Abstract
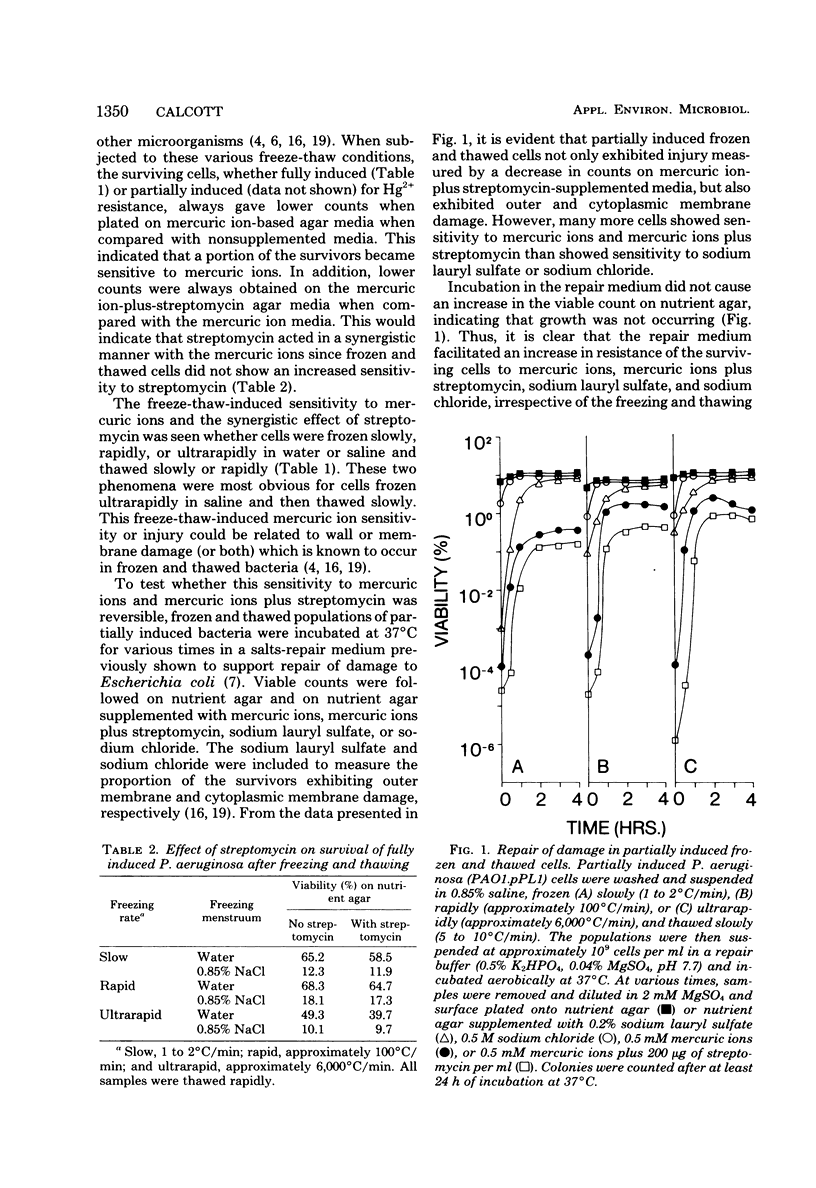
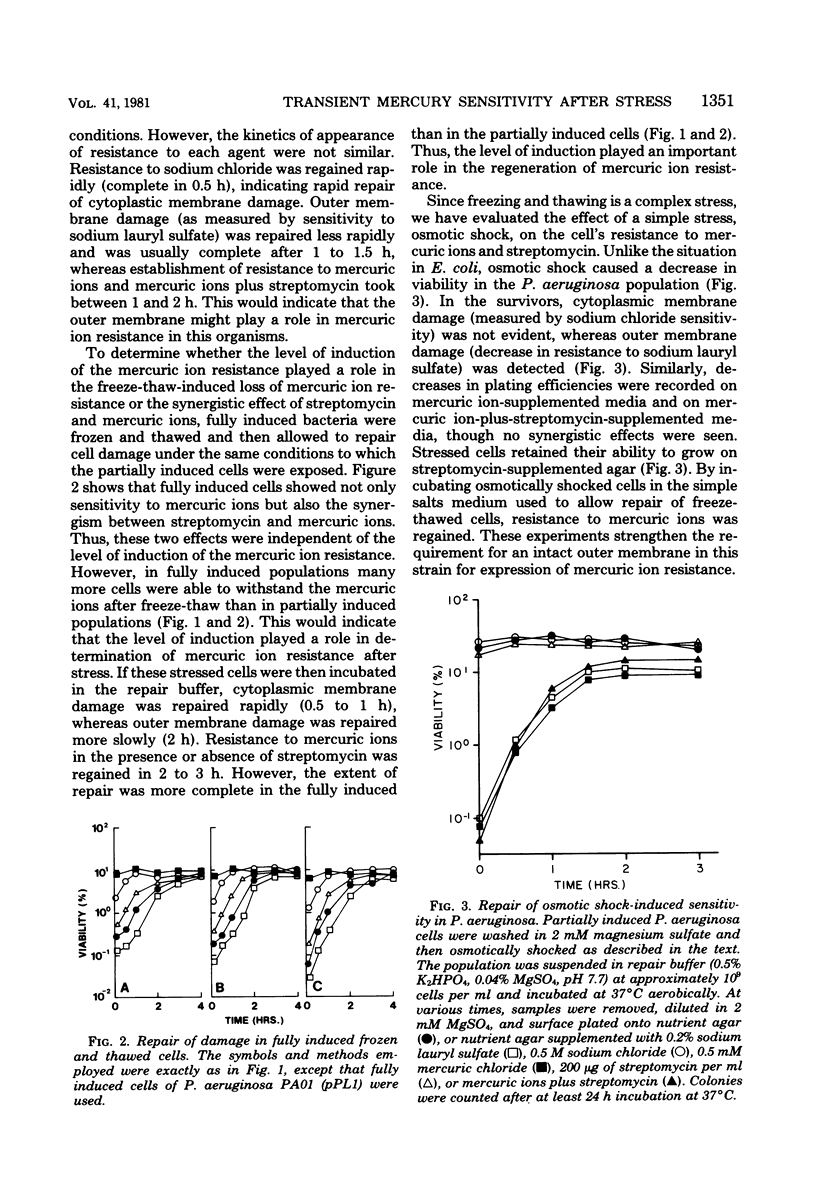
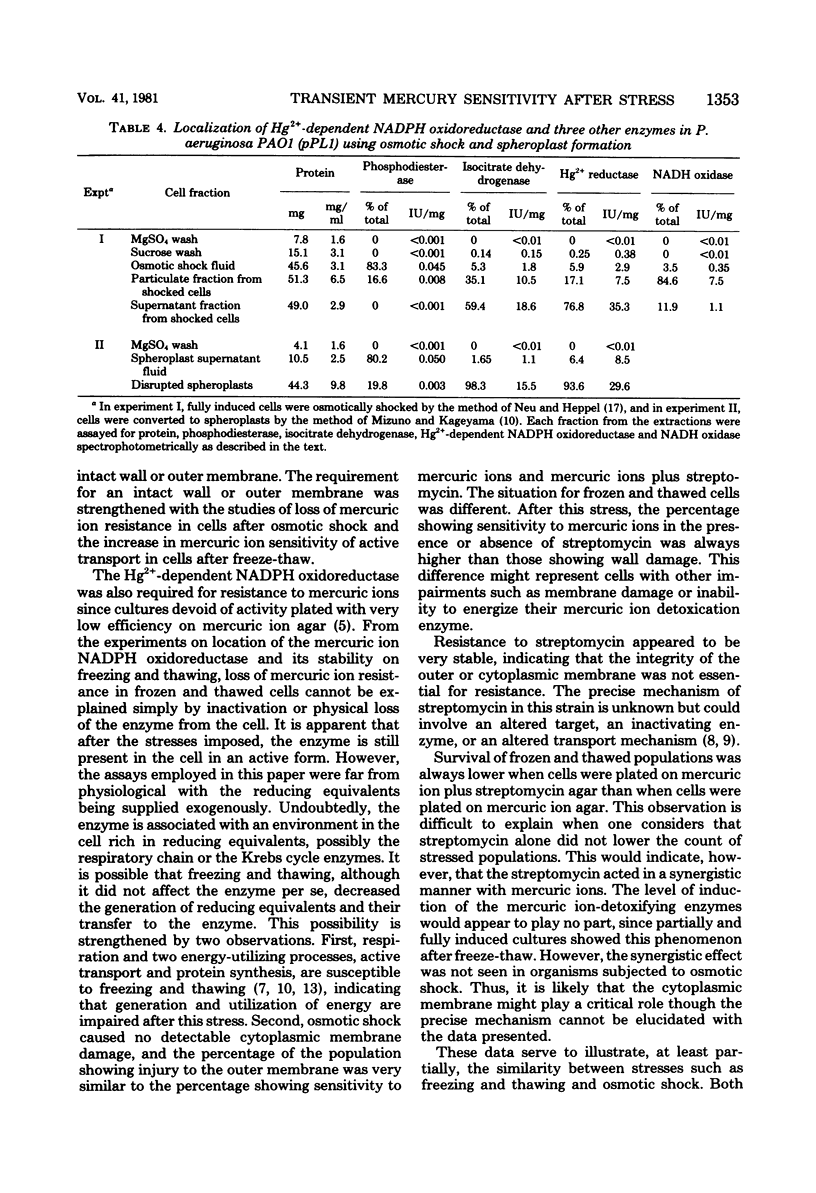
After freezing and thawing, Pseudomonas aeruginosa harboring a drug resistance plasmid (Hg2+r, Strr), became acutely sensitive to mercuric ions but not to streptomycin in the plating medium, whereas its sensitivity to both agents became more pronounced indicating a synergistic effect. This freeze-thaw-induced sensitivity was transient and capable of being repaired to a simple salts medium. Transient outer and cytoplasmic membrane damage was also observed in frozen and thawed preparations. From kinetics studies, repair of cytoplasmic membrane damage superseded repair of outer membrane damage and damage measured by mercuric ions and mercuric ions plus streptomycin. Osmotically shocked cells were also sensitive to mercuric ions, mercuric ions plus streptomycin, and sodium lauryl sulfate, but not to sodium chloride or streptomycin alone. This sensitivity was again transient and capable of repair in the same simple salts medium. Active transport of a non-metabolizable amino acid, alpha-amino isobutyric acid, was sensitive to mercuric ions and became more so after freezing and thawing. A freeze-thaw-resistant mercuric ion-dependent reduced nicotinamide adenine dinucleotide phosphate oxidoreductase was localized in the cytoplasm of this organism. This enzyme and an intact outer membrane appear to be required for mercuric ion resistance in this strain.