Abstract
There is widespread anecdotal evidence that growth hormone (GH) is used by athletes for its anabolic and lipolytic properties. Although there is little evidence that GH improves performance in young healthy adults, randomized controlled studies carried out so far are inadequately designed to demonstrate this, not least because GH is often abused in combination with anabolic steroids and insulin. Some of the anabolic actions of GH are mediated through the generation of insulin-like growth factor-I (IGF-I), and it is believed that this is also being abused. Athletes are exposing themselves to potential harm by self-administering large doses of GH, IGF-I and insulin. The effects of excess GH are exemplified by acromegaly. IGF-I may mediate and cause some of these changes, but in addition, IGF-I may lead to profound hypoglycaemia, as indeed can insulin. Although GH is on the World Anti-doping Agency list of banned substances, the detection of abuse with GH is challenging. Two approaches have been developed to detect GH abuse. The first is based on an assessment of the effect of exogenous recombinant human GH on pituitary GH isoforms and the second is based on the measurement of markers of GH action. As a result, GH abuse can be detected with reasonable sensitivity and specificity. Testing for IGF-I and insulin is in its infancy, but the measurement of markers of GH action may also detect IGF-I usage, while urine mass spectroscopy has begun to identify the use of insulin analogues.
Keywords: GH, IGF-I, insulin, sport, abuse
Introduction
It is widely believed that growth hormone (GH) has been used by sportsmen and women since the 1980s to improve their athletic performance (McHugh et al., 2005) despite being banned for many years and appearing on the World Anti-Doping Agency list of banned substances.
The actions of GH that interest athletes are anabolic and lipolytic, leading to an increase in lean body mass and reduction in fat mass. Some of the anabolic GH actions are mediated through the generation of insulin-like growth factor-I (IGF-I), and there is anecdotal evidence that this too is being abused by athletes either alone or in combination with GH. The regulation of protein synthesis involves the synergistic actions of GH and IGF-I stimulating protein synthesis, while insulin simultaneously inhibits protein breakdown (Russell Jones et al., 1993). GH stimulates protein synthesis through a mechanism that is separate and distinct from anabolic steroids and therefore it seems likely that their effects will be additive. This has led many athletes to combine GH with insulin and anabolic steroids (Sönksen, 2001).
The GH doses used by athletes are thought to be up to 10 times higher than those used by endocrinologists, and as such athletes are putting themselves at risk of harmful effects such as hypertension, diabetes and Creutzfeld–Jakob disease.
The detection of GH poses the greatest contemporary challenge to the anti-doping community. The detection of GH abuse has proved difficult for several reasons. Unlike many substances of abuse, such as synthetic anabolic steroids, GH is a naturally occurring substance. The demonstration of exogenous administration must therefore rely on finding concentrations exceeding normal physiological levels while excluding pathological causes such as acromegaly. This is made harder because GH is secreted in a pulsatile manner with exercise and stress being major stimulators of GH secretion (Prinz et al., 1983; Savine and Sönksen, 2000). Consequently, GH concentrations are often at their highest in the immediate post-competition setting when most dope testing occurs. Recombinant human GH is almost identical to pituitary GH, whereas cadaveric GH, which is in plentiful supply on the Internet, is indistinguishable from endogenously produced GH. Blood testing is needed for GH and IGF-I because less than 0.1% is excreted unchanged and even that is erratic, rendering urine testing unfeasible (Moreira-Andres et al., 1993).
The review will explore why athletes abuse GH, IGF-I and insulin and also the methodology that has been developed to catch the cheats.
GH abuse in sport
Growth hormone was first extracted from human pituitary glands in 1945 (Li et al., 1945). It was shown to promote growth in hypopituitary animals and was soon used to restore growth in children with hypopituitarism. How and where GH was first used as a doping agent is unknown, but the earliest publication to draw attention to it was Dan Duchaine's ‘Underground Steroid handbook', which emerged from California in 1982 (Duchaine, 1982). Although it contains some fundamental errors, such as the recommendation and advertisement of animal GH for use in humans, the description of the actions of GH in this article was remarkably accurate and pre-dated adult endocrinology experience by about a decade. GH was described as the ‘most expensive, most fashionable and least understood of the new athletic drugs. It has firmly established itself in power-lifting and within a few years will be a commonly used drug in all strength athletics.'
