Abstract
Why do humans survive so long past reproductive age, and why does juvenile mortality decline after birth, both contrary to the classic theory of aging? Previous work has shown formally that intergenerational transfers can explain both these patterns. Here, simulations confirm those results under weaker assumptions and explore how different social arrangements shape life-history evolution. Simulated single-sex hunter–gatherers survive, forage, reproduce, and share food with kin and nonkin in ways guided by the ethnographic literature. Natural selection acts on probabilistically occurring deleterious mutations. Neither stable population age distributions nor homogeneous genetic lineages are assumed. When food is shared only within kin groups, an infant death permits reallocation of its unneeded food to the infant's kin, offsetting the fitness cost of the death and weakening the force of selection against infant mortality. Thus, evolved infant mortality is relatively high, more so in larger kin groups. Food sharing with nonkin reduces the costs to kin of child rearing, but also reduces the resources recaptured by kin after an infant death, so evolved infant mortality is lower. Postreproductive adults transfer food to descendants, enhancing their growth and survival, so postreproductive survival is selected. The force of selection for old-age survival depends in complicated ways on the food-sharing arrangements. Population-level food sharing with nonkin leads to the classic pattern of constant low mortality up to sexual maturity and no postreproductive survival.
Keywords: aging, evolution, life-history theory, parental investment, demography
Human hunter–gatherers lived, foraged, and reared children in small, genetically diverse groups that shared food and many tasks (1–5). These social arrangements must have shaped the evolution of various aspects of human life including senescence and mortality (6–9), but the classic evolutionary theories of senescence based on mutation accumulation (10–12) or antagonistic pleiotropy (10, 11) largely ignore parental investment and other social arrangements, focusing instead on selection pressures shaped by individual fertility and mortality.
In the antagonistic pleiotropy theory, genes that have deleterious effects at older ages may nonetheless be selected because they also have beneficial effects at younger ages, which matter more for reproductive fitness (10, 11), leading to population aging. Such pleiotropic effects can arise by the chance association of age-related traits on genes or, alternatively, through biological tradeoffs between investing in early reproduction versus maintenance and survival. In optimal life-history theory, such tradeoffs are modeled explicitly (13).
The other leading approach, taken here, is mutation accumulation theory. Deleterious mutations have age-specific effects, and the force of selection against each is proportional to the share of lifetime reproduction remaining after the age affected, as shown by Hamilton (12, 14). This share declines from 1.0 at sexual maturity to zero when fertility ceases (approximately age 50 in humans). Consequently, in Hamilton's classic theory, mortality is predicted to be low and constant until sexual maturity and then to rise with age until menopause, when it increases explosively—precluding postreproductive survival (12).
Contrary to this theory, human female hunter–gatherers lived, on average, 15 or 20 years after reproduction, and their infant mortality declined strongly from initially high levels (15–17). Lee (18) formalized earlier ideas (11, 12, 19, 20) by incorporating intergenerational transfers into the classic theory, which then explains both postreproductive survival and declining juvenile mortality. Lee showed that, with intergenerational transfers, including parental investment in offspring after birth, the force of selection against a mutation that raises mortality at any age is a weighted average of the Hamilton effect [remaining reproduction after age a, F(a)] and a new intergenerational transfer effect [remaining net transfers after age a, T(a)]. If a species makes no transfers after birth, then the weight on F(a) is 1.0 and on T(a) is 0, and the classic theory holds. If a species has evolved to the optimal quantity–quality tradeoff for offspring, then the weight on F(a) is 0 and on T(a) is 1.0.
Lee's (18) analysis compared steady states for genetically homogeneous single-sex lineages. The ethnographically informed microsimulations of human hunter–gatherer evolution in this article are a concrete application of the theory, modeling the evolution of human mortality for a single-sex population living in small food-sharing groups within which intergenerational transfers take place. The simulations do not require genetic lineages or stable population age distributions, and explicitly include mutation and genetic heterogeneity [supporting information (SI) Table S1].
The formal details of the simulation setup are described in Methods. Additional details and annotated computer code written in the software language R (21) are in SI Text. The following paragraphs summarize the setup.