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bhatti A. R., DeVoe I. W., Ingram J. M. Cell division in Pseudomonas aeruginosa: participation of alkaline phosphatase. J Bacteriol. 1976 Apr;126(1):400–409. doi: 10.1128/jb.126.1.400-409.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatti A. R., DeVoe I. W., Ingram J. M. The release and characterization of some periplasm-located enzymes of Pseudomona aeruginosa. Can J Microbiol. 1976 Oct;22(10):1425–1429. doi: 10.1139/m76-211. [DOI] [PubMed] [Google Scholar]
- Calcott P. H., MacLeod R. A. Survival of Escherichia coli from freeze-thaw damage: a theoretical and practical study. Can J Microbiol. 1974 May;20(5):671–681. doi: 10.1139/m74-103. [DOI] [PubMed] [Google Scholar]
- Chakrabarty A. M. Plasmids in Pseudomonas. Annu Rev Genet. 1976;10:7–30. doi: 10.1146/annurev.ge.10.120176.000255. [DOI] [PubMed] [Google Scholar]
- Davies J., Smith D. I. Plasmid-determined resistance to antimicrobial agents. Annu Rev Microbiol. 1978;32:469–518. doi: 10.1146/annurev.mi.32.100178.002345. [DOI] [PubMed] [Google Scholar]
- Knowles C. J., Calcott P. H., MacLeod R. A. Periplasmic CO-binding c-type cytochrome in a marine bacterium. FEBS Lett. 1974 Dec 1;49(1):78–83. doi: 10.1016/0014-5793(74)80636-8. [DOI] [PubMed] [Google Scholar]
