenden A, Moreau J P, Coy D, Avis I, Cuttitta F, Mulshine J L, Moody T W. Cancer Res. 1991;51:1798–1802. [PubMed] [Google Scholar]
- 14.Brenneman D E, Gozes I. J Clin Invest. 1996;97:2299–2307. doi: 10.1172/JCI118672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Everard M J, Macaulay V M, Millar J L, Smith I E. Eur J Cancer. 1993;29A:1450–1453. doi: 10.1016/0959-8049(93)90019-c. [DOI] [PubMed] [Google Scholar]
- 16.Langdon S, Sethi T, Ritchie A, Muir M, Smyth J, Rozengurt E. Cancer Res. 1992;52:4554–4557. [PubMed] [Google Scholar]
- 17.Siegall C B, Kreitman R J, FitzGerald D J, Pastan I. Cancer Res. 1991;51:2831–2836. [PubMed] [Google Scholar]
- 18.Moody T W, Zia F, Makheia A. Peptides (Tarrytown, NY) 1993;14:241–246. doi: 10.1016/0196-9781(93)90036-g. [DOI] [PubMed] [Google Scholar]
- 19.Moody, T. W., Zia, F., Goldstein, A. L., Naylor, P. H., Sarin, E., Brenneman, D., Koros, A. M. C., Reubi, J. C., Korman, L. Y., Fridkin, M., et al. (1992) Biomed. Res. 13, Suppl. 2, 131–135.
- 20.Moody T W, Zia F, Draoui M, Brenneman D E, Fridkin M, Davidson A, Gozes I. Proc Natl Acad Sci USA. 1993;90:4345–4349. doi: 10.1073/pnas.90.10.4345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grenman R, Burk D, Virolainen E, Buick R N, Church J, Schwartz D R, Carey T E. Int J Cancer. 1989;44:131–136. doi: 10.1002/ijc.2910440123. [DOI] [PubMed] [Google Scholar]
- 22.Tanigawa N, Kern D H, Hikasa Y, Morton D L. Cancer Res. 1982;42:2159–2164. [PubMed] [Google Scholar]
- 23.Selby P, Buick R N, Tannock I. N Engl J Med. 1983;308:129–134. doi: 10.1056/NEJM198301203080304. [DOI] [PubMed] [Google Scholar]
- 24.Sondak V K, Berlelsen C A, Tanigawa N, Hildebrande-Zanki S U, Morton D L, Korn E L, Kern D H. Cancer Res. 1984;44:1725–1728. [PubMed] [Google Scholar]
- 25.Nagy A, Schally A V, Halmos G, Armatis P, Cai R-Z, Csernus V, Kovács M, Koppán M, Szepesházi K, Kahán Z. Proc Natl Acad Sci USA. 1998;95:1794–1799. doi: 10.1073/pnas.95.4.1794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sreedharan S P, Huang J-X, Cheung M-C, Goetzl E J. Proc Natl Acad Sci USA. 1995;92:2939–2943. doi: 10.1073/pnas.92.7.2939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Virgolini I, Raderer M, Kurtaran A, Angelberger P, Banyai S, Yang Q, Li S, Banyai M, Pidlich J, Niederle B, et al. N Engl J Med. 1994;331:1116–1121. doi: 10.1056/NEJM199410273311703. [DOI] [PubMed] [Google Scholar]
- 28.Brauch H, Johnson B, Hovis J, Yano T, Gazdar A, Pettengill O S, Graziano S, Sorenson G D, Poiesz B J, Minna J, et al. N Engl J Med. 1987;317:1109–1113. doi: 10.1056/NEJM198710293171803. [DOI] [PubMed] [Google Scholar]
- 29.Naylor S L, Johnson B E, Minna J D, Sakaguchi A Y. Nature (London) 1987;329:451–456. doi: 10.1038/329451a0. [DOI] [PubMed] [Google Scholar]
- 30.Killary A M, Wolf M E, Giambernardi T A, Naylor S L. Proc Natl Acad Sci USA. 1992;89:10877–10881. doi: 10.1073/pnas.89.22.10877. [DOI] [PMC free article] [PubMed] [Google Scholar]