The simulation proceeds in 5-year cycles of age and time for at least 15,000 cycles (75,000 years). Births, deaths, and mutations are stochastic: A probability for each event and individual is calculated from available information, and the actual outcome depends on random draws. Production and consumption are deterministic, although they depend on prior demographic outcomes that are themselves stochastic. There are 16 age groups, each spanning 5 years.
Daughters inherit their mother's genes, subject to probabilistic mutations in each generation. Rules, parameters, and initial age schedules are based on historical and ethnographic studies. Initial age patterns of fertility, production, and consumption are set at observed hunter–gatherer levels (16, 22, 23) and imply transfers from adults to children. Transfers, food sharing, and living arrangements are taken as given, and the focus is on simulated mortality.
By a “matriarchy,” I mean all individuals descended from a single living female, for example a grandmother, her two daughters, and their children. When the grandmother dies, this matriarchy becomes two new ones. Matriarchies may belong to larger kin groups defined by a specified maximum number of generations back to a common ancestor. K5 groups comprise related individuals up to third cousins (common great-great-grandmother), K4 up to second cousins, and K3 up to first cousins.
Food sharing is assumed to take place within these kin groups but in some specifications also within larger sharing groups that contain a number of different kin groups. Based on Binford's (1) analysis of ethnographic studies of 339 hunter–gatherer populations, I assume that a sharing group fissions if it grows beyond 25 members and fuses with another if it falls below 8 members. Ethnographic studies (2, 3) guide a variety of experimental assumptions about food-sharing behavior (SI Text), in which each kin group puts a specified share of its output into a common pool for the larger sharing group.
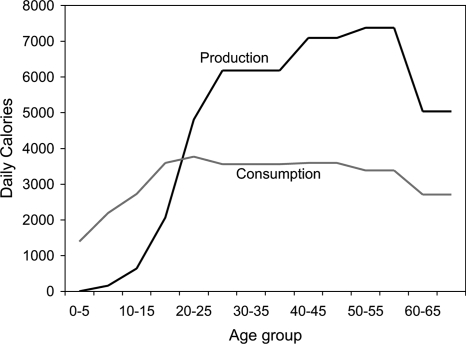
Empirical age profiles of consumption and production averaged across the sexes for three Amazon Basin hunter–gatherer/horticulturalist groups (22, 23) are plotted in Fig. 1. The simulation modifies these age profiles to reflect varying circumstances in ways I will now describe.
Fig. 1.
Per capita production and consumption by age (weighted average for three Amazon Basin hunter–gatherer groups, sexes combined) Modified from Kaplan (20) as in Lee (19). Note the long duration of juvenile dependence and the continuing production of surplus in old age.
I assume that competition for resources takes place among the total population of all sharing groups. Accordingly, in the simulation, an individual's baseline production as shown in Fig. 1 varies inversely with population size. I assume that higher consumption in childhood (age 0–4) raises an individual's adult productivity, perhaps by increasing body size or cognitive function, with 10% more childhood consumption causing 5% higher adult production. Higher consumption in the previous period also raises an individual's current productivity by providing more energy for foraging, again with 10% higher previous cycle consumption raising current productivity by 5% (Eq. 2 shows these three adjustments).
At the start of a simulation cycle, we know for each individual both the level of her consumption in the preceding cycle and the level when she was age 0–4 as well as the size of the total population summed across all groups and individuals. Based on this information, we can calculate the level of production for each individual in the current cycle as described in the previous paragraph. By summing across individuals in a group, we can also find the total level of production in that group. This must exactly equal total consumption in each closed group, because I assume that no output is saved or wasted. This total consumption is allocated to individuals at each age in proportion to the baseline consumption schedule shown in Fig. 1. The parameter γj for group j is the multiplier that makes the balance identity hold (Eq. 3), corresponding to the γ parameter in Lee (18). In this way, consumption for every individual is determined for this cycle, and it influences production by this individual in subsequent cycles. Under a 50% sharing assumption, an individual's consumption is governed by the average of these γs for her kin group and her sharing group as detailed in SI Text).
