Abstract
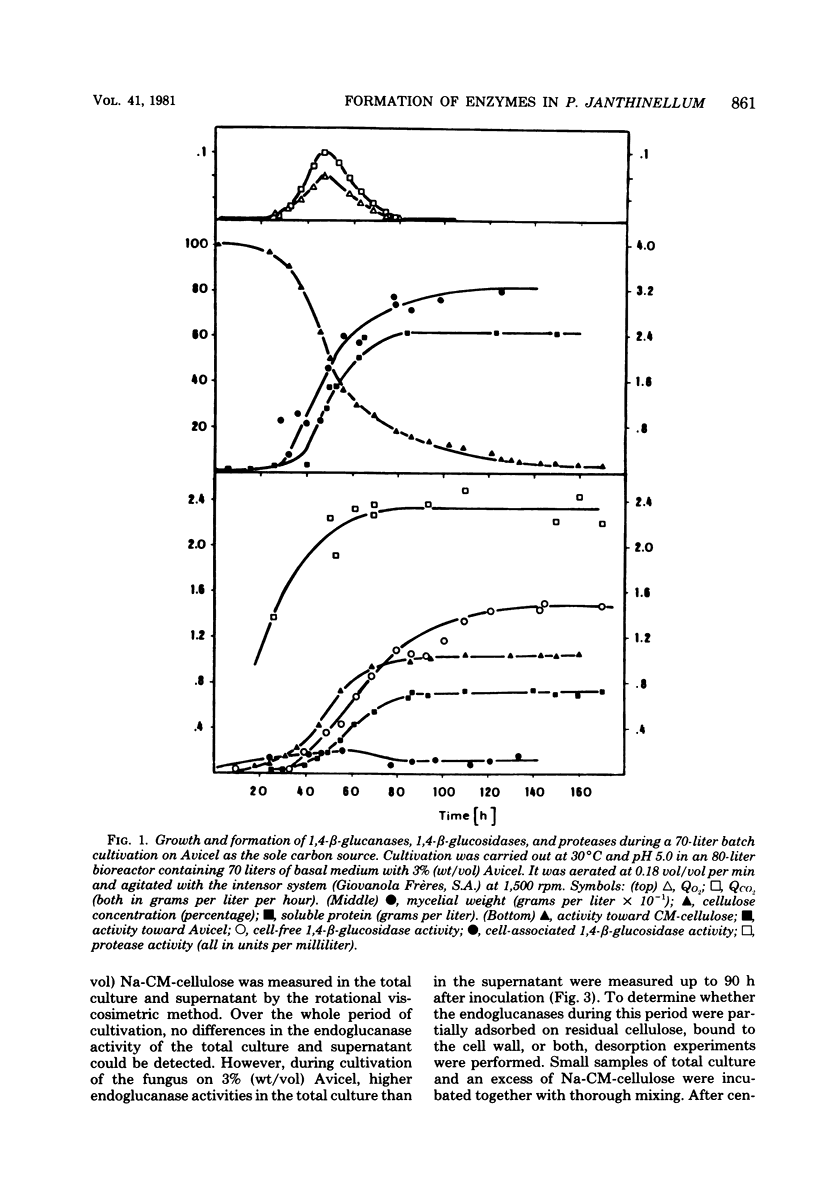
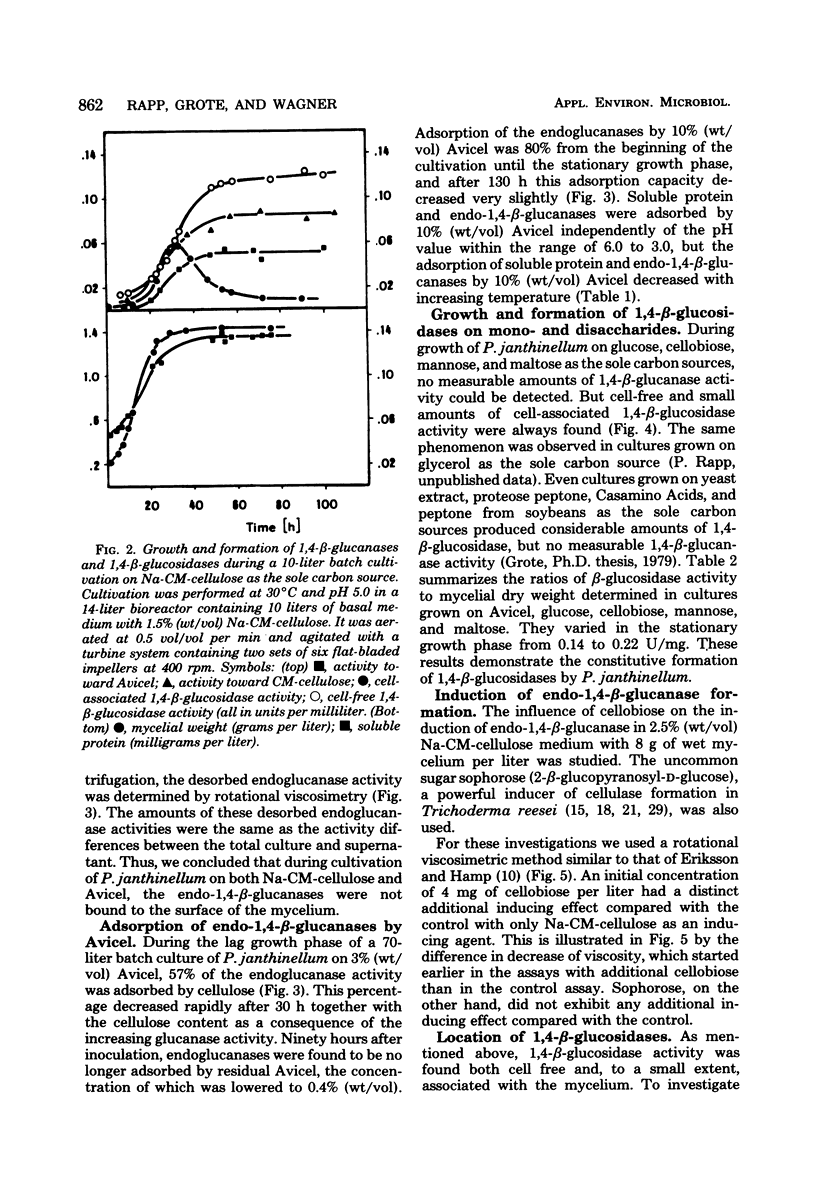
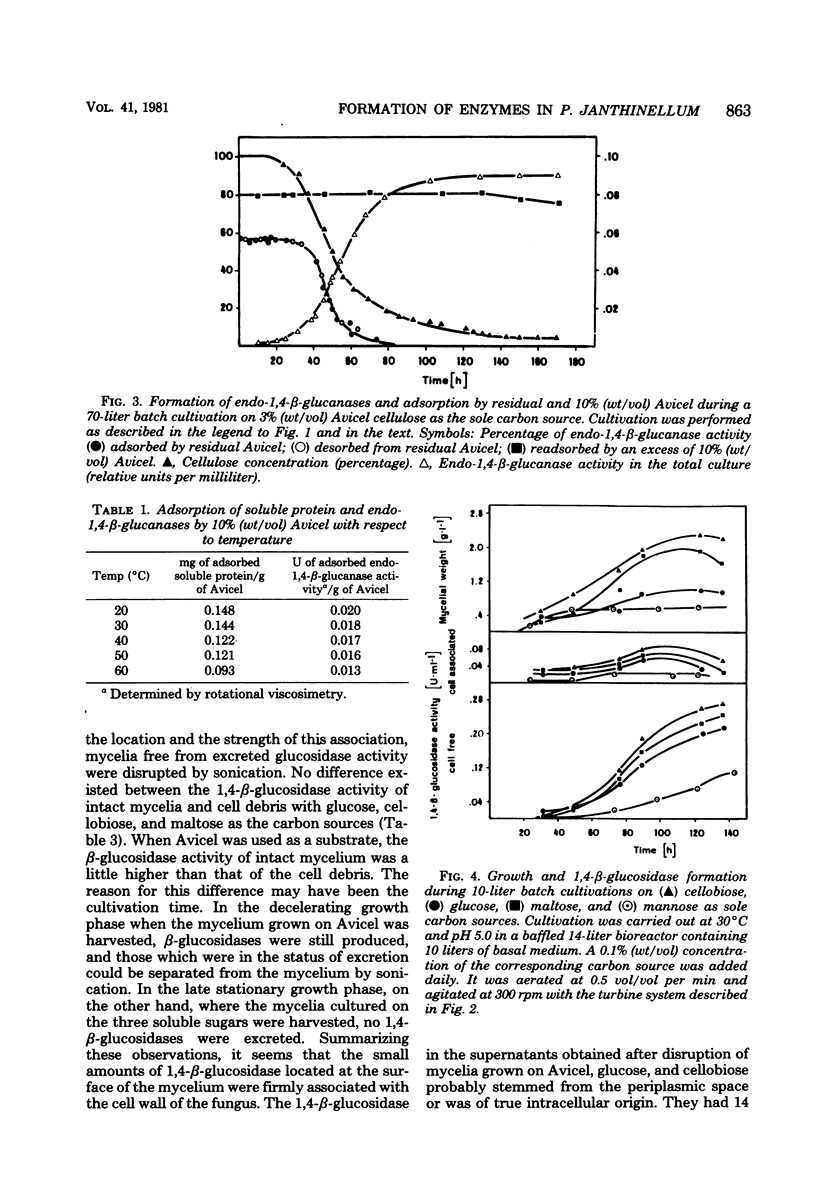
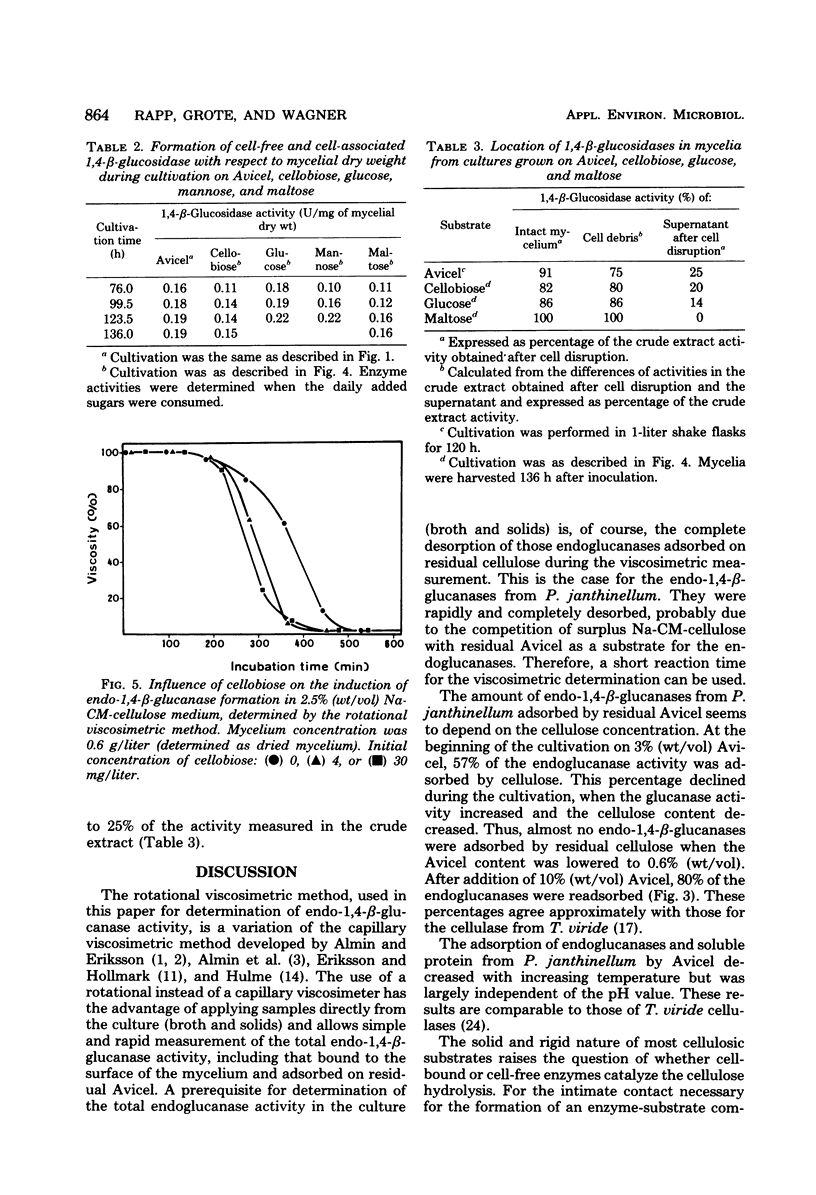
Formation and location of 1,4-β-glucanases and 1,4-β-glucosidases were studied in cultures of Penicillium janthinellum grown on Avicel, sodium carboxymethyl cellulose, cellobiose, glucose, mannose, and maltose. Endo-1,4-β-glucanases were found to be cell free, and their formation was induced by cellobiose. 1,4-β-Glucosidases, on the other hand, were formed constitutively and were primarily cell free, but with a small amount strongly associated with the cell wall. Low 1,4-β-glucosidase activities of periplasmic or intracellular origin were also found. A rotational viscosimetric method was developed to measure the total endo-1,4-β-glucanase activity of the culture (broth and solids). By this method, it was possible to determine the endo-1,4-β-glucanase activity not only in the supernatant of the culture but also on the surface of the mycelium or absorbed on residual Avicel. During a 70-liter batch cultivation of P. janthinellum, the adsorption of endo-1,4-β-glucanases by residual and newly added 10% Avicel was measured. The adsorption of soluble protein and endo-1,4-β-glucanases by Avicel was found to be largely independent of the pH value but dependent on temperature.