The most famous case of GH abuse in professional athletics came to light in 1988 after Ben Johnson won the 100 m gold medal at the Olympic Games in Seoul. He was subsequently disqualified after stanazolol was detected in his urine, but at a later hearing, both he and his coach Charley Francis admitted under oath that he had taken human GH in addition to anabolic steroids.
It is impossible to determine the precise prevalence of GH abuse among sportsmen and women, as much of our evidence comes from anecdotal reports (McHugh et al., 2005). Although initially advocated for strength disciplines, endurance athletes are also attracted to GH's lipolytic actions and reduced fat mass; in 1988, a large quantity of GH was found in a team car at the Tour de France.
There is evidence that adolescents are using GH. In a survey of two US high schools, 5% of male students admitted to having taken GH and nearly one-third knew someone who had taken GH (Rickert et al., 1992). Most GH users were unaware of its side effects and reported their first use between 14–15 years of age.
Growth hormone is an expensive drug and this has led some parents to sell GH prescribed to treat their child's GH deficiency on the black market. Officials have also misappropriated GH for their athletes. At the 1998 World Swimming Championships, Yuan Yuan, a Chinese swimmer, was stopped on entry into Perth with a suitcase full of GH that had been exported to China for therapeutic reasons.
A few athletes have admitted to taking GH. In a death-bed confession, Lyle Alzado, an American football player, admitted that 80% of American footballers have taken GH. In 2000, Australian discus champion Walter Reiterer claimed institutional and supervised usage of GH. With this in mind, it is interesting to note that 6 months before the Sydney Olympic Games, 1575 vials of GH were stolen from an importer's warehouse in Sydney.
More recently, Victor Conte, the owner of the Bay Area Laboratory Co-Operative, claimed that he had supplied GH to many high-profile American athletes including Tim Montgomery and Marion Jones. This admission came after the raid on the Bay Area Laboratory Co-Operative's headquarters on 3 September 2003, when evidence of systematic doping was found and many of the top names in athletics, baseball and American football were implicated in the scandal. Although many have denied taking GH, Tim Montgomery allegedly admitted to taking GH before a US Federal grand jury and later faced a 2-year ban for doping offences. Conte was imprisoned for 4 months for his role in the scandal (Fainaru-Wada and Williams, 2006).
The recent conviction of Sylvester Stallone, who was caught with GH in his possession on entering Australia, suggests that GH is readily available in athletic and body-building circles.
IGF-I abuse in sport
The prevalence of IGF-I abuse is probably much lower than for GH because, unlike GH, there is no readily available natural source, and therefore all IGF-I is obtained through recombinant DNA technology. Two companies currently market IGF-I, and these preparations have only recently received approval for use in humans to treat growth failure in children with severe primary IGF-I deficiency or with GH gene deletion who have developed neutralizing GH antibodies. The first product is Increlex or recombinant human IGF-I, manufactured by Tercica, and the second product manufactured by Insmed is Iplex, which differs from Increlex in that the recombinant human IGF-I is supplied bound to its major binding protein, IGFBP-3 (Kemp et al., 2006; Kemp, 2007).
Although in relatively short supply, other companies make IGF-I for cell culture and other uses, and this material could also become available to athletes. The wider availability of IGF-I, together with an appreciation of the efforts to detect GH abuse, is likely to increase its illicit use by athletes, despite being prohibited by the World Anti-Doping Agency.
Abuse of insulin in sport
We have only sketchy details about the use of insulin by professional athletes. It is alleged that short-acting insulin is being used in a haphazard way to increase muscle bulk in body builders, weight lifters and power lifters (Sönksen, 2001). After concerns raised by the Russian medical officer at the Nagano Olympic games, the International Olympic Committee immediately banned its use in those without diabetes. Athletes with insulin-requiring diabetes may use insulin with a medical exemption.