In a given simulation cycle, the net transfer an individual makes or receives is her production minus her consumption. The average value in the population of these net transfers for individuals of each age, together with demographic variables, can be used to calculate T(a) (Eq. 4), the expected net transfers remaining after age a (18).
Baseline fertility follows an empirical age schedule for a contemporary hunter–gatherer group (16). Consumption of food is assumed to affect fertility and mortality and therefore influences reproductive fitness and natural selection. Lacking good estimates for contemporary hunter–gatherers, I turn to historical studies of the effect of grain price variations on fertility and mortality for landless laborers in Europe and Asia (24, 25). I assume that a 10% increase in consumption raises fertility at each age by 10% in the next cycle (Eq. 5) and similarly reduces mortality by 10% (Eq. 6), consistent with the range of historical estimates (SI Text).
Individuals can have zero, one, or more mutant genes affecting mortality for each 5-year age group. All mutations raise the force of mortality at the relevant age by 0.02 deaths per year (0.1 per 5 years) from a baseline of .005 (an assumed 0 baseline makes little difference). This level is adjusted by individual consumption in the previous cycle, as described above. A daughter inherits her mother's genotype, described by the number of mutant genes affecting mortality at each age. However, for each 5-year age span, there is a 0.01 probability at birth that one additional mutation affecting that age span will occur, raising by one the corresponding number of mutations affecting that age span that were inherited from the mother. The probabilities of mutations for each age span are assumed to be equal and independent, and each birth can experience, at most, one mutation for each of the 16 5-year age intervals. Consequently, in the simulations, approximately one-seventh of births experience at least one deleterious mutation (0.149 = 1 − (1 − 0.99)16), below current estimated rates for humans (26, 27).
Before turning to the results, it will be useful to discuss the forces at work in the simulation and how, through natural selection, they generate an age schedule of mortality. In a given group, if the dependency ratio is favorable, then consumption will be high, raising fertility for adults and survival for all, thus raising reproductive fitness. A mutant gene raising postreproductive mortality raises the dependency ratio, thereby reducing consumption and fitness for each individual, including the carrier of the mutation and her relatives, so the mutation will tend to be deselected. The death of a child raises consumption for all in the group including her relatives, and thereby raises their fertility and reduces their mortality, partially offsetting the relatives' direct loss of fitness due to the death. Selection pressure against mortality for older children in whom more has been invested is greater than for newborns, who can be cheaply replaced. Such forces lead to a mutation–selection balance in the simulation at each age in the population (subject to qualifications discussed below), shaping age-specific mortality.
The presence of unrelated individuals in the sharing group dilutes the effect of a mutant gene on its carrier's inclusive fitness, because, through food sharing, all group members bear the cost of a high dependency ratio. However, individuals live in groups with kin, who have a higher probability of carrying this mutation than do nonkin. Other carriers in the same group will include the individual's daughters, granddaughters, and siblings, for example, because related families tend to live together. Individuals who carry this mutation will, on average, have lower fitness than individuals who do not carry it, and the mutation will tend to be selected out of the population, more or less rapidly depending on the age affected.
Results
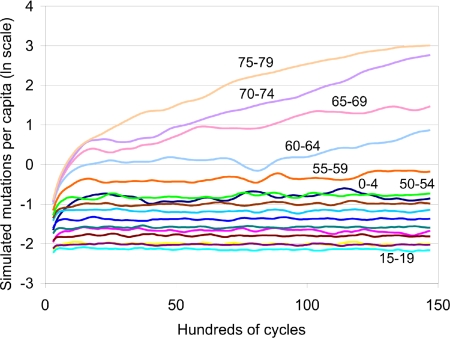
Eventually, the frequency distribution of mutations affecting each age group becomes approximately stochastically stationary, fluctuating about constant values at other than high ages (Fig. 2). At high ages, selection against mutations is weak and Muller's Ratchet (28) eventually leads all individuals to have at least one mutation, then at least two, and so on. This process actually occurs at every age, but at younger ages it occurs so slowly that we can speak of equilibrium. The Ratchet operates in single-sex (haploid) simulations with no back mutation and is therefore not relevant for real humans.
