Abstract
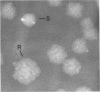
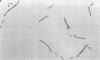
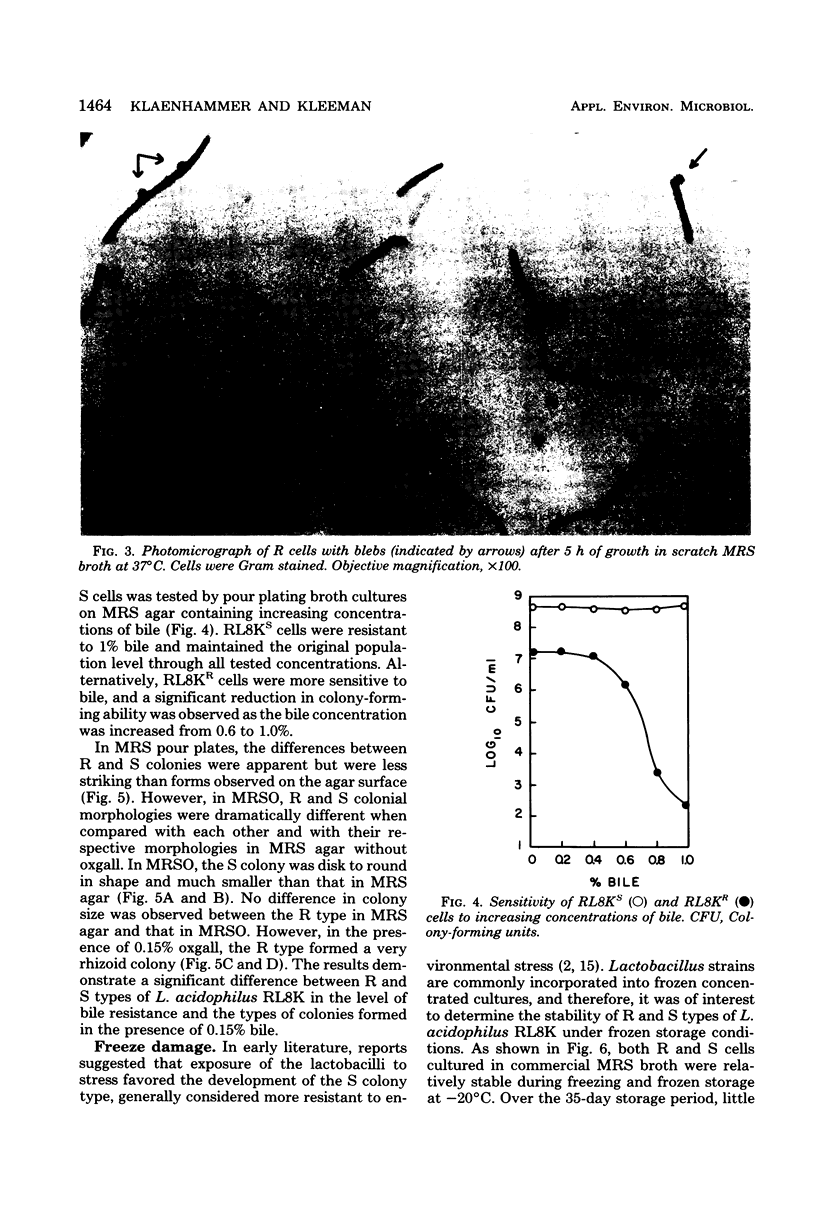
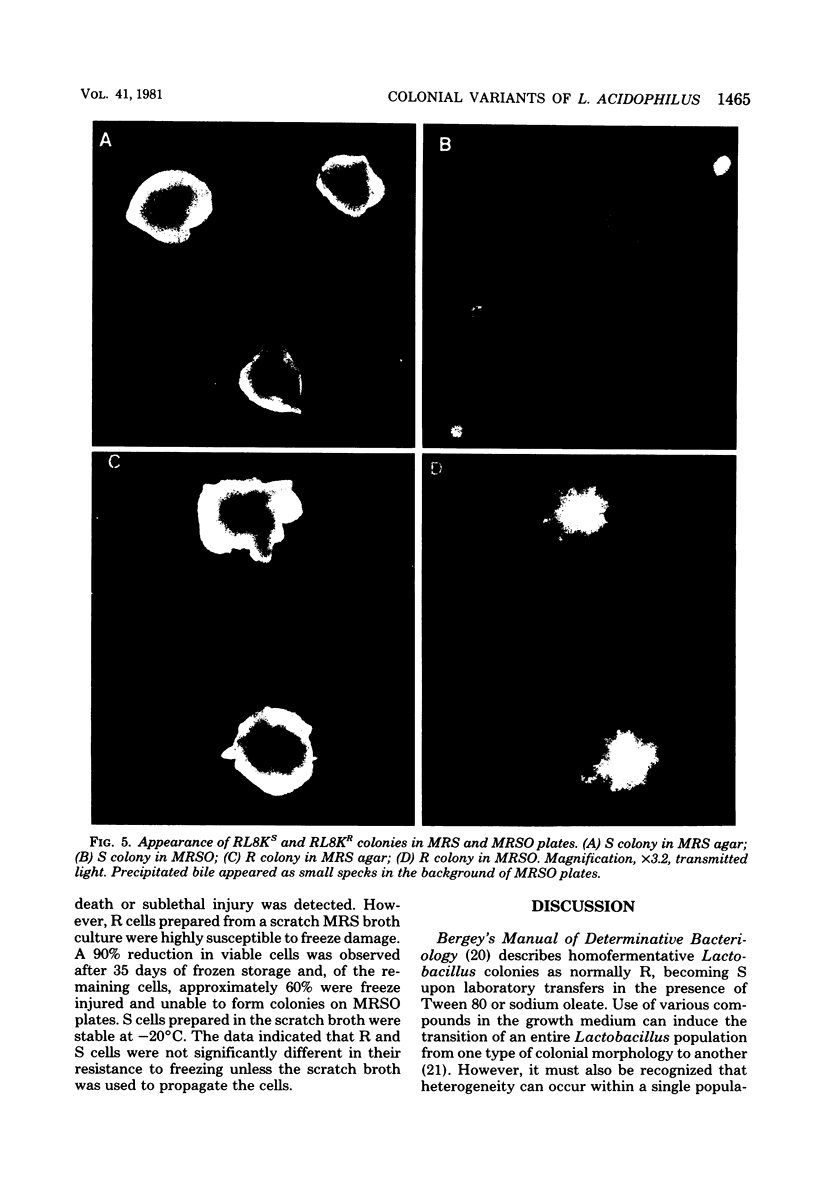
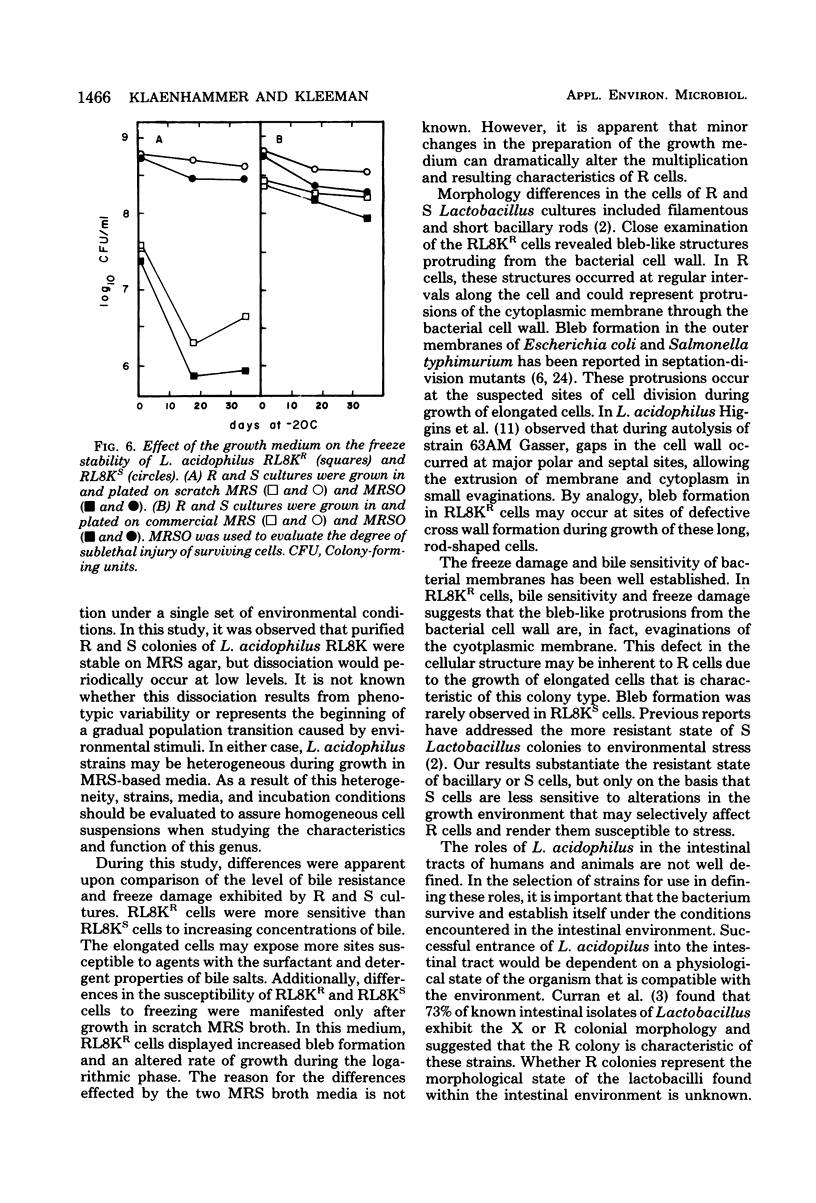
Rough (R) and smooth (S) colonial variants were isolated from a heterogeneous culture of Lactobacillus acidophilus RL8K. R and S types were stable upon repeated transfer on agar, but revertant colonies did appear after broth transfers. When propagated in commercial MRS broth, R and S cultures showed similar growth characteristics, and both cell types were insensitive to freezing and frozen storage at −20°C. Alternatively, during growth in scratch MRS broth, R cultures shifted to a reduced rate of growth during the late logarithmic phase. R cells grown under these conditions were susceptible to death by freezing and injury at −20°C. Microscopically, R cells were observed as long gram-positive rods with small nonstainable blebs protruding from the cell wall. In bile sensitivity studies of R and S cells plated on MRS agar plus oxgall, the S culture was resistant to 1% bile, whereas the R culture was sensitive to 0.6% bile. Differences in the bile resistance and freeze damage of R and S cells suggest that colonial and cellular morphologies are important considerations for the selection of Lactobacillus strains as dietary adjuncts and for the development of growth conditions for preparing frozen concentrated cultures from either cell type.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barber F. W., Frazier W. C. Dissociants of Lactobacilli. J Bacteriol. 1945 Dec;50(6):637–649. [PMC free article] [PubMed] [Google Scholar]
