Abstract
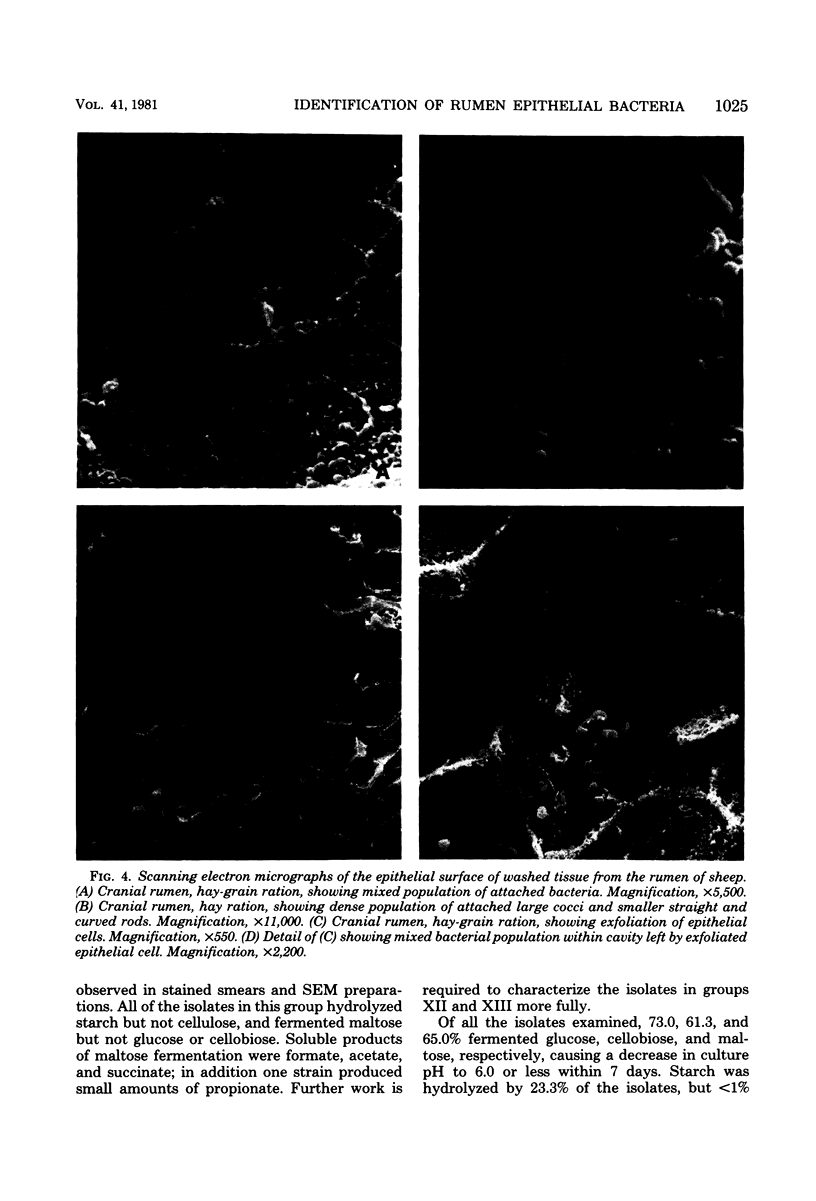
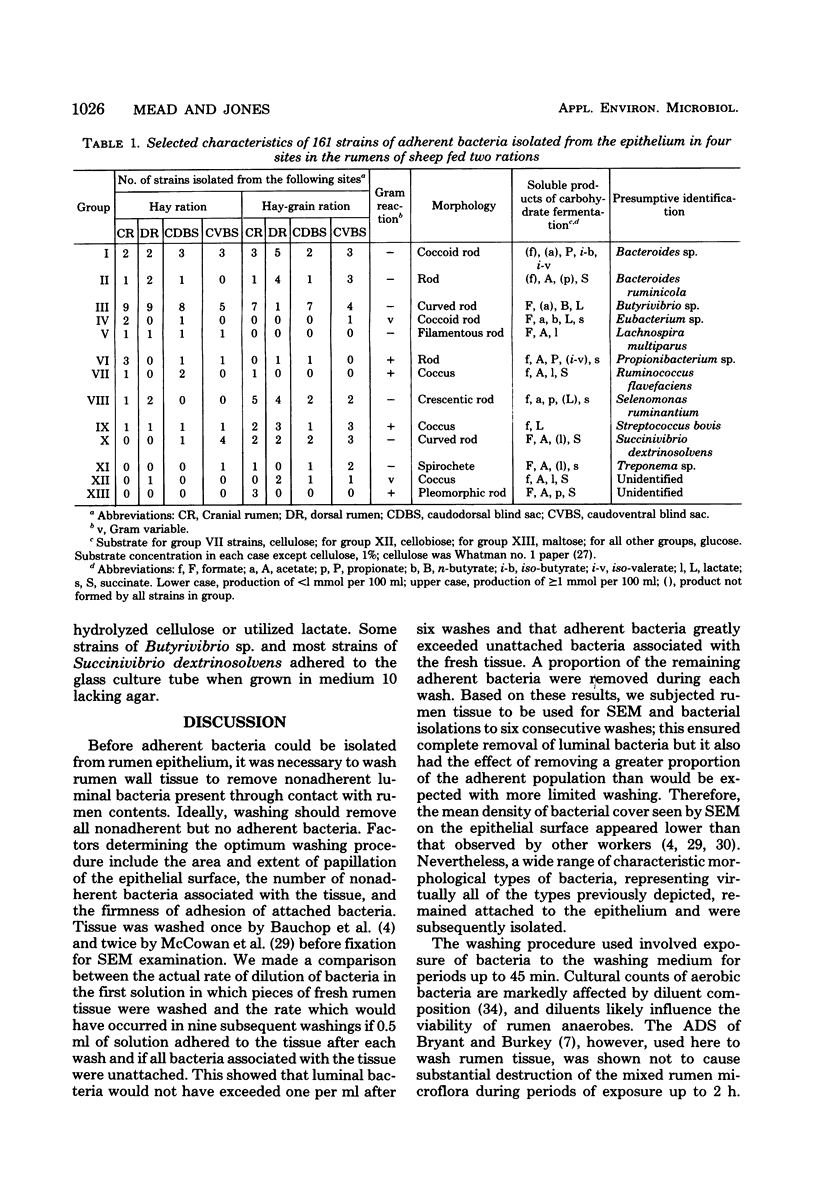
One hundred sixty-one strains of adherent bacteria were isolated under anaerobic conditions from four sites on the rumen epithelial surface of sheep fed hay or a hay-grain ration. Before isolation of bacteria, rumen tissue was washed six times in an anaerobic dilution solution, and viable bacteria suspended in the washings were counted. Calculation indicated that unattached bacteria would have been removed from the tissue by this procedure, but a slow and progressive release of attached bacteria also occurred. Nevertheless, a wide range of characteristic morphological types remained associated with the epithelium as demonstrated by scanning electron microscopy. Most of these types were represented among the isolates. Characterization and presumptive identification of the isolates showed that 95.0% belonged to previously described genera of functionally significant rumen bacteria, including Butyrivibrio sp. (31.1%), Bacteroides sp. (22.4%), Selenomonas ruminantium (9.9%), Succinivibrio dextrinosolvens (8.7%), Streptococcus bovis (8.1%), Propionibacterium sp. (4.3%), Treponema sp. (3.1%), and Eubacterium sp., Lachnospira multiparus, and Ruminococcus flavefaciens (2.5% each). Eight isolates (5.0%) were not identified. L. multiparus was recovered only from hay-fed animals; all other genera were obtained from animals fed either ration. All S. bovis strains and two strains each of Bacteroides sp. and Butyrivibrio sp. were aerotolerant; all other strains were strictly anaerobic. Bacteria representing the gram-positive, facultatively anaerobic flora associated with rumen wall tissue (R. J. Wallace, K.-J. Cheng, D. Dinsdale, and E. R. Ørskov, Nature (London) 279:424-426, 1979) were therefore not recovered by the techniques used; instead a different fraction of the adherent population was isolated. The term “epimural” is proposed to describe the flora associated with the rumen epithelium.