- Komura I., Funaba T., Izaki K. Mechanism of mercuric chloride resistance in microorganisms. II. NADPH-dependent reduction of mercuric chloride and vaporization of mercury from mercuric chloride by a multiple drug resistant strain of Escherichia coli. J Biochem. 1971 Dec;70(6):895–901. doi: 10.1093/oxfordjournals.jbchem.a129719. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lee S. K., Calcott P. H., MacLeod R. A. Relationship of cytochrome content to the sensitivity of bacteria to NaCl on freezing and thawing. Can J Microbiol. 1977 Apr;23(4):413–419. doi: 10.1139/m77-061. [DOI] [PubMed] [Google Scholar]
- Lehrbach P., Kung A. H., Lee B. T., Jacoby G. A. Plasmid modification of radiation and chemical-mutagen sensitivity in Pseudomonas aeruginosa. J Gen Microbiol. 1977 Jan;98(1):167–176. doi: 10.1099/00221287-98-1-167. [DOI] [PubMed] [Google Scholar]
- Mizuno T., Kageyama M. Separation and characterization of the outer membrane of Pseudomonas aeruginosa. J Biochem. 1978 Jul;84(1):179–191. doi: 10.1093/oxfordjournals.jbchem.a132106. [DOI] [PubMed] [Google Scholar]
- Neu H. C., Heppel L. A. The release of enzymes from Escherichia coli by osmotic shock and during the formation of spheroplasts. J Biol Chem. 1965 Sep;240(9):3685–3692. [PubMed] [Google Scholar]
- Ray B., Speck M. L. Freeze-injury in bacteria. CRC Crit Rev Clin Lab Sci. 1973 Aug;4(2):161–213. doi: 10.3109/10408367309151556. [DOI] [PubMed] [Google Scholar]
- Summers A. O., Silver S. Microbial transformations of metals. Annu Rev Microbiol. 1978;32:637–672. doi: 10.1146/annurev.mi.32.100178.003225. [DOI] [PubMed] [Google Scholar]