Physiology of the GH–IGF axis
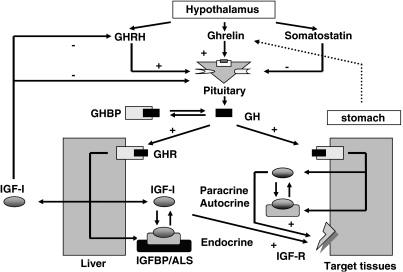
Growth hormone is the most abundant pituitary hormone and is secreted in a pulsatile manner under the control of the hypothalamic hormones, GH-releasing hormone, somatostatin and ghrelin (Figure 1).
Figure 1.
The growth hormone (GH)–insulin-like growth factor (IGF)-I axis. GH is secreted from the pituitary gland under the control of the hypothalamic hormones, somatostatin, Ghrelin and GH-releasing hormone (GHRH). GH circulates bound to its binding protein and acts through specific cell-surface receptors. The anabolic actions of GH are partially mediated by IGF-I. IGF-I acts through the IGF-I receptor in an autocrine, paracrine and classical endocrine mechanisms. Circulating IGF-I is almost entirely bound to a family of high-affinity binding proteins (IGFBPs) that coordinate and regulate the biological functions of the IGFs. IGF-I inhibits GHRH and GH secretion in a classical negative-feedback mechanism.
Regulation of GH secretion
Growth hormone-releasing hormone and ghrelin stimulate the synthesis and release of GH, whereas somatostatin is inhibitory in action (Veldhuis, 2003). Although ghrelin is secreted by the hypothalamus, the major source of ghrelin is the stomach and it is thought to be one mechanism that controls the GH response to eating (van der Lely et al., 2004). Circulating IGF-I reduces GH secretion through classical negative endocrine feedback (Carroll et al., 1997).
There are a number of physiological stimuli that increase or inhibit GH release, the most important of which are exercise and sleep (Savine and Sönksen, 2000). The highest GH peaks occur at night within the first hour of sleep during slow-wave sleep (Takahashi et al., 1968). Nutritional status regulates GH secretion both acutely and chronically; hypoglycaemia, reduced circulating free fatty acids (FFA) and higher amino-acid concentrations all increase GH secretion (Ho et al., 1988; Pombo et al., 1999), whereas in the longer term, GH secretion is increased in anorexia nervosa and decreased with obesity (Veldhuis et al., 1991; Argente et al., 1997).
Age and gender are important determinants of GH secretion (Savine and Sönksen, 2000). Secretion is highest during the pubertal growth spurt, and after the mid-20s, GH secretion decreases by 14% every decade (Toogood, 2003), and this decrease may be responsible for some of the age-related body composition changes (Holt et al., 2001). Women have higher baseline GH secretion but the pulses are not as high and are less erratic than in men (van den Berg et al., 1996).
The actions of GH and IGF-I
Growth hormone exerts its multiple metabolic and anabolic actions through binding to specific GH receptors that are found on every cell of the body (Holt, 2004). Following binding to the GH receptor, the tyrosine kinase Janus kinase 2 is activated and multitude of signalling cascades are initiated that result in the wide variety of biological responses, including cellular proliferation, differentiation and migration, prevention of apoptosis, cytoskeletal reorganization and regulation of metabolic pathways (Lanning and Carter-Su, 2006). Although a detailed description of these signalling cascades is beyond the scope of this review, it is worth remarking on the number of signalling proteins and pathways activated by GH, which include JAKs, signal transducers and activators of transcription, the mitogen-activated protein kinase pathway, and the phosphatidylinositol 3′-kinase pathway. Although these pathways are well described, the inter-relationship between the different pathways is not fully understood.