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Almin K. E., Eriksson K. E. Enzymic degradation of polymers. I. Viscometric method for the determination of enzymic activity. Biochim Biophys Acta. 1967 Jul 11;139(2):238–247. doi: 10.1016/0005-2744(67)90028-9. [DOI] [PubMed] [Google Scholar]
- Almin K. E., Eriksson K. E. Influence of carboxymethyl cellulose properties on the determination of cellulase activity in absolute terms. Arch Biochem Biophys. 1968 Mar 20;124(1):129–134. doi: 10.1016/0003-9861(68)90311-1. [DOI] [PubMed] [Google Scholar]
- Almin K. E., Eriksson K. E., Jansson C. Enzymic degradation of polymers. II. Viscometric determination of cellulase activity in absolute terms. Biochim Biophys Acta. 1967 Jul 11;139(2):248–253. doi: 10.1016/0005-2744(67)90029-0. [DOI] [PubMed] [Google Scholar]
- Berg B., Pettersson G. Location and formation of cellulases in Trichoderma viride. J Appl Bacteriol. 1977 Feb;42(1):65–75. doi: 10.1111/j.1365-2672.1977.tb00670.x. [DOI] [PubMed] [Google Scholar]
- Berghem L. E., Pettersson L. G. The mechanism of enzymatic cellulose degradation. Purification of a cellulolytic enzyme from Trichoderma viride active on highly ordered cellulose. Eur J Biochem. 1973 Aug 1;37(1):21–30. doi: 10.1111/j.1432-1033.1973.tb02952.x. [DOI] [PubMed] [Google Scholar]
- Boretti G., Garofano L., Montecucchi P., Spalla C. Cellulase production with Penicillium iriense (n.sp.). Arch Mikrobiol. 1973 Aug 21;92(3):189–200. doi: 10.1007/BF00411199. [DOI] [PubMed] [Google Scholar]
