Abstract
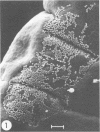
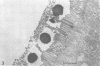
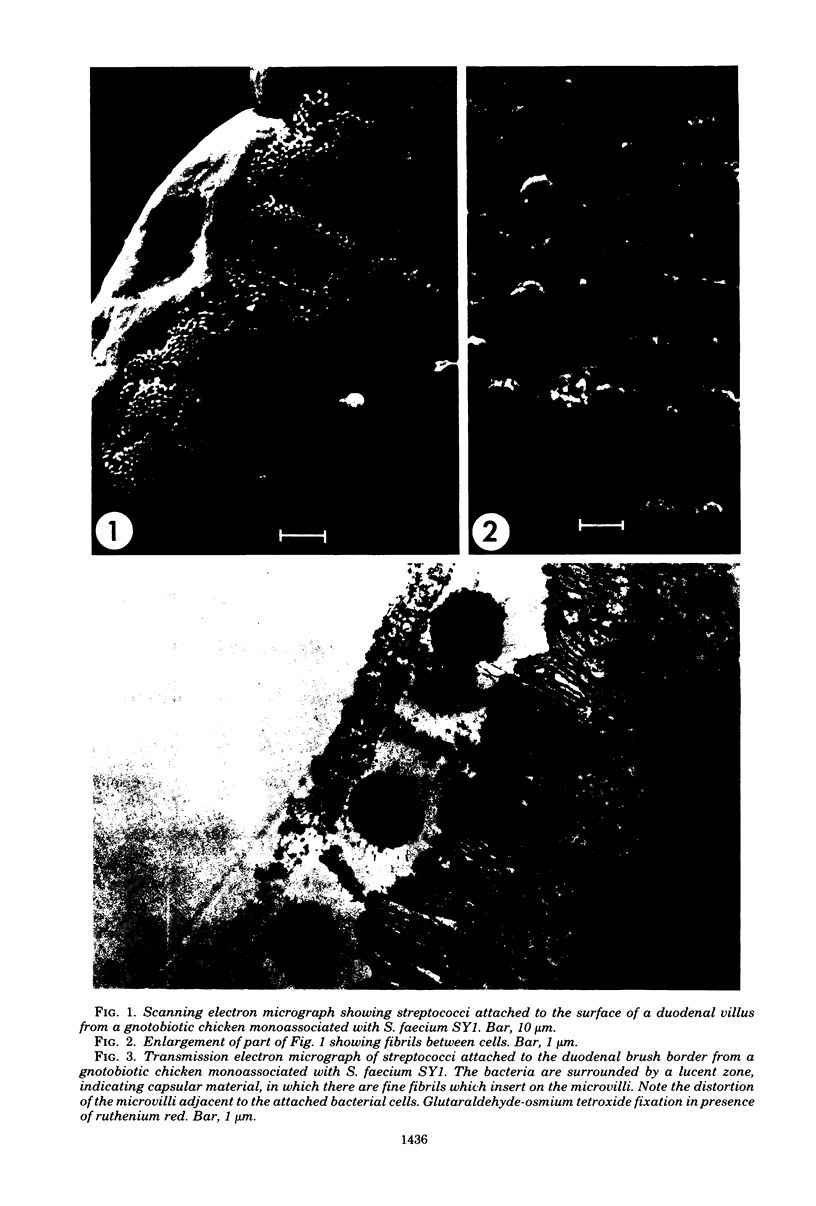
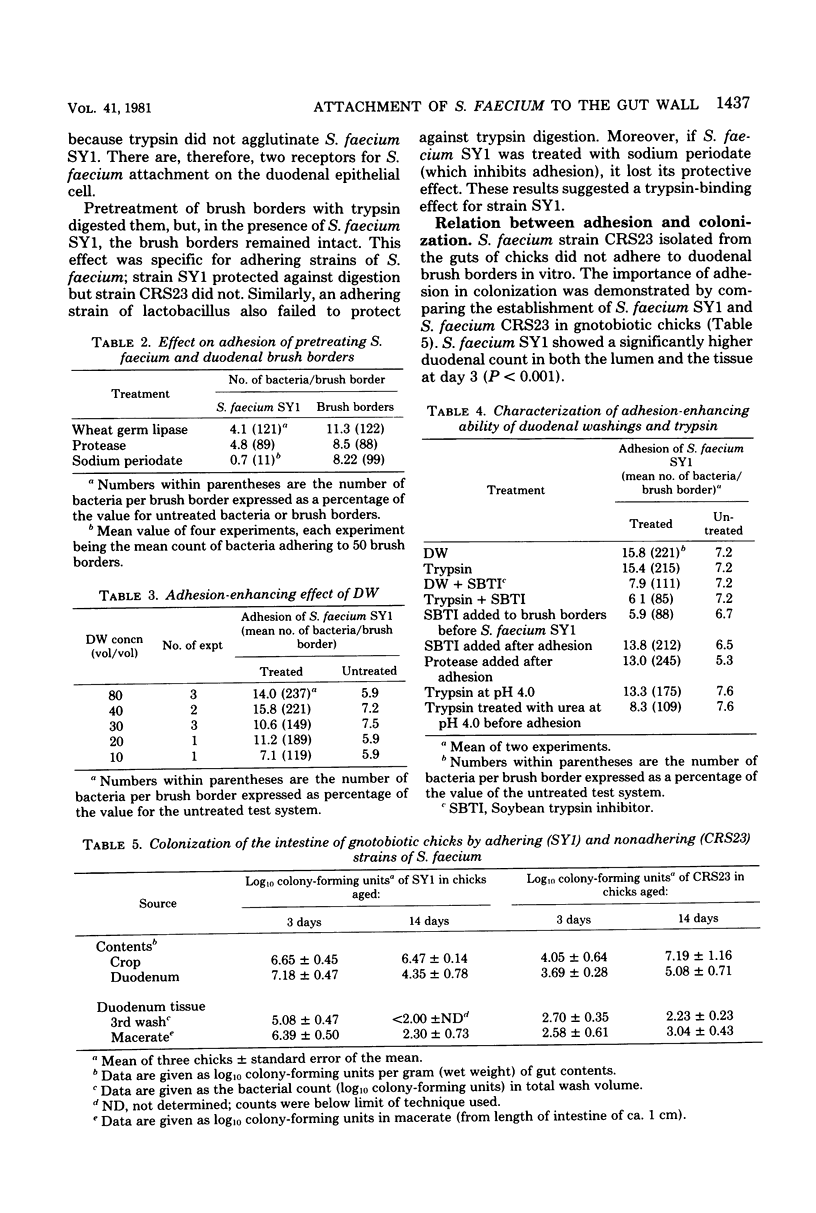
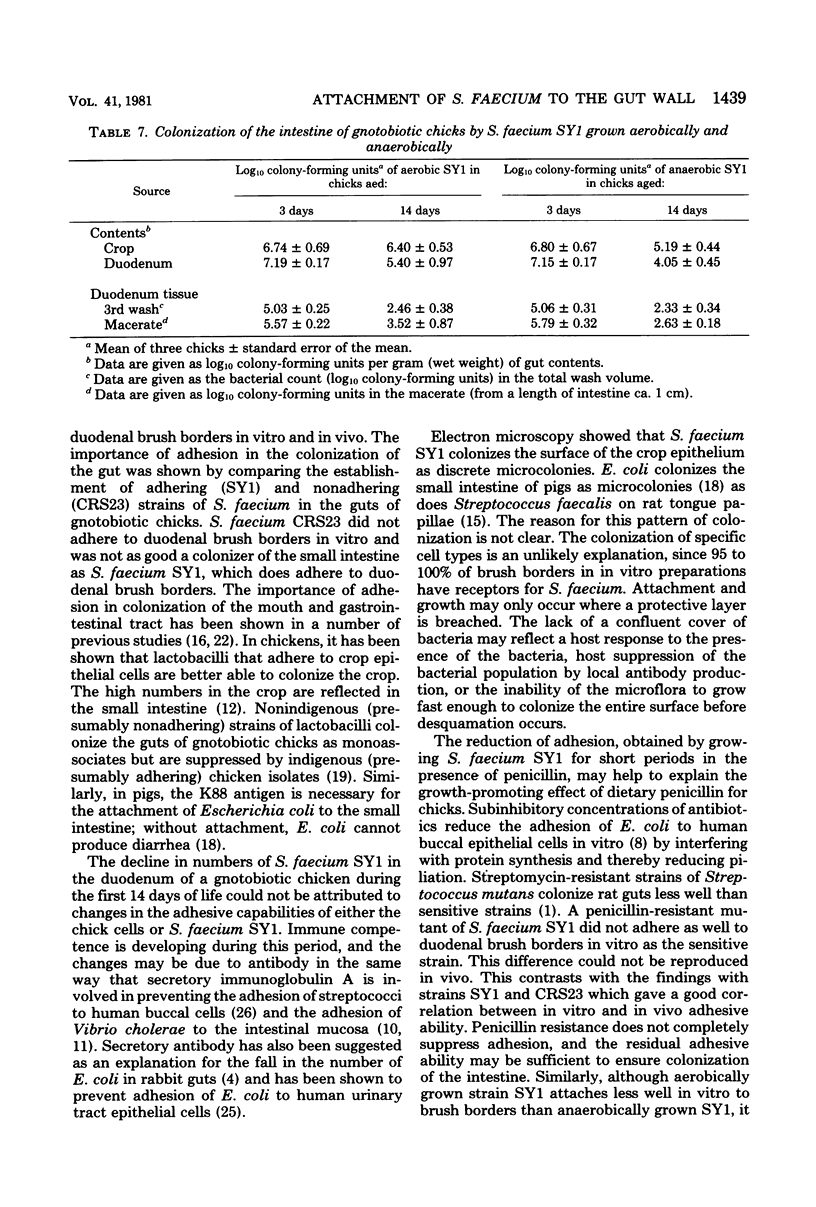
The counts of Streptococcus faecium SY1 in the duodenums of gnotobiotic chicks exceeded the counts in their crops, indicating that multiplication was occurring in the anterior small intestine. This growth was related to adhesion to the gut wall which could be demonstrated by viable counts of macerated washed duodenal tissue. Scanning electron microscopy demonstrated that adhesion occurred in restricted areas on the surface of the villus, and transmission studies showed the presence of a thick extracellular layer on the bacterium. Attachment of S. faecium SY1 was confirmed in vitro by using chicken duodenal brush borders. The washings, produced during the preparation of the brush borders, increased the number of S. faecium adhering to the brush borders. This enhancing effect was due to the presence of trypsin in the duodenal washings. However, the effect was not dependent on the enzymatic activity of the trypsin molecule. The initial adhesion was not prevented by pretreatment of the brush borders with soy bean trypsin inhibitor. There were, therefore, two adhesion systems operating, only one of which was dependent on trypsin. Pretreatment of brush borders with trypsin digested them, but they remained intact in the presence of S. faecium SY1, indicating that the enzymatic activity was being inhibited. This effect was specific for the adhering strain of S. faecium SY1; the nonadhering S. faecium strain CRS23 and an adhering strain of Lactobacillus sp. were inactive, as was strain SY1 when adhesion was prevented by including sodium periodate in the test system. The