Growth hormone exerts most of its anabolic actions through the generation of circulating IGF-I (the somatomedin hypothesis) (Le Roith et al., 2001), the majority of which is produced in the liver. IGF-I may also act in a paracrine or autocrine fashion in response to GH action at other target tissues. Transgenic animals, in which the IGF-I gene has been selectively deleted in the liver and whose serum IGF-I is marked reduced, have led some to question the somatomedin hypothesis as these animals appear to grow normally (Sjogren et al., 1999; Yakar et al., 1999), even though with the development of insulin resistance (Sjogren et al., 2001; Yakar et al., 2001). It is known, however, that the total amount of circulating IGF-I in an adult is not required for normal growth, as neonates have much lower circulating levels but grow very rapidly. The precise mechanisms regulating IGF-I bioavailability to the tissues are not yet understood but certainly involve IGF-binding proteins (IGFBPs). As IGFBP distribution in liver IGF-I knockout mice, as in newborn humans, is quite different from that in healthy adults, it has been argued that these data do not really challenge the view that endocrine IGF-I has a role in growth regulation.
Effects on whole body physiology
The physiological effects of GH are best examined by considering the condition of adult GH deficiency. Studies of hypopituitary individuals with appropriate replacement of thyroid, steroid and sex steroid replacement have shown that GH plays a pivotal role in body composition, well-being, physical performance and cardiovascular health (Cuneo et al., 1992; Carroll et al., 2000). In the absence of GH, lean tissue is lost and fat accumulates, and correspondingly waist-to-hip ratio increases as visceral fat increases (Table 1).
Table 1.
Clinical features of GH deficiency and effect of GH replacement
| Effect of GH deficiency | Effect of GH replacement |
|---|---|
| Body composition | |
| Increased body fat | Decreased fat mass |
| Increased waist–hip ratio | Decreased waist–hip ratio |
| Increased visceral fat mass | Decreased visceral fat |
| Decreased lean body mass | Increased lean body mass |
| Decreased bone mineral density | Increased bone mineral density |
| Physical performance | |
| Decreased muscle mass | Increased muscle mass |
| Decreased muscle strength | Increased muscle strength |
| Decreased maximal exercise performance | Increased maximal exercise performance |
| Decreased maximum oxygen uptake | Increased maximal oxygen uptake, maximum power output, maximum heart rate and anaerobic threshold |
| Decreased maximum heart rate | Increased red cell mass |
| Psychological well-being | |
| Decreased ability to cope with daily life | Increased energy levels |
| Increased level of perceived health problems | Increased ability to participate in physical activities without tiring |
| Decreased physical and mental energy | Increased emotional reaction and social isolation scores |
| Decreased concentration skills | Increased perceived quality of life |
| Decreased initiative | Increased self-esteem |
| Increased social isolation | Decreased sleep requirement |
| Decreased self-esteem | |
| Decreased sex life | |
| Increased sleep requirement | |
| Cardiovascular system | |
| Increased prevalence of cardiovascular events | Increased left ventricular mass |
| Increased hypertension | Increased stroke volume |
| Decreased left ventricular mass | Increased cardiac output and resting heart rate |
| Decreased fibre shortening | Decreased diastolic blood pressure |
Effects on intermediate metabolism
Protein metabolism
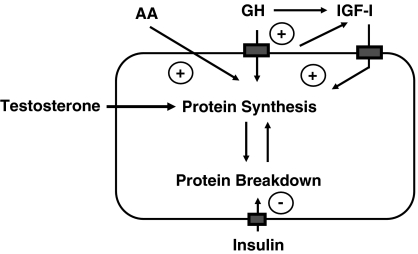
Protein synthesis and degradation are each regulated by multiple hormonal and nutritional factors, and protein turnover in individual tissues and the whole body is in a state of constant flux. Insulin, GH and IGF-I have synergistic anabolic effects on protein metabolism (Figure 2) (Sönksen, 2001).
Figure 2.
The synergistic action between insulin, IGF-I and GH in regulating protein synthesis. Without insulin, GH loses much (if not all) of its anabolic action. GH and IGF-I stimulate protein synthesis directly, whereas insulin is anabolic through inhibition of protein breakdown. The anabolic action of both GH and IGF-I appears to be mediated through induction of amino-acid transporters in the cell membrane. It is not yet clear how much of the action of IGF-I is through locally generated IGF-I (‘autocrine' and ‘paracrine') or through circulating IGF-I that is largely derived from the liver.