Full text
PDF


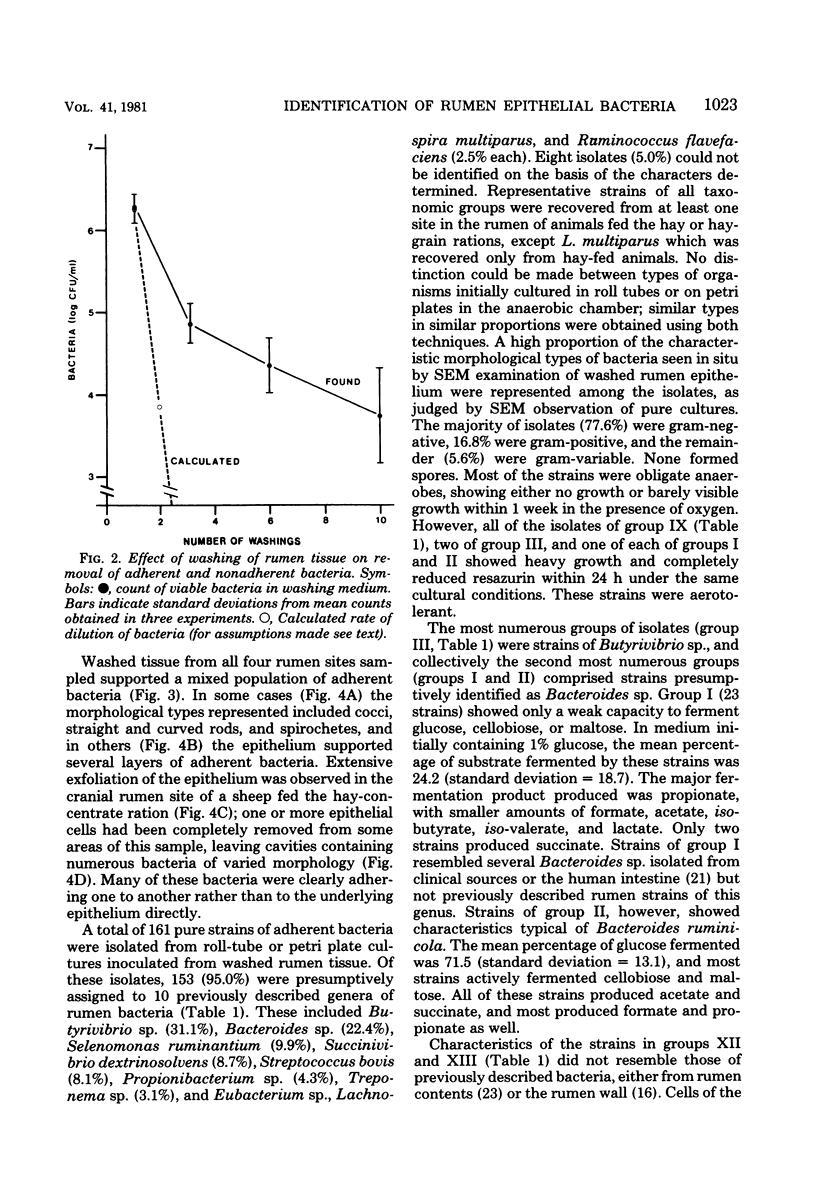
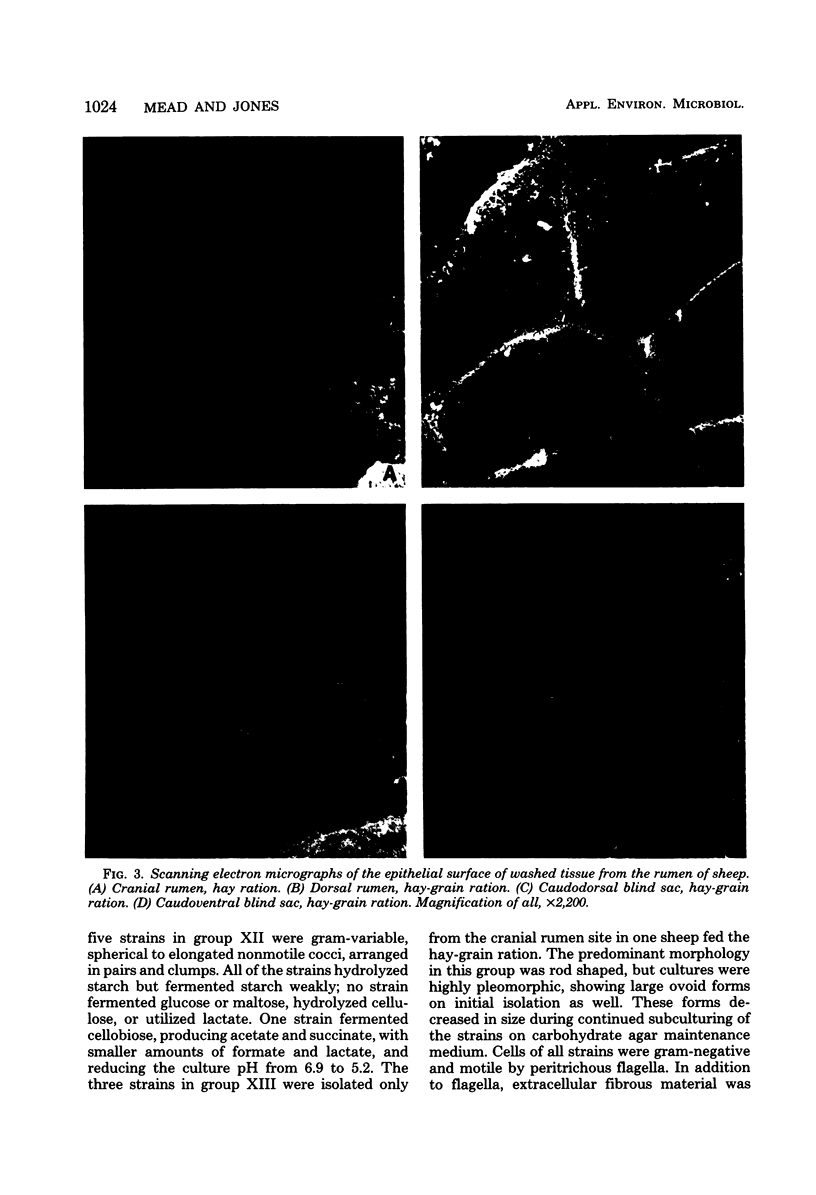


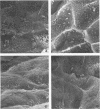
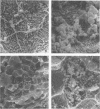
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akin D. E. Ultrastructure of rumen bacterial attachment to forage cell walls. Appl Environ Microbiol. 1976 Apr;31(4):562–568. doi: 10.1128/aem.31.4.562-568.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BRYANT M. P. Bacterial species of the rumen. Bacteriol Rev. 1959 Sep;23(3):125–153. doi: 10.1128/br.23.3.125-153.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BRYANT M. P., ROBINSON I. M. Some nutritional characteristics of predominant culturable ruminal bacteria. J Bacteriol. 1962 Oct;84:605–614. doi: 10.1128/jb.84.4.605-614.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BRYANT M. P., SMALL N. The anaerobic monotrichous butyric acid-producing curved rod-shaped bacteria of the rumen. J Bacteriol. 1956 Jul;72(1):16–21. doi: 10.1128/jb.72.1.16-21.1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balch W. E., Wolfe R. S. New approach to the cultivation of methanogenic bacteria: 2-mercaptoethanesulfonic acid (HS-CoM)-dependent growth of Methanobacterium ruminantium in a pressureized atmosphere. Appl Environ Microbiol. 1976 Dec;32(6):781–791. doi: 10.1128/aem.32.6.781-791.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balish E., Brown J. F., Wilkins T. D. Transparent plastic incubator for the anaerobic glove box. Appl Environ Microbiol. 1977 Mar;33(3):525–527. doi: 10.1128/aem.33.3.525-527.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauchop T., Clarke R. T., Newhook J. C. Scanning electron microscope study of bacteria associated with the rumen epithelium of sheep. Appl Microbiol. 1975 Oct;30(4):668–675. doi: 10.1128/am.30.4.668-675.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldwell D. R., Bryant M. P. Medium without rumen fluid for nonselective enumeration and isolation of rumen bacteria. Appl Microbiol. 