Abstract
Background and purpose: The age-related decline in vasorelaxation is largely due to ceramide-induced induction of phosphatase 2A (PP2A), which limits nitric oxide synthase (eNOS) phosphorylation at stimulatory sites. We hypothesized that ceramide accumulation was from an age-related loss of endothelial glutathione (GSH) and subsequent activation of neutral sphingomyelinase (nSMase), an enzyme whose activity increases when GSH is limited.
Experimental approach: Old (30-32 mo) F344xBN rats were given (R)-α-lipoic acid (LA), an agent known to induce GSH synthesis. Vasorelaxation was measured in aortic rings; GSH and ceramide levels, activity of nSMase and eNOS phosphorylation (by Western blot) was measured in aortic endothelial cells, isolated from the same aortas.
Key results: In old animals, endothelium-dependent relaxation in aortic rings was decreased, GSH levels and its redox state in aortic endothelia were over 30% lower and nSMase activity and endothelial ceramide levels were three-fold increased, relative to young (2-4 mo) rats. LA treatment of old animals improved relaxation in aortic rings, reversed the changes in endothelial GSH, in nSMase activities and in ceramide levels. Similar effects on GSH levels and nSMase activity in old rats were also induced by treatment with GSH monoethylester. Activation (by phosphorylation) of eNOS was decreased by about 50% in old rats and this age-related decrease was partially reversed by LA treatment.
Conclusions and implications: Decreased endothelial GSH was partly responsible for the age-related loss of vascular endothelial function and LA might be therapeutically evaluated to treat endothelial dysfunction.
Keywords: ageing, endothelium, endothelial function, lipoic acid, nitric oxide, glutathione
Introduction
Ageing leads to a profound and specific loss of vasodilation in conduit arteries from all mammalian species tested. Much of this decline is due to vascular endothelial cell dysfunction, as many studies now show that relaxation of smooth muscle remains intact with age (Koga et al., 1988; Gerhard et al., 1996). Because endothelial-derived nitric oxide (NO) is the most important vasorelaxing factor produced by the endothelium, research is focusing on the potential role of NO in the age-associated loss of vascular tone. Indeed, NO levels decline in old rats, thus reducing vascular smooth muscle relaxation (Tschudi et al., 1996; Chou et al., 1998; van der Loo et al., 2000; Smith et al., 2006a). Possibly, NO loss is associated with the age-related increases in oxidative stress and conversion of NO to nitrogen species incapable of inducing vasorelaxation. However, several features of rat aortic endothelial nitric oxide synthase (eNOS), including its distribution in the plasma membrane, its phosphorylation state and activity, diminish significantly with age (Smith et al., 2006a, 2006b), greatly limiting NO synthesis and availability as a vasorelaxant. In turn, both lower eNOS activity and NO degradation may be involved in the loss in vascular tone associated with age.
Recently, we observed that the loss of eNOS phosphorylation is related to higher ceramide-activated protein phosphatase 2A (PP2A) activity, which is driven by a marked increase in ceramide in aged endothelia (Smith et al., 2006a). Elevated ceramide levels are consequent on chronic activation of sphingomyelinases with neutral pH optima (nSMase). Although the mechanism(s) leading to nSMase activation is yet to be investigated, decreased glutathione (GSH) levels are known to specifically induce nSMases (Liu and Hannun, 1997; Tsyupko et al., 2001). Notably, nSMase induction and decreased GSH are also associated with heightened levels of inflammatory markers. Bearing in mind that the marked loss in endothelial GSH in aged rat aorta correlates with hyperactivation of nSMase (Smith et al., 2006a), age-related GSH deficits may induce a ceramide overload and eventual downstream decline in eNOS activity.
In light of the indirect, but potent adverse effect of low endothelial GSH concentrations on eNOS activity, increasing GSH concentrations in aged endothelia should limit ceramide-induced PP2A activity, its dephosphorylation of eNOS and, ultimately, impairment of vasomotion. Unfortunately, increasing endothelial GSH through its direct dietary supplementation is impractical owing to limited intact GSH absorption from the gastrointestinal tract and uptake into endothelial cells (Grattagliano et al., 1995). However, agents that induce phase II detoxification genes increase GSH synthetic capacity through heightened expression of γ-glutamylcysteine ligase, the rate-controlling enzyme for GSH synthesis (Griffith, 1999; Kondo et al., 1999). One such inducer of phase II enzymes, (R)-α-lipoic acid (LA), reverses the age-related loss of GSH and its redox status in the heart, liver and brain of old rats (Sen et al., 1997; Packer, 1998; Powell et al., 2001; Bharat et al., 2002; Moini et al., 2002; Suh et al., 2003, 2004). LA, in itself an antioxidant, also protects isolated hepatocytes from oxidative insult from tert-butylhydroperoxide, a model alkylhydroperoxide known to be detoxified in a GSH-dependent manner (Hagen et al., 2000). Hence, we hypothesized that pharmacological treatment with LA may improve endothelial NO-dependent vasomotor function in the elderly via amelioration of endothelial GSH status (Smith and Hagen, 2003).
Materials and methods
All animal procedures were in accordance with the Oregon State University Guidelines for animal experimentation. Fischer 344 × Brown Norway (F344 × BN) rats were used as the experimental animals. This is a well-characterized rat strain that is approved for ageing studies by the National Institutes on Aging (NIH/NIA). Young (2–4 months) and old (32–34 months) rats were used for all studies. Although F344 × BN rats do not develop atherosclerosis, they do exhibit the same age-related decline in vascular function as do humans and other mammalian species (Hynes and Duckles, 1987).
Treatments
(R)-α-lipoic acid (ASTA Medica AG, Frankfurt, Germany) was administered to old rats (40 mg kg−1, i.p.) twice over 24 h. Sequential injections were given at 0 and 12 h, followed by killing (decapitation, after anaesthesia with CO2) and removal of tissues at 24 h. In addition to saline controls, some old rats were given glutathione monoethylester (GE) (40 mg kg−1; Sigma, St Louis, MO, USA), which is a stable, esterified form of GSH known to elevate tissue and cellular GSH levels (Levy et al., 1993; Grattagliano et al., 1995). For studies in which GE was used, a similar dosing and temporal protocol as used for LA was followed.