Growth hormone causes nitrogen retention, as shown by decreased urinary excretion rates of urea, creatinine and ammonium. In healthy humans, acute administration of GH modestly stimulates muscle and whole body protein synthesis (Fryburg et al., 1991). When GH is infused locally into the brachial artery, forearm muscle protein synthesis increases, whereas systemic IGF-I concentrations and whole body protein turnover are unchanged, indicating that GH stimulates protein synthesis directly as well as indirectly through IGF-I (Fryburg et al., 1991).
Insulin-like growth factor-I has an anabolic effect on protein metabolism, inhibiting whole body protein breakdown and stimulating protein synthesis (Fryburg, 1994). This effect is dependent on serum insulin and an adequate supply of amino acids. After IGF-I is administered systemically, serum insulin and amino acids decrease, the former through decreased production and the latter as a result of increased clearance from the blood. This attenuates the stimulation of whole body protein synthesis, but if the amino acids and insulin are replaced during the administration, the effect on protein synthesis by IGF-I is clearly seen (Russell Jones et al., 1994; Jacob et al., 1996).
Protein breakdown is inhibited by physiological concentrations of plasma insulin, the ‘Chalonic' action of insulin (Umpleby and Sönksen, 1985; Tessari et al., 1986). In contrast, the rates of protein degradation are increased in cardiac and skeletal muscles in situations where insulin concentrations are low such as type 1 diabetes or starvation (Charlton and Nair, 1998). Although the rate of protein synthesis is reduced by 40–50% in young diabetic rats, a physiological anabolic effect of insulin on protein synthesis has not been confirmed in humans (Liu and Barrett, 2002).
The similarities between IGF-I and insulin suggest that these proteins act in a coordinated manner to regulate protein turnover. There are interesting differences, however, in their respective dose–response curves. Low physiological insulin concentrations inhibit protein breakdown and increase glucose disposal into skeletal muscle, whereas higher, non-physiological concentrations are required to stimulate protein synthesis (Louard et al., 1992). In contrast, increases in IGF-I that have no effect on glucose uptake stimulate protein synthesis, and higher concentrations are required to inhibit protein breakdown (Fryburg, 1994). During the last decade, there have been advances in the understanding of the intracellular signalling mechanisms of insulin and IGF-I, many of which are shared, such as insulin receptor substrate 1. The precise mechanism by which these similar but divergent pathways interact is not fully understood, but IGF-I, similar to GH, most likely acts through stimulating amino-acid uptake.
Lipolysis
The administration of GH to humans, either by continuous infusion or by a bolus injection, leads to stimulation of lipolysis and increased fasting FFA concentrations with the peak effect around 2–3 h after the injection (Hansen, 2002). These findings are in keeping with the changes in FFAs following physiological stimuli of GH secretion. In young healthy subjects, the nocturnal or exercise-induced peak of GH precedes the peak of FFAs by 2 h (Moller et al., 1995). Furthermore, during times of fasting or energy restriction, the lipolytic effect of GH is enhanced, although the effect is suppressed by co-administration of food or glucose (Moller et al., 2003). Acromegaly is associated with increased circulating FFAs, increased muscle uptake of FFAs and increased lipid oxidation.
Although GH receptors are abundantly expressed on adipocytes, it has been suggested that GH has a permissive effect on catecholamine-induced lipolysis (Hansen, 2002). In vitro studies have shown that GH has no direct lipolytic effect on human fat cells, but markedly increases the maximal lipolysis induced by catecholamines (Marcus et al., 1994).
Glucose homeostasis
The first observation that GH had an effect on glucose metabolism came in the 1930s when it was observed that hypophysectomy ameliorated the hyperglycaemia of experimental diabetes in dogs (Houssay and Biasotti, 1930). In both healthy subjects and those with type 1 diabetes, GH increases fasting hepatic glucose output, by increasing hepatic gluconeogenesis and glycogenolysis, and decreases peripheral glucose utilization through the inhibition of glycogen synthesis and glucose oxidation (Tamborlane et al., 1979; Bak et al., 1991; Fowelin et al., 1991, 1993, 1995). Patients with acromegaly develop insulin resistance and hyperinsulinaemia (Sönksen et al., 1967), and up to 40% become diabetic (Ezzat et al., 1994; Colao et al., 2000).