- Curran H. R., Rogers L. A., Whittier E. O. The Distinguishing Characteristics of Lactobacillus acidophilus. J Bacteriol. 1933 Jun;25(6):595–621. doi: 10.1128/jb.25.6.595-621.1933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DEIBEL R. H., DOWNING M., NIVEN C. F., Jr, SCHWEIGERT B. S. Filament formation by Lactobacillus leichmannii when desoxyribosides replace vitamin B12 in the growth medium. J Bacteriol. 1956 Feb;71(2):255–256. doi: 10.1128/jb.71.2.255-256.1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller R. The importance of Lactobacilli in maintaining normal microbial balance in the crop. Br Poult Sci. 1977 Jan;18(1):85–94. doi: 10.1080/00071667708416332. [DOI] [PubMed] [Google Scholar]
- Fung J. C., MacAlister T. J., Weigand R. A., Rothfield L. I. Morphogenesis of the bacterial division septum: identification of potential sites of division in lkyD mutants of Salmonella typhimurium. J Bacteriol. 1980 Aug;143(2):1019–1024. doi: 10.1128/jb.143.2.1019-1024.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilliland S. E., Speck M. L. Use of the Minitek system for characterizing lactobacilli. Appl Environ Microbiol. 1977 Jun;33(6):1289–1292. doi: 10.1128/aem.33.6.1289-1292.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldin B. R., Swenson L., Dwyer J., Sexton M., Gorbach S. L. Effect of diet and Lactobacillus acidophilus supplements on human fecal bacterial enzymes. J Natl Cancer Inst. 1980 Feb;64(2):255–261. doi: 10.1093/jnci/64.2.255. [DOI] [PubMed] [Google Scholar]