Aortic ring myography
Segments of thoracic aorta were cleaned of adherent connective tissue, cut into 3–5 mm long rings and suspended in an organ-bath chamber containing Krebs–Henseleit solution (composition, (mM): NaCl, 118.0; KCl, 2.8; KH2PO4, 1.2; MgSO4, 1.2; (+)-glucose, 5.0; NaHCO3, 25.0; CaCl2, 2.5) pH 7.2, gassed with 95% O2 and 5% CO2 and maintained at 37 °C. Tissues were mounted on an isometric force-displacement transducer (Kent Scientific, Torrington, CT, USA) and changes in isometric forces were continuously recorded. Rings were gradually stretched to 1–1.5 g and allowed to equilibrate for 90 min. Maximal contractility was evaluated by the addition of KCl 60 mM. After washing and further equilibration, the rings were contracted with 3 × 10−7 M phenylephrine. After stabilization (10–15 min), relaxation was assessed by the cumulative addition of ACh (10−10–10−4 M). Sodium nitroprusside (10−10–10−5 M) was used to evaluate endothelium-independent vasorelaxation. In some studies, the specificity of ACh-dependent stimulation of endothelial NO was determined by rubbing the vessel ring to denude the endothelium prior to myography.
Preparation of vascular endothelium samples
Freshly isolated aortae from male F344 × BrN rats were perfused with Hank's Buffered Saline Solution, pH 7.4. For some experiments, protease and phosphatase inhibitors (proprietary inhibitor cocktail; Sigma) were included. Endothelia were isolated by freezing and scraping the aorta using a modification of the method described by Ryan and Maxwell (1986). Briefly, the washed and cleaned aortae were opened longitudinally and adhered to poly-L-lysine-coated glass, and then rapidly frozen over liquid nitrogen. After freezing, the endothelial surface was carefully scraped from the luminal surface of the vessel segments with a surgical scalpel and collected into homogenization buffer. Immunostaining (Simmons et al., 2004) was employed to determine that the endothelial isolate was not significantly contaminated with smooth muscle cells. On the basis of positive expression of Factor VIII antigen (von Willebrand's factor) and negligible presence of α-smooth muscle actin, the isolated fraction was comprised of relatively pure aortic endothelium (data not shown).
Glutathione measurement
Endothelial preparations were lysed in 10% (w v−1, final concentration) perchloric acid. The acid-soluble fractions containing GSH and glutathione disulphide (GSSG) were derivatized with iodoacetic acid (40 mM). The resulting carboxymethyl derivatives were further derivatized with dansyl chloride (75 mM) and separated using the method of Jones et al. (1998), by high-performance liquid chromatography using fluorescent detection (Hitachi L7000, San Jose, CA, USA; Ex/Em: 330/515 nm). Quantification was achieved relative to GSH and GSSG standards. γ-Glutamyl-glutamate was used as an internal control to assess the completeness of derivatization.
Sphingomyelinase assay
Endogenous neutral and acidic SMase and ceramidase activities were estimated by incubation of endothelial membrane fractions with fluorescent nitrobenzofuran (NBD)-derivatized substrates (NBD-sphingomyelin, NBD-ceramide; Molecular Probes, Eugene, OR, USA) in vitro. Briefly, endothelial membrane fractions were incubated with either NBD-sphingomyelin or NBD-ceramide in phosphate-buffered saline, pH 7.4, containing 5 mM MgCl for 1 h at 37 °C. The reaction was terminated by addition of ethanol and the resulting solution was separated by high-performance liquid chromatography with fluorescence detection (455/530 nm; Ex/Em). Liberated NBD-ceramide (from SMase activity) and NBD-fatty acid (from ceramidase activity) were quantified according to NBD-ceramide NBD-fatty acid standards. To confirm specificity of the assay, an inhibitor of neutral SMase (GW4869, 50 μM) and acidic SMase (desipramine, 50 μM) was used as negative control.
Ceramide measurement
Free ceramides in the endothelium were estimated using a modification of the DAG kinase assay (Bielawska et al., 2001). Briefly, lipids were extracted from a known amount of endothelial protein and dried under N2. The dried lipids were solubilized by bath sonication in a detergent solution (7.5% (w v−1) n-octyl-β-D-glucopyranoside and 5 mM 1,2-dioleoyl-sn-glycero-3-[phospho-rac-(1-glycerol)]) and incubated with 5 U recombinant bacterial DAG kinase and 4 μCi [γ-32P]ATP for 2 h at 25 °C. The reaction was quenched by addition of ice-cold methanol and the lipids were extracted and dried under N2. The resultant [γ-32P]-labelled phospholipids were separated by TLC. [γ-32P]-labelled 1-phospho-ceramide bands were visualized by autoradiography and scraped from the TLC plates and quantified by scintillation counting. As the DAG kinase reaction is an entirely in vitro method, synthetic C6-ceramide was included in all reactions as an internal standard to control for completeness of the reaction. Endogenous ceramide levels were quantified according to external standards, which consisted of synthetic C16- and C18-ceramides in the DAG kinase reactions, in place of sample lipids.
Immunochemical analysis of endothelial proteins
Vascular endothelium was isolated from young and old rats as described above. Protein homogenates were quantified by a modification of Lowry's method (Peterson, 1977). Samples were separated by sodium dodecyl sulphate polyacrylamide gel electrophoresis and analysed by western blot. The immunodetected proteins (anti-eNOS, anti-phospho-eNOS, anti-Akt, anti-phospho-Akt; Cell Signaling Technology, Beverly, MA, USA; anti-β-actin; Sigma) were quantified by horseradish peroxidase-linked secondary antibodies and subsequent chemiluminescent detection.
Statistical methods and data analysis
Experimental data are presented as the mean±s.d. of the mean unless otherwise stated. For multiple comparisons, experimental means were compared using ANOVA with Bonferroni's post-hoc analysis. Differences were considered significant if P-values were less than 0.05. For graphical representation of some data, box-and-whisker plots were used. The centre line of the box represents the median assay value, the box extends from the 25th to 75th percentile and the bars extend to show the highest and lowest values of the data set.