colonizations of the gut by strains of S. faecium of differing adhesive abilities were compared. The nonadhering strain CRS23 showed reduced ability to colonize the duodenum, but the penicillin-resistant mutant of S. faecium SY1, which had reduced adhesive ability but could still attach to a lesser degree, was able to colonize the duodenum as efficiently as the parent strain.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bammann L. L., Clark W. B., Gibbons R. J. Impaired colonization of gnotobiotic and conventional rats by streptomycin-resistant strains of Streptococcus mutans. Infect Immun. 1978 Dec;22(3):721–726. doi: 10.1128/iai.22.3.721-726.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooker B. E., Fuller R. Adhesion of Lactobacilli to the chicken crop epithelium. J Ultrastruct Res. 1975 Jul;52(1):21–31. doi: 10.1016/s0022-5320(75)80019-0. [DOI] [PubMed] [Google Scholar]
- COATES M. E., FULLER R., HARRISON G. F., LEV M., SUFFOLK S. F. A comparison of the growth of chicks in the Gustafsson germ-free apparatus and in a conventional environment, with and without dietary supplements of penicillin. Br J Nutr. 1963;17:141–150. doi: 10.1079/bjn19630015. [DOI] [PubMed] [Google Scholar]
- Cantey J. R., Hosterman D. S. Characterization of colonization of the rabbit gastrointestinal tract by Escherichia coli RDEC-1. Infect Immun. 1979 Dec;26(3):1099–1103. doi: 10.1128/iai.26.3.1099-1103.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DYER J. R. Use of periodate oxidations in biochemical analysis. Methods Biochem Anal. 1956;3:111–152. doi: 10.1002/9780470110195.ch5. [DOI] [PubMed] [Google Scholar]