Although the effect on glucose homeostasis would appear to be disadvantageous for the athletes, it is worth bearing in mind that long-standing adult GH deficiency is associated with insulin resistance and that any acute effect on glucose homeostasis does not take into account changes in IGF-I, which also affects insulin sensitivity (Salomon et al., 1994).
Intravenous IGF-I causes hypoglycaemia in rats by stimulating peripheral glucose uptake, glycolysis and glycogen synthesis, although having only a minimal effect on hepatic glucose production (Jacob et al., 1989). In dogs, however, IGF-I has been shown to suppress hepatic glucose output, but to a lesser degree than insulin at the doses used, as well as increasing peripheral glucose utilization (Shojaee Moradie et al., 1995). As hepatic expression of the IGF-I receptor is reportedly low (Stefano et al., 2006), it is possible that this effect of IGF-I on hepatic glucose output is by an indirect mechanism. For example, IGF-I may bind the insulin receptor with low affinity (Holt et al., 2003). Alternatively, it may improve whole body insulin sensitivity by inhibiting the secretion of GH.
The effects of an intravenous IGF-I infusion in humans are similar to those described in animals and lead to hypoglycaemia (Zenobi et al., 1992). Insulin sensitivity increases with respect to glucose by IGF-I through increased peripheral glucose uptake and decreased hepatic glucose production (Boulware et al., 1994; Russell Jones et al., 1995). A subcutaneous infusion of IGF-I also causes hypoglycaemia, but the effect is slower in onset than insulin and decreases more slowly after the infusion was stopped, because of the presence of the IGF-binding proteins.
Bone metabolism
Growth hormone has profound effects on bone metabolism. GH deficiency is associated with osteopaenia, which is reversed by GH replacement (Gomez et al., 2000). Male subjects with osteoporosis have reduced GH peaks and low serum IGF-I concentrations, and furthermore, IGF-I concentrations correlate well with estimates of bone mineral density (Patel et al., 2005). In addition to a direct effect on bone stimulating the cycle of bone growth, there is evidence to suggest that GH and IGF-I may modify intestinal calcium absorption and serum 1,25 hydroxy vitamin D concentrations. Patients with acromegaly have increased intestinal calcium absorption (Lund et al., 1981), and GH therapy in pigs increases intestinal calcium absorption, probably through increased production of serum 1,25 hydroxy vitamin D (Chipman et al., 1980). GH replacement therapy in adult GHD leads to a short-term increase in serum 1,25 hydroxy vitamin D concentrations (Burstein et al., 1983). These data suggest that GH increases intestinal calcium absorption by its effects on renal 25 (OH)D 1-α-hydroxylase activity, but other mechanisms may also be involved (Halloran and Spencer, 1988).
Why do athletes abuse GH?
The importance of GH in adult physiology as well as in children was confirmed beyond doubt in 1989 by two independent studies undertaken in the UK and Denmark (Jorgensen et al., 1989; Salomon et al., 1989). Both groups undertook double-blind, placebo-controlled trials in adults with hypopituitarism given appropriate replacement therapy with everything except GH. The studies showed remarkably congruous results after 6 months of GH treatment.
The most impressive finding was a change in and normalization of body composition with an average 6 kg increase in lean body mass, largely accounted by an increase in skeletal muscle, and a concomitant loss of fat. The body composition changes were accompanied by improvements in quality of life, particularly in the area of ‘increased energy' and performance enhancements (McGauley et al., 1990; Cuneo et al., 1991a, 1991b). Longer studies over the first 3 years of GH replacement showed that exercise performance continued to improve (Jorgensen et al., 1994).
Does GH enhances performance in normal healthy young adults?
There has been considerable debate about the ability of GH to translate these body composition and other metabolic changes into improved performance (Rennie, 2003). Despite the theoretical benefits of GH, sceptics point to the condition of acromegaly and negative clinical studies as evidence of a lack of benefit.