Fig. 2.
The time path of average number of mutations per simulated individual by year of simulation by age affected by mutation (501 point-centered moving average, natural log scale) for matriarchy specification with a population of 100,000. Shown are the first 15,000 cycles or 75,000 years of this simulation. Average frequencies for ages up to 55–59 stabilize by 15,000 years, but above age 60, frequencies keep rising. The ages corresponding to the lowest 10 lines at the far right in ascending order are 15–19, 20–24, 10–14, 25–29, 5–9, 30–34, 35–39, 40–44, 45–49, and 0–4. The other lines are labeled on the figure.
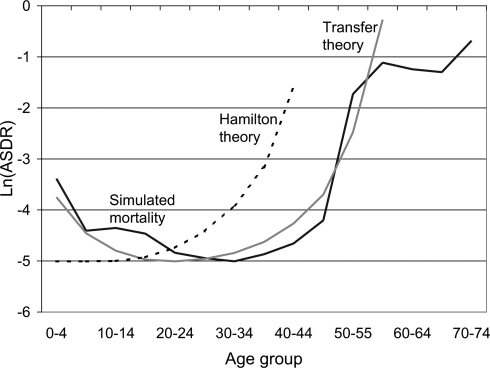
Fig. 3 plots the evolved age-specific mortality for the case of matriarchies that consume all of their own output. Forty-five percent of the simulated matriarchies have only one member, and their average size is 1.9, so their population-age distributions are highly unstable. Because maternal death often entails the death of dependent children, child mortality is elevated, as seen particularly at ages 5–19. The mother of a child age 0–4 is very likely still alive. Nonetheless, the evolved pattern of mortality is close to the prediction of the transfer theory (18) until the oldest ages, with declining juvenile mortality and substantial postreproductive survival, unlike the Hamilton prediction that is also shown. We would not expect an exact fit, because the transfer theory assumes stable populations and genetic homogeneity. SI Text discusses confidence intervals for the simulation.
Fig. 3.
The matriarchal case. The age pattern of evolved mortality after 150,000 years (30,000 simulation cycles) compared with theoretical predictions based on Hamilton (remaining lifetime net fertility) and transfers (remaining lifetime net transfers). The shapes but not levels of theoretical benchmarks are calculated from the simulated fertility, mortality, and transfers: proportional to ln[1/F(a)] for the classic or Hamilton theory and to ln[1/T(a)] for the transfer theory. The simulation line plots the natural log of age-specific death rates over the last 500 cycles of a 30,000-cycle simulation. The simulation line is qualitatively similar to the transfer theory except at the highest ages and different from the Hamilton theory.
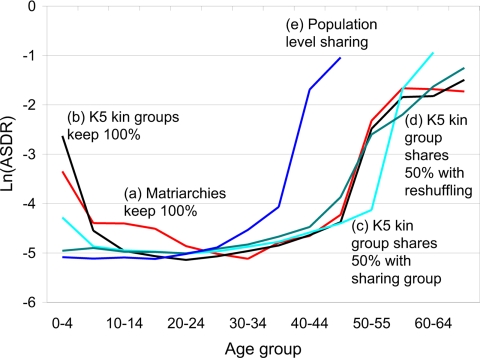
Different social arrangements for living and for sharing food lead to different age patterns of evolved mortality as shown in Fig. 4. Line a repeats the matriarchy case of Fig. 3 for comparison purposes. Line b shows the outcome when individuals live in K5 groups, which consume 100% of the K5 output. These groups have a mean size of 4.6, and only 11% are singleton, so their age distributions are closer to stable. Infant mortality is higher in b than in a because of weaker selection against mutations at earlier ages. It is more likely that an infant who dies in a K5, as opposed to a Matriarchy, has surviving relatives, particularly children, in whom the food that would have been consumed by the dead infant can be invested, partially offsetting the loss. This more common offset in the K5 groups reduces the force of selection against infant mortality relative to the Matriarchies, and hence, mortality at ages 0–4 is higher. If the mother dies in a K5 group, it is more likely that another adult can feed the dependent young, so mortality is lower at ages 5–19. Women after 50 are more likely to have younger relatives in whom to invest, hence selection is stronger against their death, and mortality is lower in b than in a.