- Deshpande V., Eriksson K. E., Pettersson B. Production , purification and partial characterization of 1,4-beta-glucosidase enzymes from Sporotrichum pulverulentum. Eur J Biochem. 1978 Sep 15;90(1):191–198. doi: 10.1111/j.1432-1033.1978.tb12590.x. [DOI] [PubMed] [Google Scholar]
- Eriksson K. E., Hamp S. G. Regulation of Endo-1,4-beta-glucanase production in Sporotrichum pulverulentum. Eur J Biochem. 1978 Sep 15;90(1):183–190. doi: 10.1111/j.1432-1033.1978.tb12589.x. [DOI] [PubMed] [Google Scholar]
- Eriksson K. E., Hollmark B. H. Kinetic studies of the action of cellulase upon sodium carboxymethyl cellulose. Arch Biochem Biophys. 1969 Sep;133(2):233–237. doi: 10.1016/0003-9861(69)90450-0. [DOI] [PubMed] [Google Scholar]
- Eriksson K. E., Pettersson G. Studies on cellulolytic enzymes. V. Some structural properties of the cellulase from Penicillium notatum. Arch Biochem Biophys. 1968 Mar 20;124(1):160–166. doi: 10.1016/0003-9861(68)90316-0. [DOI] [PubMed] [Google Scholar]
- Hulme M. A. Viscometric determination of carboxymethylcellulase in standard international units. Arch Biochem Biophys. 1971 Nov;147(1):49–54. doi: 10.1016/0003-9861(71)90308-0. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Loewenberg J. R., Chapman C. M. Sophorose metabolism and cellulase induction in Trichoderma. Arch Microbiol. 1977 May 13;113(1-2):61–64. doi: 10.1007/BF00428581. [DOI] [PubMed] [Google Scholar]