Results
LA significantly restored the endothelium-dependent vasodilation otherwise lost with age
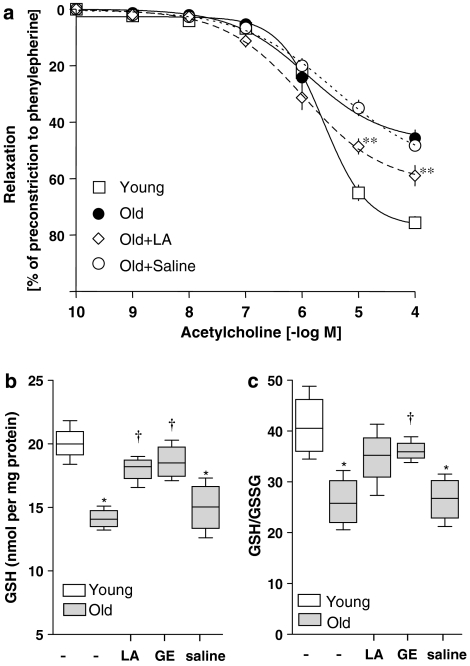
After a period of equilibration and pre-contraction with phenylephrine, dose-dependent relaxation response to ACh was measured. Aortic vessel rings from old rats treated only with saline displayed significant relaxation at 10−7 M ACh, which paralleled the relaxation curve of untreated old animals (Figure 1a). However, in contrast to vessel segments from young rats, which relax to nearly 80% of maximal tension at 10−4 M ACh, relaxation of vessel rings from old rats was severely attenuated and reached only ∼40% at 10−4 M ACh.
Figure 1.
Lipoic acid (LA) partially restored aortic endothelial vasomotor function and glutathione (GSH), which otherwise declines with age. Old rats were treated with either LA (40 mg kg−1 i.p.) or saline vehicle and aortas were isolated 24 h post-treatment. (a) Aortic ring myography was employed to determine endothelium-dependent relaxation in response to ACh. Results showed that maximal relaxation of vessels from old animals was significantly impaired. However, vasodilation in old animals treated with LA was significantly improved compared with untreated or saline-treated old animals (means±s.d.; n=4). (b) GSH levels and the GSH/GSSG ratio were monitored by high-performance liquid chromatography. LA significantly improved the age-associated loss of GSH (n=4 animals per group). (c) Age leads to a significant (P⩽0.05) decline in the endothelial GSH redox ratio (GSH/GSSG), and LA treatment induced a partial recovery of this loss. However, GSH/GSSG ratios were still significantly lower than those seen in young untreated rats. No effect on GSH/GSSG was observed in the saline-injected controls. In (b) and (c), data are shown as box-and-whisker plots; the central line of the box represents the median, the box extends from the 25th to 75th percentile and the bars extend to show the highest and lowest values of the data set. **Statistical significance compared with young, old or old saline-treated animals (P⩽0.05). *Statistical significance compared with young group (P⩽0.01) and †compared with the old (P⩽0.05).
In contrast, vessel segments from LA-treated animals were far more responsive to ACh treatment (Figure 1a). Vessel rings from LA-treated animals relaxed significantly more than those from saline-treated controls and the level of relaxation became significant at ACh doses above 10−6 M. However, LA treatment did not completely reverse the age-related loss of vasomotor function even at the highest ACh dose given.
LA reversed the age-related loss of endothelial GSH status
Figure 1b shows that the GSH content of aortic endothelial cells taken from old rats was nearly 33% less than that of endothelial cells from young rats of the same strain, a significant (P⩽0.05) age-related loss (data shown as box-and-whisker plots). To find out whether dietary agents that induce GSH synthesis in other organs also improve the age-related decline in endothelial GSH and its redox status, old rats were treated with LA. We found that aortic endothelial cells from LA-treated rats displayed significantly higher reduced GSH levels compared with untreated old animals (mean±s.d.; 20.1±0.7 vs 14.1±0.4 nmol per mg protein, respectively). In addition, there was a trend for LA to increase the GSH redox (GSH/GSSG) ratio in old animals (26±2.5 vs 34.8±2.9 in rats treated with or without LA, respectively), but the improvement did not reach statistical significance (Figure 1c).
LA modulated sphingolipid signalling in the aged endothelium
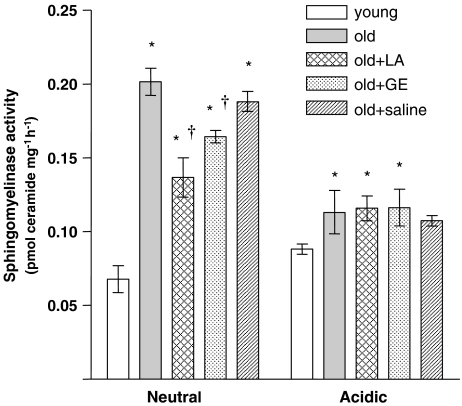
Because pharmacological administration of LA partially reversed the age-associated loss of intracellular GSH, we hypothesized that LA might also ameliorate elevated nSMase activity previously observed in aged endothelium (Smith et al., 2006a), as other studies showed that low GSH levels are a potent trigger for inducing nSMase activity. Age more than doubled endothelial nSMase activity (Figure 2), but nSMase activities in old rats were 30% lower following LA treatment compared with saline-treated controls (Figure 2). However, no age-associated differences in SMase activity with acidic pH optima were evident (Figure 2).
Figure 2.
The role of thiols in sphingomyelinase dysregulation in the aged endothelium. Enzymatic activities of SMases with neutral (nSMase) and acidic (aSMase) pH optima were measured in endothelial preparations from young and old animals, and from old animals treated with (R)-α-lipoic acid (LA), glutathione monoethylester (GE) or saline (n=4 animals per treatment group). For nSMase, enzymatic activity in the old endothelium was nearly three times the activity seen in the young. aSMase activity also increased with age; however, this increase was not as marked as that in nSMase. LA treatment significantly decreased nSMase activity, with no effect on the acidic isoform. GE also lowered nSMase activity, though to a lesser extent than LA. The observation that GE worked in a similar manner to LA suggests that the effects of LA are due to increase in endothelial glutathione. *Statistical significance compared with the young sample group (P⩽0.01). †Statistical significance compared with the old sample group (P⩽0.05).