1966 Sep;14(5):794–801. doi: 10.1128/am.14.5.794-801.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng K. J., Akin D. E., Costerton J. W. Rumen bacteria: interaction with particulate dietary components and response to dietary variation. Fed Proc. 1977 Feb;36(2):193–197. [PubMed] [Google Scholar]
- Cheng K. J., Costerton J. W. Ultrastructure of Butyrivibrio fibrisolvens: a gram-positive bacterium. J Bacteriol. 1977 Mar;129(3):1506–1512. doi: 10.1128/jb.129.3.1506-1512.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng K. J., McCowan R. P., Costerton J. W. Adherent epithelial bacteria in ruminants and their roles in digestive tract function. Am J Clin Nutr. 1979 Jan;32(1):139–148. doi: 10.1093/ajcn/32.1.139. [DOI] [PubMed] [Google Scholar]
- Cheng K. J., Wallace R. J. The mechanism of passage of endogenous urea through the rumen wall and the role of ureolytic epithelial bacteria in the urea flux. Br J Nutr. 1979 Nov;42(3):553–557. doi: 10.1079/bjn19790147. [DOI] [PubMed] [Google Scholar]
- Dinsdale D., Cheng K. J., Wallace R. J., Goodlad R. A. Digestion of epithelial tissue of the rumen wall by adherent bacteria in infused and conventionally fed sheep. Appl Environ Microbiol. 1980 May;39(5):1059–1066. doi: 10.1128/aem.39.5.1059-1066.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fay J. P., Cheng K. J., Costerton J. W. Production of alkaline phosphatase by epithelial cells and adherent bacteria of the bovine rumen and abomasum. Can J Microbiol. 1979 Aug;25(8):932–936. doi: 10.1139/m79-140. [DOI] [PubMed] [Google Scholar]
- HUNGATE R. E. The anaerobic mesophilic cellulolytic bacteria. Bacteriol Rev. 1950 Mar;14(1):1–49. doi: 10.1128/br.14.1.1-49.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann S. O. An improved method for determining celluloytic activity in anerobic bacteria. J Appl Bacteriol. 1968 Jun;31(2):241–244. doi: 10.1111/j.1365-2672.1968.tb00363.x. [DOI] [PubMed] [Google Scholar]