Fig. 4.
Evolved mortality depends on food-sharing arrangements. All lines show the natural log of average age-specific death rates (ASDR) over the last 500 cycles of a 15,000-cycle simulation. Each simulation is done under a different assumption about food sharing as discussed and as labeled. “Keeps 100%” means that the kin group does not share at all with a broader sharing group. “Shares 50%” means that the indicated kin group keeps 50% of their food production for consumption within the kin group and puts 50% in the common pool. “Population level sharing” means that all production is put in a common pool for the whole population. “Reshuffling” means the K5 groups are recombined into new sharing groups after each cycle.
Table 1.
Baseline assumptions
| Item | Specification |
| Fertility | Ache, normalized to GRR = 1 (16); Elastm,γ = +1.0 |
| Mortality | Initially 0 until 80, then 1.0; Elastasdr,γ = −1.0; ε = 0.005; δ = 0.1 |
| Production | Average of three hunter–gatherer groups (22, 23); Elasty,γ in childhood = 0.5, in previous period (t − 1) = 0.5 |
| Density dependence | Multiply production age schedule by (E/N)1.0, Elasty,(E/N) = 1.0 |
| Consumption | Base = average of three hunter–gatherer groups (22, 23); Elastc,γ = 1.0 |
| Mutation frequency | For each of 16 ages affected, 0.01 risk per birth. |
| Environment, E | Set for equilibrium population size near 100,000 or sometimes 10,000 |
| Group formation | Fusion if falls below 8, fission if rises above 25, keep kin together at fission, families fuse randomly. |
| Food sharing | Families consume a specified share of their output e.g. 50% and share 50%; this share differs by simulation |
| Age and time | 5-year age groups, 5-year time steps per cycle |
| Sequencing | γ Measured at end of age–time interval |
| Deaths occur at end of interval | |
| Births occur after death at end of interval | |
| Length of simulation | At least 15,000 cycles or 75,000 years; some up to 45,000 cycles |
In c, K5 kin groups live in sharing groups of 8–25 members, and each shares 50% of its output with the sharing group, keeping 50% for its own K5 kin. Child mortality is substantially lower than in a and b. This is not because the larger group size avoids randomly high dependency ratios but is due rather to lower evolved mortality: The number of mutations affecting 0–4 under c is less than 1/10 the number under b. Why? Under c, a child is half supported by the sharing group, which makes child rearing cheaper to its kin group. However, the flip side is that if the child dies, then only half the resources freed by its death are recaptured by its kin group, whereas in a and b all of them can be. This makes its death more costly in c and leads to stronger selection and lower child mortality.
Case d is like c, except now the particular K5 groups combined into the sharing groups are reshuffled after each simulation cycle. This reshuffling would not matter if relatedness within the sharing groups were fully described by the K5 kin structures. Fig. 4 shows that reshuffling does matter, which indicates that the unshuffled groups in c have additional relatedness more distant than the K5 groups but nonetheless important for selection. Opportunities for investment in more distant relatives also explain why mortality at 50–54 is lower in c than in d. In d child mortality is completely flat, whereas postreproductive mortality is similar to a and b. Evidently, reshuffling of groups reduces relatedness enough so that the death of a child is uniformly costly across age, whereas the kin selection benefits of postreproductive survival remain strong.
Finally, in e, all sharing is at the level of the largely unrelated total population, so mortality collapses to the Hamilton case. The fitness effect of an individual's adult survival and released resources after infant death are diluted by 10,000 and are negligible. Large-scale sharing would occur in nature only among closely related populations, so this simulation is presented purely for purposes of contrast and not as a realistic possibility.
More than 100 alternative specifications were investigated. The results are summarized in SI Text. For example, equilibrium mortality should be proportional to the rate at which mutations occur divided by the force of selection against them and independent of the incremental effect of the mutations (14). Experiments confirm these predictions.