To determine whether the LA-mediated decline in nSMase activity stemmed from its GSH-inducing action, GE was used as a positive control to directly augment cellular endothelial GSH levels. GE treatment of old rats elevated endothelial GSH over 30% (18.6±0.7 vs 14.1±0.4 pmol per mg protein; Figure 1b) and also increased the GSH/GSSG ratio by 42% relative to old control animals (from 26±2.5 to 36.1±1 pmol per mg protein, Figure 1c). Moreover, nSMase activity fell by more than 25% 24 h after GE administration to old rats relative to saline-treated controls (Figure 2). Like LA treatment, GE did not completely reverse increased nSMase activity.
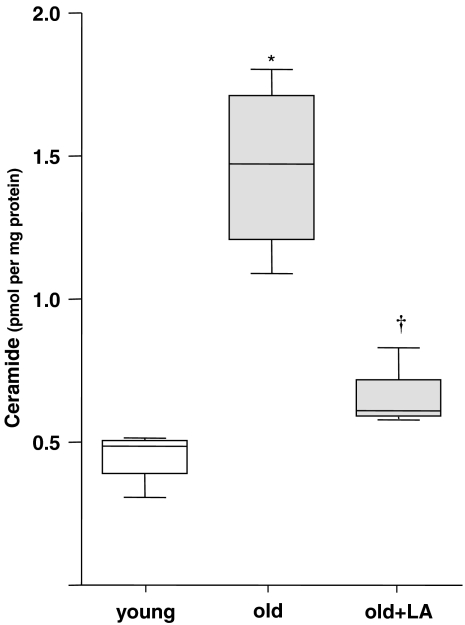
Because heightened nSMase activities would also be expected to directly affect ceramide values, overall endothelial ceramide content was quantified in young and old aortic endothelia. As anticipated, we observed an over 2.5-fold increase in ceramide on an age basis (Figure 3), which suggests a general imbalance in ceramide metabolism in ageing endothelia. To assess the extent to which ceramides were elevated due to nSMase activity, as well as the effects of reversing the loss in GSH, old rats were treated with LA and endothelial ceramides assessed 24 h later. Compared with the old control rats, LA significantly lowered free ceramide levels by 46% (Figure 3; 0.66±0.06 vs 1.46±0.15 pmol per mg protein in old controls), which was no longer significantly different from that found in young endothelium.
Figure 3.
(R)-α-lipoic acid (LA) lowered the age-associated increase in ceramide compared with saline controls. Ceramides were measured after administration of LA or saline to old animals. After treatment of old animals with LA, the ceramide levels were no longer different from those in untreated young animals. *Statistical significance compared with the young sample group (P⩽0.01). †Statistical significance compared with the old sample group (P⩽0.01; n=4).
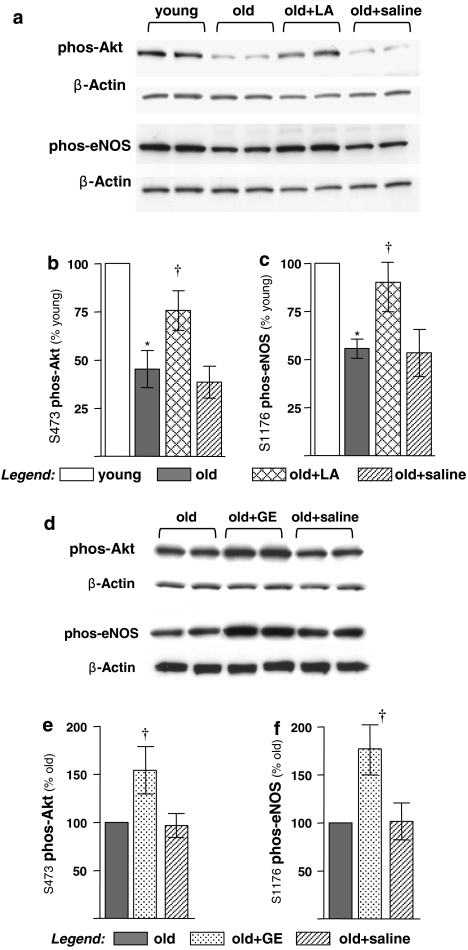
LA restored age-related losses in eNOS and Akt phosphorylation
The age-dependent increase in ceramide levels may adversely influence eNOS activity by inducing PP2A, a phosphatase known to govern activation of Akt, which in turn phosphorylates eNOS at stimulatory sites. We thus hypothesized that Akt would be less active in ageing endothelia, but LA would reverse this loss. Western blot analysis revealed that Akt phosphorylation at S473, an activation site, markedly declined with age (Figures 4a and b), which was in keeping with our previously published reports (Smith and Hagen, 2003; Smith et al., 2006a). However, LA significantly reversed the low level of S473 phosphorylation in old rats, although not to the levels observed in young control animals (Figures 4a and b). Treatment with GE induced a reversal similar to that observed with LA, indicating that the improvement was through a thiol-, probably GSH-, mediated mechanism (Figures 4d and e).
Figure 4.
(R)-α-lipoic acid (LA) and glutathione monoethylester (GE) partially restored the age-associated inactivation of endothelial nitric oxide synthase (eNOS) and Akt in the aortic endothelium. Endothelial plasma membrane fractions were prepared from young and old rats, or old rats treated with either LA, GE or saline. The phosphorylation status of eNOS (S1176) and Akt (S473) was analysed by western blot. (a) LA significantly improved the age-related decline in both Akt and eNOS phosphorylation. (b) Digital quantification of western blots reveals that Akt phosphorylation (S473) declines by 52% with age, which is significantly restored by LA administration. (c) Age leads to a 46% decline in eNOS phosphorylation, which is almost completely restored by LA. (d) GE increases endothelial Akt and eNOS phosphorylation in aortas from old rats. (e) Digital quantification of the blots illustrates that GE causes a 55% increase in Akt phosphorylation over untreated or saline-treated old animals. (f) Similar improvements in eNOS phosphorylation are also observed by GE administration to old animals. GE treatment led to an 80% increase in eNOS phosphorylation in old animals compared with either untreated or saline-treated animals. (n=4) *Statistical significance compared with young controls (P⩽0.01). †Statistical significance compared with old controls (P⩽0.02).