- Marples R. R. Effects of soaps, germicides and disinfectants on the skin flora. Soc Appl Bacteriol Symp Ser. 1974;3(0):35–46. [PubMed] [Google Scholar]
- McCowan R. P., Cheng K. J., Bailey C. B., Costerton J. W. Adhesion of bacteria to epithelial cell surfaces within the reticulo-rumen of cattle. Appl Environ Microbiol. 1978 Jan;35(1):149–155. doi: 10.1128/aem.35.1.149-155.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCowan R. P., Cheng K. J., Costerton J. W. Adherent bacterial populations on the bovine rumen wall: distribution patterns of adherent bacteria. Appl Environ Microbiol. 1980 Jan;39(1):233–241. doi: 10.1128/aem.39.1.233-241.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rittenburg J. H., Bayer R. C., Stern M. D. A technique for preparing ciliated rumen protozoa for scanning electron microscopy. J Anim Sci. 1977 Apr;44(4):710–712. doi: 10.2527/jas1977.444710x. [DOI] [PubMed] [Google Scholar]
- STRAKA R. P., STOKES J. L. Rapid destruction of bacteria in commonly used diluents and its elimination. Appl Microbiol. 1957 Jan;5(1):21–25. doi: 10.1128/am.5.1.21-25.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salanitro J. P., Muirhead P. A. Quantitative method for the gas chromatographic analysis of short-chain monocarboxylic and dicarboxylic acids in fermentation media. Appl Microbiol. 1975 Mar;29(3):374–381. doi: 10.1128/am.29.3.374-381.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace R. J., Cheng K. J., Dinsdale D., Orskov E. R. An independent microbial flora of the epithelium and its role in the ecomicrobiology of the rumen. Nature. 1979 May 31;279(5712):424–426. doi: 10.1038/279424a0. [DOI] [PubMed] [Google Scholar]
- Ziolecki A., Tomerska H., Wojciechowicz M. Characteristics of pure cultures of Borrelia sp. isolated from the bovine rumen. Acta Microbiol Pol B. 1975;7(1):45–50. [PubMed] [Google Scholar]