It is an important limitation that the model is single-sex with no recombination, but there is nonetheless considerable genetic heterogeneity within kin groups and sharing groups. A daughter has an 85% probability of being genetically identical to her mother [1 − (1 − 0.01)16], because there are 16 age groups, and there is a 0.01 chance for each of these 16 age groups that a new mutation affecting it will occur at birth. Similarly, each birth has a 73% chance of being identical to a sister. First, second and third cousins have probabilities of 53%, 39%, and 28% chance of being genetically identical. On average, a single Matriarchy accounts for only ≈12% of a sharing group's population, and a K5 forms ≈29% of a typical sharing group. “Migration” into sharing groups occurs when one falls below eight members and fuses with another small group, which happens to 3% of groups each cycle, or every 5.5 generations on average. In some simulations, the kin units are reshuffled into new sharing groups every cycle as in d of Fig. 4. Such increased genetic heterogeneity reduces the downward slope of juvenile mortality but has little effect on adult and postreproductive mortality.
Discussion
This analysis provides a framework for applying mutation-accumulation theory to humans and other species that have parental care or are social. However, positively selected beneficial mutations that move the organism toward its optimal life history (29–31) are also important and were not considered here. Elsewhere, Chu, Chien, and Lee (13) formally analyze the role of intergenerational transfers in life-history optimization and reach conclusions that are closely related to those of Lee (18), but the more complex social arrangements considered here would be difficult or impossible to consider through formal analysis of this sort.
The stability assumption has been used in formal analyses of intergenerational transfers not only in Lee (18) but in other treatments as well (13, 29–31). Indeed, it is hard to see how formal analysis of intergenerational energy flows within a social group could proceed without it. Fortunately, we have seen that, for the most part, the nonstable simulations confirm the results based on the stable assumption. However, there are interesting deviations. Age-specific mortality depends on both the cumulated mutations affecting this age and the average value of γ for individuals at this age. The birth of infants testifies to the living presence of their mother in the recent past, but, as children age, the probability that their mother has died also rises. In Fig. 2, we saw that the age affected by the lowest number of mutations was 15–19, but in Fig. 3, we saw that mortality is not particularly low at this age; five age groups have lower. The reason is that, when the mother dies, these children have low consumption and are likely to die as well, despite the low number of mutations affecting this age. In a stable population, this would not happen, because transfers from other ages would make up for the loss of the mother's support. In Fig. 4, the age profile of mortality at these older child ages for the K5 grouping was very low because the K5 age distribution is closer to the stable one.
It is striking that the simulations produce a very human age pattern of mortality, with postreproductive survival and declining mortality after birth, as in Lee (18). But we have been able to go well beyond this important consequence of intergenerational transfers to explore the nuanced effects of variations in social arrangements as described above. These effects depend crucially on the size and depth of the kin group within which sharing occurs, on the degree of interrelatedness within it, and on the extent of sharing with nonkin. The approach could be used to explore many other issues at the interface of culture and evolution.
Methods
The annotated computer code is in SI Text.
For simplicity, equations are given below for the case of 100% food sharing within each group. Differing shares are used in the actual simulations. A γg is calculated for each sharing group as described, and a γf is calculated for each kin group in exactly the same way. The γ for an individual is then βγf + (1 − β)γg, where β is the fraction of food retained in the family (see SI Text).
i,j indexes individual i in group j; x = age. mi,j(x) = fertility of individual; α is its elasticity with respect to γ; μi,j(x) = force of mortality, assumed constant between x and x + 1 = −ln(1 − qi,j(x)), where q is the proportion dying between age x and x + 1, also called asdr (age specific death rate); ξ is the elasticity of mortality with respect to γ; N = total population, summed over all groups j; E = environment; t − 1 = one period ago, otherwise t is suppressed; superscript “child” is average for age 0–4; ci,j(x), yi,j(x) = consumption and production; ĉ(x), ŷ(x) = standard (baseline) consumption and production plotted in Fig. 1; m̂(x) = standard (baseline) fertility for Ache (16); γj = consumption multiplier for group j; τi,j(x) = net transfers = γij(x) − cij(x); T(a) = survival weighted lifetime net transfers after age a; δ = increase in mortality due to a single mutation; ε = background level of mortality not due to mutations; r = the population growth rate; in stochastic equilibrium, this averages 0.