Endothelial nitric oxide synthase phosphorylation in control and LA-treated old rats was also monitored using western blot analysis. As with Akt, eNOS phosphorylation status at S1176 declined significantly with age and was not affected by saline injection (Figures 4a and c). However, LA reversed the loss in eNOS phosphorylation, but not to the level seen in young control rats (Figures 4a and c). As a positive control, GE was as effective as LA in lowering eNOS phosphorylation state, indicating that GSH levels directly influence eNOS activity (Figures 4d and f).
Discussion
Impaired vasomotion consequent to the loss of endothelial function is a common feature of age-related cardiovascular disease. Although the precise nature of such age-induced loss of vasomotion is still equivocal and, most likely, multifactorial, reduced NO production by endothelial cells is a major contributor. Recent evidence points to elevated activity of nSMase in endothelium from old rats when compared with endothelium from young (Smith et al., 2006a). This process, triggered by age-associated increased circulating concentrations of inflammatory markers, leads to higher ceramide levels and, in turn, disregulation of eNOS phosphorylation patterns and subcellular distribution. LA increases NO biosynthesis in cultured endothelial cells (Jones et al., 2002; Visioli et al., 2002) and improves endothelial function in a variety of conditions, including obesity (Lee et al., 2005), cyclosporine treatment (Lexis et al., 2006), diabetes (Bojunga et al., 2004; Sena et al., 2007) and metabolic syndrome (Sola et al., 2005). Therefore, we theorized that LA administration might partially restore endothelium-dependent vasorelaxation of aortic rings isolated from old rats. As shown in Figure 1a, acute LA administration to aged rats significantly, albeit not completely, restored proper vasomotion, as evaluated by ACh-induced vasorelaxation of aortic rings. The observed age-associated loss of vasomotion is in line with results by van der Loo et al. (2000). It should be also noted that endothelial function is not irreversibly altered in the ageing rats.
(R)-α-lipoic acid has been proposed as a highly effective compound for increasing intracellular GSH in various contexts, including endothelial (Bogani et al., 2007) and ageing cells (Bast and Haenen, 1988; Han et al., 1997; Hagen et al., 1999; Bharat et al., 2002; Moini et al., 2002). As we demonstrated a correlation between loss of vasomotion and age-associated loss of antioxidants, namely GSH, we sought to determine whether endothelial GSH status in the aged animal might be sensitive to pharmacological manipulation by LA. Indeed, LA administration to aged rats leads to marked increases in both endothelial GSH concentrations and the GSH/GSSG ratio. These effects were of lower magnitude than those of GE, but still suggestive of a thiol-dependent mechanism. Indeed, nSMase activity, which is stimulated by low GSH levels, is elevated in old endothelium when compared with young (Smith et al., 2006a). As pharmacological administration of LA partially reversed the age-associated loss of intracellular GSH in the endothelium, it is plausible that LA treatment might be able to reverse the activation of nSMase in the aged endothelium via augmentation of GSH status. This notion was reinforced by the observation that administration of GE also lowered membrane nSMase activity (Figure 2).
Loss of eNOS activity is largely due to a ceramide-triggered hyperactivation of PP2A and subsequent deactivation of eNOS. As LA treatment improved GSH in the aged endothelium and significantly lowered nSMase activity, we measured free ceramide levels in the endothelium of aged rats after administration of LA, which restored ceramide to the levels close to those typically found in young endothelium. The effects of LA on ceramide levels might be a consequence of the action of LA on cellular free fatty acid levels. In fact, ageing and several age-related pathologies are characterized by diminished mitochondrial efficiency and accumulation of free fatty acids due to altered β-oxidation. LA has been shown to improve mitochondrial efficiency and metabolic function by improving β-oxidation and lowering free fatty acid levels. However, an important source of cellular ceramide is its de novo synthesis, which can be upregulated in response to excess free fatty acid concentrations (van Blitterswijk et al., 2003). Thus, LA may have effects on cellular ceramide levels independently of nSMase modulation. The effects of age and LA on ceramide concentrations appear to be confined to nSMase, as endothelial ceramidase activity levels were not altered by either age or LA administration (data not shown). Also, it is noteworthy that SMase activities per se are not generally activated on an age basis, but that only those SMases with neutral pH optima are affected.
The age-associated increase in free ceramide (Smith et al., 2006a) suggests that phosphatase hyperactivation is responsible for the deactivation of Akt and, subsequently, eNOS. Thus, we assessed the steady-state phosphorylation of eNOS and Akt at serine 1176 and serine 473 (respectively). Phosphorylation at these sites stimulates Akt activity. In agreement with previously published data (Smith and Hagen, 2003), we showed that treatment of old animals with LA partially restored the age-associated decline in phosphorylation status of both Akt S473 (Figures 4a and b) and eNOS S-1176 (Figures 4a and c), as also reported by Artwohl et al. (2007). Interestingly, GE administration had a similar effect on the phosphorylation status of Akt and eNOS in old animals, further reinforcing the hypothesis that many of the beneficial effects of LA on vasomotion are due to its GSH-enhancing ability.
In this study, the LA-driven restoration of ceramide concentrations in old rats to levels close to those observed in young animals was not accompanied by complete reinstatement of vasorelaxation, suggesting that other mechanisms may also play a role in the age-associated loss of endothelial function, as previously suggested (Maytin et al., 1999; van der Loo et al., 2000; Drew and Leeuwenburgh, 2002). Examples include premature degradation of NO, loss of eNOS cofactors, loss of growth factors, and cell–cell and cell–matrix associations, which regulate Akt-dependent activation of eNOS (Belmin et al., 1993; Hashimoto et al., 1999; Heymes et al., 2000; Aviv, 2002; Csiszar et al., 2003; Rashid et al., 2004).
One limitation of this study is that it does not provide information on the effects of a chronic treatment with LA. Indeed, prolonged administration of LA (or GE) might prevent—to a large extent—the appearance of age-associated endothelial dysfunction.