- HOLDEN J. T., HOLMAN J. Abnormal cellular morphology associated with a vitamin B6 deficiency in Lactobacillus arabinosus. J Bacteriol. 1957 Apr;73(4):592–593. doi: 10.1128/jb.73.4.592-593.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins M. L., Coyette J., Shockman G. D. Sites of cellular autolysis in Lactobacillus acidophilus. J Bacteriol. 1973 Dec;116(3):1375–1382. doi: 10.1128/jb.116.3.1375-1382.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojima M., Suda S., Hotta S., Hamada K. Induction of pleomorphy and calcium ion deficiency in Lactobacillus bifidus. J Bacteriol. 1970 Apr;102(1):217–220. doi: 10.1128/jb.102.1.217-220.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopeloff L. M., Etchells J. L., Kopeloff N. Bacteriological Changes in Acidophilus Milk at Room and Ice-Box Temperatures. J Bacteriol. 1934 Nov;28(5):489–500. doi: 10.1128/jb.28.5.489-500.1934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDONALD I. J., FRAZIER W. C. Variation in morphology of colonies of lactobacilli. J Bacteriol. 1951 May;61(5):627–637. doi: 10.1128/jb.61.5.627-637.1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROGOSA M., MITCHELL J. A. Induced colonial variation of a total population among certain lactobacilli. J Bacteriol. 1950 Feb;59(2):303–308. doi: 10.1128/jb.59.2.303-308.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz U., Asmus A., Frank H. Autolytic enzymes and cell division of Escherichia coli. J Mol Biol. 1969 May 14;41(3):419–429. doi: 10.1016/0022-2836(69)90285-x. [DOI] [PubMed] [Google Scholar]