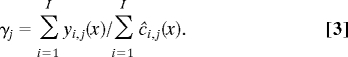 |
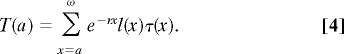 |
Supplementary Material
Acknowledgments.
Timothy Miller and Carl Boe programmed the simulations, contributed to their design, and commented on this paper. Additional advice or comment was received from Hillard Kaplan, Michael Gurven, Tommy Bengtsson, Steve Orzack, Daniel Levitis, Joel Cohen, Samuel Pavard, Brian Charlesworth, and Arthur Robson. Remaining errors are my responsibility alone. This work was supported by National Institute on Aging Grant P01 AG022500.
Footnotes
The author declares no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0710234105/DCSupplemental.
References
- 1.Binford LR. Constructing Frames of Reference. Berkeley, CA: Univ of California Press; 2001. [Google Scholar]
- 2.Gurven M. To give and to give not: The behavioral ecology of human food transfers. Behav Brain Sci. 2004;27:543–583. [Google Scholar]
- 3.Kaplan H, Gurven M. The natural history of food sharing and cooperation: A review and a new multi-individual approach to the negotiation of norms. In: Gintis H, Bowles S, Boyd R, Fehr E, editors. Moral Sentiments and Material Interests: The Foundations of Cooperation in Economic Life. Cambridge, MA: MIT Press; 2005. pp. 75–114. [Google Scholar]
- 4.Mace R, Sear R. Are humans cooperative breeders? In: Voland E, Chasiotis A, Schiefenhövel W, editors. Grandmotherhood: The Evolutionary Significance of the Second Half of Female Life. New Brunswick, NJ: Rutgers Univ Press; 2005. pp. 143–159. [Google Scholar]
- 5.Hrdy SB. Cooperative breeders with an ace in the hole. In: Voland E, Chasiotis A, Schiefenhövel W, editors. Grandmotherhood: The Evolutionary Significance of the Second Half of Female Life. New Brunswick, NJ: Rutgers Univ Press; 2005. pp. 295–317. [Google Scholar]
- 6.Beise J. The helping and the helpful grandmother: The role of maternal and paternal grandmothers in child mortality in the seventeenth- and eighteenth-century population of French settlers in Quebec, Canada. In: Voland E, Chasiotis A, Schiefenhövel W, editors. Grandmotherhood: The Evolutionary Significance of the Second Half of Female Life. New Brunswick, NJ: Rutgers Univ Press; 2005. pp. 215–238. [Google Scholar]
- 7.Lahdenpera M, Lummaa V, Helle S, Tremblay M, Russell AF. Fitness benefits of prolonged post-reproductive lifespan in women. Nature. 2004;428:178–181. doi: 10.1038/nature02367. [DOI] [PubMed] [Google Scholar]
- 8.Pavard S, Sibert A, Heyer E. The effect of maternal care on child's survival: A demographic, genetic and evolutionary perspective. Evolution (Lawrence, Kans) 2007;61:1153–1161. doi: 10.1111/j.1558-5646.2007.00086.x. [DOI] [PubMed] [Google Scholar]
- 9.Hawkes K, Blurton Jones N. Human age structures, paleodemography, and the grandmother hypothesis. In: Voland E, Chasiotis A, Schiefenhövel W, editors. Grandmotherhood: The Evolutionary Significance of the Second Half of Female Life. New Brunswick, NJ: Rutgers Univ Press; 2005. pp. 118–140. [Google Scholar]
- 10.Medawar PB. An Unsolved Problem of Biology. London: Lewis; 1952. [Google Scholar]
- 11.Williams GC. Natural selection, and the evolution of senescence. Evolution (Lawrence, Kans) 1957;11:398–411. [Google Scholar]
- 12.Hamilton WD. The moulding of senescence by natural selection. J Theor Biol. 1966;12:12–45. doi: 10.1016/0022-5193(66)90184-6. [DOI] [PubMed] [Google Scholar]