In summary, our data show that LA partially, but significantly restores proper vasomotion in the aged rat, largely via restoration of intracellular GSH concentrations and redox status, even though direct actions on phosphorylation/dephosphorylation pathways might also contribute, for example through thiol-mediated stimulation of receptor tyrosine kinases and PI3 kinase pathways (Yaworsky et al., 2000; Konrad et al., 2001; Artwohl et al., 2007). Even though a balanced diet is the first preventive measure to be implemented, these data contribute to the body of literature reporting vasomodulatory actions of LA and provide further reasons for its assessment in clinical settings.
Acknowledgments
This research was supported by Grants 2R01AG017141—06A2 and P01 AT002034-01 from the NIH (TMH) and in part by an American Heart Association pre-doctoral fellowship (0110213Z; ARS). We thank Jeffrey Monette for his expertize in development and adaptation of ceramide quantitation and the SMase-ceramidase assay.
Abbreviations
- eNOS
endothelial nitric oxide synthase
- GSH
glutathione
- GSSG
oxidized glutathione
- GE
glutathione monoethylester
- PP2A
protein phosphatase 2A
- SMase
sphingomyelinase
- LA
(R)-α-lipoic acid
Conflict of interest
The authors state no conflict of interest.
References
- Artwohl M, Muth K, Kosulin K, De Martin R, Holzenbein T, Rainer G, et al. R-(+)-{alpha}-lipoic acid inhibits endothelial cell apoptosis and proliferation: involvement of Akt and retinoblastoma protein/E2F-1. Am J Physiol Endocrinol Metab. 2007;293:E681–E689. doi: 10.1152/ajpendo.00584.2006. [DOI] [PubMed] [Google Scholar]
- Aviv A. Chronology versus biology: telomeres, essential hypertension, and vascular aging. Hypertension. 2002;40:229–232. doi: 10.1161/01.hyp.0000027280.91984.1b. [DOI] [PubMed] [Google Scholar]
- Bast A, Haenen GR. Interplay between lipoic acid and glutathione in the protection against microsomal lipid peroxidation. Biochim Biophys Acta. 1988;963:558–561. doi: 10.1016/0005-2760(88)90326-8. [DOI] [PubMed] [Google Scholar]
- Belmin J, Corman B, Merval R, Tedgui A. Age-related changes in endothelial permeability and distribution volume of albumin in rat aorta. Am J Physiol. 1993;264:H679–H685. doi: 10.1152/ajpheart.1993.264.3.H679. [DOI] [PubMed] [Google Scholar]
- Bharat S, Cochran BC, Hsu M, Liu J, Ames BN, Andersen JK. Pre-treatment with R-lipoic acid alleviates the effects of GSH depletion in PC12 cells: implications for Parkinson's disease therapy. Neurotoxicology. 2002;23:479–486. doi: 10.1016/s0161-813x(02)00035-9. [DOI] [PubMed] [Google Scholar]
- Bielawska A, Perry DK, Hannun YA. Determination of ceramides and diglycerides by the diglyceride kinase assay. Anal Biochem. 2001;298:141–150. doi: 10.1006/abio.2001.5342. [DOI] [PubMed] [Google Scholar]
- Bogani P, Canavesi M, Hagen TM, Visioli F, Bellosta S. Thiol supplementation inhibits metalloproteinase activity independent of glutathione status. Biochem Biophys Res Commun. 2007;363:651–655. doi: 10.1016/j.bbrc.2007.09.018. [DOI] [PubMed] [Google Scholar]
- Bojunga J, Dresar-Mayert B, Usadel KH, Kusterer K, Zeuzem S. Antioxidative treatment reverses imbalances of nitric oxide synthase isoform expression and attenuates tissue-cGMP activation in diabetic rats. Biochem Biophys Res Commun. 2004;316:771–780. doi: 10.1016/j.bbrc.2004.02.110. [DOI] [PubMed] [Google Scholar]
- Chou TC, Yen MH, Li CY, Ding YA. Alterations of nitric oxide synthase expression with aging and hypertension in rats. Hypertension. 1998;31:643–648. doi: 10.1161/01.hyp.31.2.643. [DOI] [PubMed] [Google Scholar]
- Csiszar A, Ungvari Z, Koller A, Edwards JG, Kaley G. Aging-induced proinflammatory shift in cytokine expression profile in coronary arteries. FASEB J. 2003;17:1183–1185. doi: 10.1096/fj.02-1049fje. [DOI] [PubMed] [Google Scholar]
- Drew B, Leeuwenburgh C. Aging and the role of reactive nitrogen species. Ann N Y Acad Sci. 2002;959:66–81. doi: 10.1111/j.1749-6632.2002.tb02084.x. [DOI] [PubMed] [Google Scholar]
- Gerhard M, Roddy MA, Creager SJ, Creager MA. Aging progressively impairs endothelium-dependent vasodilation in forearm resistance vessels of humans. Hypertension. 1996;27:849–853. doi: 10.1161/01.hyp.27.4.849. [DOI] [PubMed] [Google Scholar]
- Grattagliano I, Wieland P, Schranz C, Lauterburg BH. Disposition of glutathione monoethyl ester in the rat: glutathione ester is a slow release form of extracellular glutathione. J Pharmacol Exp Ther. 1995;272:484–488. [PubMed] [Google Scholar]
- Griffith OW. Biologic and pharmacologic regulation of mammalian glutathione synthesis. Free Radic Biol Med. 1999;27:922–935. doi: 10.1016/s0891-5849(99)00176-8. [DOI] [PubMed] [Google Scholar]
- Hagen TM, Ingersoll RT, Lykkesfeldt J, Liu J, Wehr CM, Vinarsky V, et al. (R)-alpha-lipoic acid-supplemented old rats have improved mitochondrial function, decreased oxidative damage, and increased metabolic rate. FASEB J. 1999;13:411–418. doi: 10.1096/fasebj.13.2.411. [DOI] [PubMed] [Google Scholar]