- Edén C. S., Hansson H. A. Escherichia coli pili as possible mediators of attachment to human urinary tract epithelial cells. Infect Immun. 1978 Jul;21(1):229–237. doi: 10.1128/iai.21.1.229-237.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenstein B. I., Beachey E. H., Ofek I. Influence of sublethal concentrations of antibiotics on the expression of the mannose-specific ligand of Escherichia coli. Infect Immun. 1980 Apr;28(1):154–159. doi: 10.1128/iai.28.1.154-159.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans D. G., Evans D. J., Jr, Tjoa W. S., DuPont H. L. Detection and characterization of colonization factor of enterotoxigenic Escherichia coli isolated from adults with diarrhea. Infect Immun. 1978 Feb;19(2):727–736. doi: 10.1128/iai.19.2.727-736.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freter R. Interactions between mechanisms controlling the intestinal microflora. Am J Clin Nutr. 1974 Dec;27(12):1409–1416. doi: 10.1093/ajcn/27.12.1409. [DOI] [PubMed] [Google Scholar]
- Fubara E. S., Freter R. Protection against enteric bacterial infection by secretory IgA antibodies. J Immunol. 1973 Aug;111(2):395–403. [PubMed] [Google Scholar]
- Gibbons R. A., Jones G. W., Sellwood R. An attempt to identify the intestinal receptor for the K88 adhesin by means of a haemagglutination inhibition test using glycoproteins and fractions from sow colostrum. J Gen Microbiol. 1975 Feb;86(2):228–240. doi: 10.1099/00221287-86-2-228. [DOI] [PubMed] [Google Scholar]
- Gibbons R. J., Houte J. V. Bacterial adherence in oral microbial ecology. Annu Rev Microbiol. 1975;29:19–44. doi: 10.1146/annurev.mi.29.100175.000315. [DOI] [PubMed] [Google Scholar]
- Gibbons R. J., Spinell D. M., Skobe Z. Selective adherence as a determinant of the host tropisms of certain indigenous and pathogenic bacteria. Infect Immun. 1976 Jan;13(1):238–246. doi: 10.1128/iai.13.1.238-246.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HARRIS J. I. Effect of urea on trypsin and alpha-chymotrypsin. Nature. 1956 Mar 10;177(4506):471–473. doi: 10.1038/177471a0. [DOI] [PubMed] [Google Scholar]