- MANDELS M., PARRISH F. W., REESE E. T. Sophorose as an inducer of cellulase in Trichoderma viride. J Bacteriol. 1962 Feb;83:400–408. doi: 10.1128/jb.83.2.400-408.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MANDELS M., REESE E. T. Induction of cellulase in fungi by cellobiose. J Bacteriol. 1960 Jun;79:816–826. doi: 10.1128/jb.79.6.816-826.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nisizawa T., Suzuki H., Nakayama M., Nisizawa K. Inductive formation of cellulase by sophorose in Trichoderma viride. J Biochem. 1971 Sep;70(3):375–385. doi: 10.1093/oxfordjournals.jbchem.a129652. [DOI] [PubMed] [Google Scholar]
- Nisizawa T., Suzuki H., Nisizawa K. Catabolite repression of cellulase formation in Trichoderma viride. J Biochem. 1972 Jun;71(6):999–1007. doi: 10.1093/oxfordjournals.jbchem.a129872. [DOI] [PubMed] [Google Scholar]
- Olutiola P. O. A cellulase complex in culture filtrates of Penicillium citrinum. Can J Microbiol. 1976 Aug;22(8):1153–1159. doi: 10.1139/m76-167. [DOI] [PubMed] [Google Scholar]
- Peitersen N., Medeiros J., Mandels M. Adsorption of Trichoderma cellulase on cellulose. Biotechnol Bioeng. 1977 Jul;19(7):1091–1094. doi: 10.1002/bit.260190710. [DOI] [PubMed] [Google Scholar]