- Hagen TM, Vinarsky V, Wehr CM, Ames BN. (R)-alpha-lipoic acid reverses the age-associated increase in susceptibility of hepatocytes to tert-butylhydroperoxide both in vitro and in vivo. Antioxid Redox Signal. 2000;2:473–483. doi: 10.1089/15230860050192251. [DOI] [PubMed] [Google Scholar]
- Han D, Handelman G, Marcocci L, Sen CK, Roy S, Kobuchi H, et al. Lipoic acid increases de novo synthesis of cellular glutathione by improving cystine utilization. Biofactors. 1997;6:321–338. doi: 10.1002/biof.5520060303. [DOI] [PubMed] [Google Scholar]
- Hashimoto M, Hossain S, Masumura S. Effect of aging on plasma membrane fluidity of rat aortic endothelial cells. Exp Gerontol. 1999;34:687–698. doi: 10.1016/s0531-5565(99)00025-x. [DOI] [PubMed] [Google Scholar]
- Heymes C, Habib A, Yang D, Mathieu E, Marotte F, Samuel J, et al. Cyclo-oxygenase-1 and -2 contribution to endothelial dysfunction in ageing. Br J Pharmacol. 2000;131:804–810. doi: 10.1038/sj.bjp.0703632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hynes MR, Duckles SP. Effect of increasing age on the endothelium-mediated relaxation of rat blood vessels in vitro. J Pharmacol Exp Ther. 1987;241:387–392. [PubMed] [Google Scholar]
- Jones DP, Carlson JL, Samiec PS, Sternberg P, Jr, Mody VC, Jr, Reed RL, et al. Glutathione measurement in human plasma. Evaluation of sample collection, storage and derivatization conditions for analysis of dansyl derivatives by HPLC. Clin Chim Acta. 1998;275:175–184. doi: 10.1016/s0009-8981(98)00089-8. [DOI] [PubMed] [Google Scholar]
- Jones W, Li X, Qu ZC, Perriott L, Whitesell RR, May JM. Uptake, recycling, and antioxidant actions of alpha-lipoic acid in endothelial cells. Free Radic Biol Med. 2002;33:83–93. doi: 10.1016/s0891-5849(02)00862-6. [DOI] [PubMed] [Google Scholar]
- Koga T, Takata Y, Kobayashi K, Takishita S, Yamashita Y, Fujishima M. Ageing suppresses endothelium-dependent relaxation and generates contraction mediated by the muscarinic receptors in vascular smooth muscle of normotensive Wistar–Kyoto and spontaneously hypertensive rats. J Hypertens Suppl. 1988;6:S243–S245. doi: 10.1097/00004872-198812040-00073. [DOI] [PubMed] [Google Scholar]
- Kondo T, Higashiyama Y, Goto S, Iida T, Cho S, Iwanaga M, et al. Regulation of gamma-glutamylcysteine synthetase expression in response to oxidative stress. Free Radic Res. 1999;31:325–334. doi: 10.1080/10715769900300891. [DOI] [PubMed] [Google Scholar]
- Konrad D, Somwar R, Sweeney G, Yaworsky K, Hayashi M, Ramlal T, et al. The antihyperglycemic drug alpha-lipoic acid stimulates glucose uptake via both GLUT4 translocation and GLUT4 activation: potential role of p38 mitogen-activated protein kinase in GLUT4 activation. Diabetes. 2001;50:1464–1471. doi: 10.2337/diabetes.50.6.1464. [DOI] [PubMed] [Google Scholar]
- Lee WJ, Lee IK, Kim HS, Kim YM, Koh EH, Won JC, et al. Alpha-lipoic acid prevents endothelial dysfunction in obese rats via activation of AMP-activated protein kinase. Arterioscler Thromb Vasc Biol. 2005;25:2488–2494. doi: 10.1161/01.ATV.0000190667.33224.4c. [DOI] [PubMed] [Google Scholar]
- Levy EJ, Anderson ME, Meister A. Transport of glutathione diethyl ester into human cells. Proc Natl Acad Sci USA. 1993;90:9171–9175. doi: 10.1073/pnas.90.19.9171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lexis LA, Fenning A, Brown L, Fassett RG, Coombes JS. Antioxidant supplementation enhances erythrocyte antioxidant status and attenuates cyclosporine-induced vascular dysfunction. Am J Transplant. 2006;6:41–49. doi: 10.1111/j.1600-6143.2005.01154.x. [DOI] [PubMed] [Google Scholar]
- Liu B, Hannun YA. Inhibition of the neutral magnesium-dependent sphingomyelinase by glutathione. J Biol Chem. 1997;272:16281–16287. doi: 10.1074/jbc.272.26.16281. [DOI] [PubMed] [Google Scholar]
- Liu H, Wang H, Shenvi S, Hagen TM, Liu RM. Glutathione metabolism during aging and in Alzheimer disease. Ann N Y Acad Sci. 2004;1019:346–349. doi: 10.1196/annals.1297.059. [DOI] [PubMed] [Google Scholar]
- Maytin M, Leopold J, Loscalzo J. Oxidant stress in the vasculature. Curr Atheroscler Rep. 1999;1:156–164. doi: 10.1007/s11883-999-0012-z. [DOI] [PubMed] [Google Scholar]
- Moini H, Tirosh O, Park YC, Cho KJ, Packer L. R-alpha-lipoic acid action on cell redox status, the insulin receptor, and glucose uptake in 3T3-L1 adipocytes. Arch Biochem Biophys. 2002;397:384–391. doi: 10.1006/abbi.2001.2680. [DOI] [PubMed] [Google Scholar]
- Packer L. alpha-Lipoic acid: a metabolic antioxidant which regulates NF-kappa B signal transduction and protects against oxidative injury. Drug Metab Rev. 1998;30:245–275. doi: 10.3109/03602539808996311. [DOI] [PubMed] [Google Scholar]
- Peterson GL. A simplification of the protein assay method of Lowry et al. which is more generally applicable. Anal Biochem. 1977;83:346–356. doi: 10.1016/0003-2697(77)90043-4. [DOI] [PubMed] [Google Scholar]
- Powell LA, Nally SM, Mcmaster D, Catherwood MA, Trimble ER. Restoration of glutathione levels in vascular smooth muscle cells exposed to high glucose conditions. Free Radic Biol Med. 2001;31:1149–1155. doi: 10.1016/s0891-5849(01)00648-7. [DOI] [PubMed] [Google Scholar]