- Jones G. W., Rutter J. M. Role of the K88 antigen in the pathogenesis of neonatal diarrhea caused by Escherichia coli in piglets. Infect Immun. 1972 Dec;6(6):918–927. doi: 10.1128/iai.6.6.918-927.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morishita Y., Mitsuoka T., Kaneuchi C., Yamamoto S., Ogata M. Specific establishment of lactobacilli in the digestive tract of germ-free chickens. Jpn J Microbiol. 1971 Nov;15(6):531–538. doi: 10.1111/j.1348-0421.1971.tb00615.x. [DOI] [PubMed] [Google Scholar]
- Ofek I., Mirelman D., Sharon N. Adherence of Escherichia coli to human mucosal cells mediated by mannose receptors. Nature. 1977 Feb 17;265(5595):623–625. doi: 10.1038/265623a0. [DOI] [PubMed] [Google Scholar]
- Onderdonk A. B., Moon N. E., Kasper D. L., Bartlett J. G. Adherence of Bacteroides fragilis in vivo. Infect Immun. 1978 Mar;19(3):1083–1087. doi: 10.1128/iai.19.3.1083-1087.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sellwood R., Gibbons R. A., Jones G. W., Rutter J. M. Adhesion of enteropathogenic Escherichia coli to pig intestinal brush borders: the existence of two pig phenotypes. J Med Microbiol. 1975 Aug;8(3):405–411. doi: 10.1099/00222615-8-3-405. [DOI] [PubMed] [Google Scholar]
- Svanborg-Edén C., Svennerholm A. M. Secretory immunoglobulin A and G antibodies prevent adhesion of Escherichia coli to human urinary tract epithelial cells. Infect Immun. 1978 Dec;22(3):790–797. doi: 10.1128/iai.22.3.790-797.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh J. University of michigan: letting go the contract lab. Science. 1972 Aug 25;177(4050):677–680. doi: 10.1126/science.177.4050.677. [DOI] [PubMed] [Google Scholar]