- 13.Chu CY, Hung-Ken Chien C, Lee RD. Explaining the optimality of U-shaped age-specific mortality. Theor Popul Biol. 2008;73:171–180. doi: 10.1016/j.tpb.2007.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Charlesworth B. Evolution in Age-Structured Populations. Cambridge, UK: Cambridge Univ Press; 1994. [Google Scholar]
- 15.Gurven M, Kaplan H. Longevity among hunter–gatherers: A cross-cultural examination. Pop Dev Rev. 2007;33:1–45. [Google Scholar]
- 16.Hill K, Hurtado AM. Ache Life History: The Ecology and Demography of a Foraging People. New York: Aldine De Gruyter; 1996. [Google Scholar]
- 17.Lee R. In: Between Zeus and the Salmon: The Biodemography of Longevity. Wachter KW, Finch CE, editors. Washington, DC: Natl Acad Sci Press; 1997. pp. 212–233. [PubMed] [Google Scholar]
- 18.Lee R. Rethinking the evolutionary theory of aging: Transfers, not births, shape senescence in social species. Proc Natl Acad Sci USA. 2003;100:9637–9642. doi: 10.1073/pnas.1530303100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fisher RA. The Genetical Theory of Natural Selection. Oxford: Oxford Univ Press; 1930. [Google Scholar]
- 20.Packer C. The ecology of menopause. In: Robine J-M, Kirkwood TBL, Allard M, editors. Sex and Longevity: Sexuality, Gender, Reproduction, Parenthood. Springer: Berlin; 2001. pp. 91–101. [Google Scholar]
- 21.R Development Core Team. R: A Language and Environment for Statistical Computing. Vienna: R Foundation for Statistical Computing; 2008. www.R-project.org. [Google Scholar]
- 22.Lee R. A cross-cultural perspective on intergenerational transfers and the economic lifecycle. In: Mason A, Tapinos G, editors. Sharing the Wealth: Demographic Change and Economic Transfers between Generations. Oxford: Oxford Univ Press; 2000. pp. 17–56. [Google Scholar]
- 23.Kaplan H. Evolutionary and wealth flows theories of fertility: Empirical tests and new models. Pop Dev Rev. 1994;20:753–791. [Google Scholar]
- 24.Bengtsson T, Campbell C, Lee JZ. Life Under Pressure: Mortality and Living Standards in Europe and Asia, 1700–1900. Cambridge, MA: MIT Press; 2004. [Google Scholar]
- 25.Lee R, Steckel RH. Life under pressure: An appreciation and appraisal. Historical Methods. 2006;39:171–176. [Google Scholar]
- 26.Eyre-Walker A, Keightley PD. High genomic deleterious mutation rates in hominids. Nature. 1999;397:344–347. doi: 10.1038/16915. [DOI] [PubMed] [Google Scholar]
- 27.Nachman MW, Crowell SL. Estimate of the mutation rate per nucleotide in humans. Genetics. 2000;156:297–304. doi: 10.1093/genetics/156.1.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gordo I, Charlesworth B. The degeneration of asexual haploid populations and the speed of Muller's Ratchet. Genetics. 2000;154:1379–1387. doi: 10.1093/genetics/154.3.1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chu CYC, Lee R. The co-evolution of intergenerational transfers and longevity: An optimal life history approach. Theor Popul Biol. 2006;69:193–201. doi: 10.1016/j.tpb.2005.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kaplan HS, Robson AJ. The Emergence of Humans: The coevolution of intelligence and longevity with intergenerational transfers. Proc Natl Acad Sci USA. 2002;99:10221–10226. doi: 10.1073/pnas.152502899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Robson AJ, Kaplan HS. The evolution of human life expectancy and intelligence in hunter–gatherer economies. Am Econ Rev. 2003;93:150–169. doi: 10.1257/000282803321455205. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.