- Pettersson G., Eaker D. L. Studies on cellulolytic enzymes. IV. Chemical and physiochemical characterization of a cellulase for Penicillium notatum. Arch Biochem Biophys. 1968 Mar 20;124(1):154–159. doi: 10.1016/0003-9861(68)90315-9. [DOI] [PubMed] [Google Scholar]
- Pettersson G. Studies on cellulolytic enzymes. 3. Isolation of a cellulase from Penicillium notatum. Arch Biochem Biophys. 1968 Feb;123(2):307–311. doi: 10.1016/0003-9861(68)90139-2. [DOI] [PubMed] [Google Scholar]
- Smith M. H., Gold M. H. Phanerochaete chrysosporium beta-Glucosidases: Induction, Cellular Localization, and Physical Characterization. Appl Environ Microbiol. 1979 May;37(5):938–942. doi: 10.1128/aem.37.5.938-942.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sternberg D., Mandels G. R. Induction of cellulolytic enzymes in Trichoderma reesei by sophorose. J Bacteriol. 1979 Sep;139(3):761–769. doi: 10.1128/jb.139.3.761-769.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai H., Tsai J. H., Yu P. H. Effects of yeast proteinase and its inhibitor on the inactivation of tryptophan synthase from Saccharomyces cerevisiae and Neurospora crassa. Eur J Biochem. 1973 Dec 3;40(1):225–232. doi: 10.1111/j.1432-1033.1973.tb03190.x. [DOI] [PubMed] [Google Scholar]
- Updegraff D. M. Semimicro determination of cellulose in biological materials. Anal Biochem. 1969 Dec;32(3):420–424. doi: 10.1016/s0003-2697(69)80009-6. [DOI] [PubMed] [Google Scholar]