- Rashid G, Benchetrit S, Fishman D, Bernheim J. Effect of advanced glycation end-products on gene expression and synthesis of TNF-alpha and endothelial nitric oxide synthase by endothelial cells. Kidney Int. 2004;66:1099–1106. doi: 10.1111/j.1523-1755.2004.00860.x. [DOI] [PubMed] [Google Scholar]
- Rikans LE, Moore DR. Influence of aging on rat liver enzymes involved in glutathione synthesis and degradation. Arch Gerontol Geriatr. 1991;13:263–270. doi: 10.1016/0167-4943(91)90048-u. [DOI] [PubMed] [Google Scholar]
- Ryan US, Maxwell G. Isolation, culture, and subculture of bovine pulmonary artery endothelial cells: Mechanical methods. Methods Cell Sci. 1986;10:3–5. [Google Scholar]
- Sen CK, Roy S, Han D, Packer L. Regulation of cellular thiols in human lymphocytes by alpha-lipoic acid: a flow cytometric analysis. Free Radic Biol Med. 1997;22:1241–1257. doi: 10.1016/s0891-5849(96)00552-7. [DOI] [PubMed] [Google Scholar]
- Sena CM, Nunes E, Louro T, Proenca T, Fernandes R, Boarder MR, et al. Effects of alpha-lipoic acid on endothelial function in aged diabetic and high-fat fed rats Br J Pharmacol 2007 10.1038/sj.bjp.0707474e-pub ahead of print 1 October 2007, doi [DOI] [PMC free article] [PubMed]
- Simmons CA, Zilberberg J, Davies PF. A rapid, reliable method to isolate high quality endothelial RNA from small spatially-defined locations. Ann Biomed Eng. 2004;32:1453–1459. doi: 10.1114/b:abme.0000042360.57960.2b. [DOI] [PubMed] [Google Scholar]
- Smith AR, Hagen TM. Vascular endothelial dysfunction in aging: loss of Akt-dependent endothelial nitric oxide synthase phosphorylation and partial restoration by (R)-alpha-lipoic acid. Biochem Soc Trans. 2003;31:1447–1449. doi: 10.1042/bst0311447. [DOI] [PubMed] [Google Scholar]
- Smith AR, Visioli F, Frei B, Hagen TM. Age-related changes in endothelial nitric oxide synthase phosphorylation and nitric oxide dependent vasodilation: evidence for a novel mechanism involving sphingomyelinase and ceramide-activated phosphatase 2A. Aging Cell. 2006a;5:391–400. doi: 10.1111/j.1474-9726.2006.00232.x. [DOI] [PubMed] [Google Scholar]
- Smith AR, Visioli F, Hagen TM. Plasma membrane-associated endothelial nitric oxide synthase and activity in aging rat aortic vascular endothelia markedly decline with age. Arch Biochem Biophys. 2006b;454:100–105. doi: 10.1016/j.abb.2006.02.017. [DOI] [PubMed] [Google Scholar]
- Sola S, Mir MQ, Cheema FA, Khan-Merchant N, Menon RG, Parthasarathy S, et al. Irbesartan and lipoic acid improve endothelial function and reduce markers of inflammation in the metabolic syndrome: results of the Irbesartan and Lipoic Acid in Endothelial Dysfunction (ISLAND) study. Circulation. 2005;111:343–348. doi: 10.1161/01.CIR.0000153272.48711.B9. [DOI] [PubMed] [Google Scholar]
- Suh JH, Shenvi SV, Dixon BM, Liu H, Jaiswal AK, Liu RM, et al. Decline in transcriptional activity of Nrf2 causes age-related loss of glutathione synthesis, which is reversible with lipoic acid. Proc Natl Acad Sci USA. 2004;101:3381–3386. doi: 10.1073/pnas.0400282101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suh JH, Wang H, Liu R-M, Liu J, Hagen TM. (R)-alpha-lipoic acid reverses the age-related loss in GSH redox status in post-mitotic tissues: evidence for increased cysteine requirement for GSH synthesis. Arch Biochem Biophys. 2004;423:126–135. doi: 10.1016/j.abb.2003.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tschudi MR, Barton M, Bersinger NA, Moreau P, Cosentino F, Noll G, et al. Effect of age on kinetics of nitric oxide release in rat aorta and pulmonary artery. J Clin Invest. 1996;98:899–905. doi: 10.1172/JCI118872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsyupko AN, Dudnik LB, Evstigneeva RP, Alessenko AV. Effects of reduced and oxidized glutathione on sphingomyelinase activity and contents of sphingomyelin and lipid peroxidation products in murine liver. Biochemistry (Mosc) 2001;66:1028–1034. doi: 10.1023/a:1012381928535. [DOI] [PubMed] [Google Scholar]
- Van Blitterswijk WJ, Van Der Luit AH, Veldman RJ, Verheij M, Borst J. Ceramide: second messenger or modulator of membrane structure and dynamics. Biochem J. 2003;369:199–211. doi: 10.1042/BJ20021528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Der Loo B, Labugger R, Skepper JN, Bachschmid M, Kilo J, Powell JM, et al. Enhanced peroxynitrite formation is associated with vascular aging. J Exp Med. 2000;192:1731–1744. doi: 10.1084/jem.192.12.1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visioli F, Smith A, Zhang W, Keaney JF, Jr, Hagen T, Frei B. Lipoic acid and vitamin C potentiate nitric oxide synthesis in human aortic endothelial cells independently of cellular glutathione status. Redox Rep. 2002;7:223–227. doi: 10.1179/135100002125000604. [DOI] [PubMed] [Google Scholar]
- Yaworsky K, Somwar R, Ramlal T, Tritschler HJ, Klip A. Engagement of the insulin-sensitive pathway in the stimulation of glucose transport by alpha-lipoic acid in 3T3-L1 adipocytes. Diabetologia. 2000;43:294–303. doi: 10.1007/s001250050047. [DOI] [PubMed] [Google Scholar]