Abstract
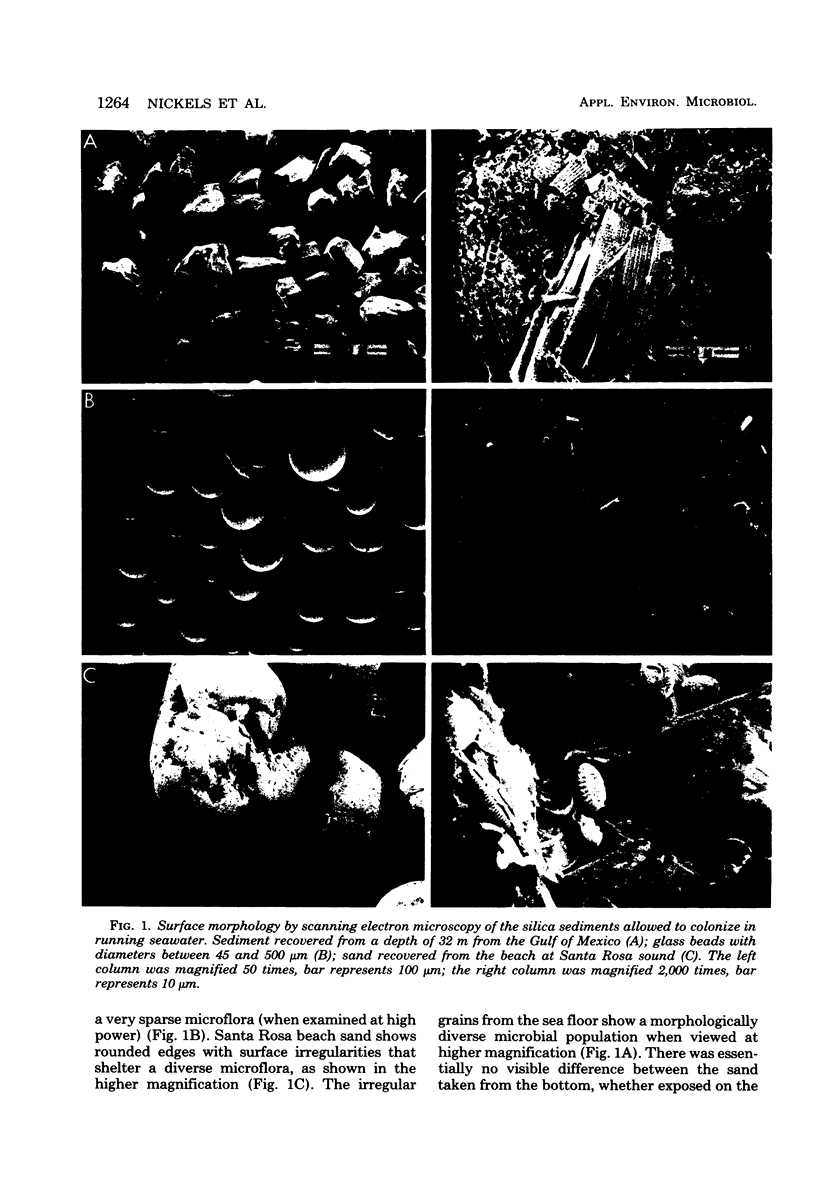
Microbiota colonizing silica grains of the same size and water pore space, but with a different microtopography, showed differences in biomass and community structure after 8 weeks of exposure to running seawater. The absence of surface cracks and crevices resulted in a marked diminution of the total microbial biomass measured as lipid phosphate and total extractable palmitic acid. With increasing smoothness of the sand grain surface, examination of the community structure showed a marked decrease in procaryotes and algal microeucaryotes, with a relative increase in microeucaryotic grazers. A comparison of the colonizing sediment incubated in running seawater or at 32 m on the sea floor with a sediment core showed a decreased bacterial biomass with a different community structure and a decreased total microeucaryotic population of both grazers and algae. The quantitative differences in microbial biomass and community structure between the microcosms and the actual benthic population in the core were determined.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Batoosingh E., Anthony E. H. Direct and indirect observations of bacteria on marine pebbles. Can J Microbiol. 1971 May;17(5):655–664. doi: 10.1139/m71-106. [DOI] [PubMed] [Google Scholar]
- Bobbie R. J., Morrison S. J., White D. C. Effects of substrate biodegradability on the mass and activity of the associated estuarine microbiota. Appl Environ Microbiol. 1978 Jan;35(1):179–184. doi: 10.1128/aem.35.1.179-184.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bobbie R. J., White D. C. Characterization of benthic microbial community structure by high-resolution gas chromatography of Fatty Acid methyl esters. Appl Environ Microbiol. 1980 Jun;39(6):1212–1222. doi: 10.1128/aem.39.6.1212-1222.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis W. M., White D. C. Fluorometric determination of adenosine nucleotide derivatives as measures of the microfouling, detrital, and sedimentary microbial biomass and physiological status. Appl Environ Microbiol. 1980 Sep;40(3):539–548. doi: 10.1128/aem.40.3.539-548.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fazio S. D., Mayberry W. R., White D. C. Muramic Acid assay in sediments. Appl Environ Microbiol. 1979 Aug;38(2):349–350. doi: 10.1128/aem.38.2.349-350.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattori T., Hattori R. The physical environment in soil microbiology: an attempt to extend principles of microbiology to soil microoganisms. CRC Crit Rev Microbiol. 1976 May;4(4):423–461. doi: 10.3109/10408417609102305. [DOI] [PubMed] [Google Scholar]
- Hill I. R., Gray T. R. Application of the fluorescent-antibody technique to an ecological study of bacteria in soil. J Bacteriol. 1967 Jun;93(6):1888–1896. doi: 10.1128/jb.93.6.1888-1896.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kates M. Bacterial lipids. Adv Lipid Res. 1964;2:17–90. [PubMed] [Google Scholar]
- King J. D., White D. C. Muramic acid as a measure of microbial biomass in estuarine and marine samples. Appl Environ Microbiol. 1977 Apr;33(4):777–783. doi: 10.1128/aem.33.4.777-783.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison S. J., White D. C. Effects of grazing by estuarine gammaridean amphipods on the microbiota of allochthonous detritus. Appl Environ Microbiol. 1980 Sep;40(3):659–671. doi: 10.1128/aem.40.3.659-671.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stotzky G. Activity, ecology, and population dynamics of microorganisms in soil. CRC Crit Rev Microbiol. 1972 Nov;2(1):59–137. doi: 10.3109/10408417209108383. [DOI] [PubMed] [Google Scholar]
- Tsernoglou D., Anthony E. H. Particle size, water-stable aggregates, and bacterial populations in lake sediments. Can J Microbiol. 1971 Feb;17(2):217–227. doi: 10.1139/m71-038. [DOI] [PubMed] [Google Scholar]
- White D. C., Cox R. H. Indentification and localization of the fatty acids in Haemophilus parainfluenzae. J Bacteriol. 1967 Mar;93(3):1079–1088. doi: 10.1128/jb.93.3.1079-1088.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zobell C. E. The Effect of Solid Surfaces upon Bacterial Activity. J Bacteriol. 1943 Jul;46(1):39–56. doi: 10.1128/jb.46.1.39-56.1943. [DOI] [PMC free article] [PubMed] [Google Scholar]