Acromegaly—nature's experiment of GH excess
It has been argued that acromegaly, where there is over-secretion of GH usually from a pituitary adenoma, provides evidence that excess GH is not performance enhancing, as it is not associated with athletic prowess. Indeed, acromegaly is usually associated with muscle weakness rather than excessive strength (Table 2) (McNab and Khandwala, 2005). It needs to be appreciated, however, that acromegaly frequently remains undiagnosed for many years and the clinical presentation at diagnosis may not reflect earlier stages of the disease. Many patients if questioned carefully will give a history of increased strength in the first few years of their condition. Indeed, we know a rower who competed at an elite level during the early stages of his acromegaly. Not only was he one of the strongest crew, but he could also tolerate harder training sessions than his colleagues and recovered more quickly afterwards (PHS, unpublished data).
Table 2.
Clinical features of acromegaly
| Musculoskeletal (acromegaly unless indicated) |
| Increased stature (gigantism) |
| Protruding mandible (prognathia) |
| Teeth separation on lower jaw |
| Big tongue (macroglossia) |
| Enlarged forehead (frontal bossing) |
| Large hands and feet |
| May cause carpal tunnel syndrome |
| Osteoarthritis from abnormal joint loading |
| Cardiovascular |
| Dilated cardiomyopathy |
| Hypertension |
| Metabolic |
| Impaired glucose tolerance or diabetes |
| Skin |
| Thickened, greasy skin |
| Excessive sweating |
| General |
| Headaches |
| Tiredness |
Although only a clinical case report, it illustrates that the timing and degree of GH excess are important for the physiological effect. Prolonged massive GH excess coupled frequently with deficiencies of other pituitary hormones, such as ACTH, may tell us little about the effects of lesser GH excess earlier in the natural history of the illness.
Can clinical trials provide us with the answer?
Although there have been many negative studies in this area, traditional randomized controlled trials that have the power to examine differences of 20–30% are ill suited to detect the much smaller differences that determine whether an individual wins a gold medal or not. Despite these difficulties, one trial showed that, in healthy, elderly men, the combination of GH and testosterone led to a 20% improvement in fitness as measured by maximal oxygen uptake (Giannoulis et al., 2006), which was larger than with either compound alone. Very recently, a well designed and executed study in past abusers of anabolic steroids showed for the first time an ergogenic effect of GH in healthy young athletes (Graham et al., 2008).
A further problem with the designs of our clinical trials is that they are designed to test one or at most two interventions. In reality, for the reasons described above, GH is frequently used by athletes in combination with insulin and anabolic steroids in varying concentrations during differing training and dietary regimens. It is impossible to control for all these variables within a single trial, and therefore it seems likely that the athletes using the ‘n=1' design are probably best placed to address the question whether GH is performance enhancing. This certainly appeared to be a potent weapon when abused by the former East German coaches (Franke and Berendonk, 1997).
A definitive answer to this question will probably never be found, as it would be difficult to obtain ethics approval for a suitable study. It should be remembered, however, that there were similar arguments about anabolic steroids 20 years ago that have subsequently been shown to have performance benefits.
Pharmacology of GH, IGF-I and insulin administration
The half-life of endogenously secreted GH is around 13 min (Sohmiya and Kato, 1992). It is rapidly cleared from the body by the liver, kidney and peripheral tissues through interaction with GH receptors. If recombinant human GH (rhGH) is administered intravenously, the half-life is similar to endogenously secreted GH (Refetoff and Sönksen, 1970; Haffner et al., 1994). In practice, however, the pharmacokinetics of exogenously administered GH differs, as it is given by intermittent, usually daily, subcutaneous injection. Following injection, GH concentrations increase and reach a maximum concentration after about 2–6 h, depending on the age and gender of the recipient (Kearns et al., 1991; Janssen et al., 1999). The estimated bioavailability of rhGH is 50–70%, because of degradation at the site of injection. This may explain why intramuscular injection results in a higher maximal and area-under-the-curve GH concentration than subcutaneous injection (Keller et al., 2007). Thereafter, rhGH is rapidly cleared and GH is usually undetectable in women 12 hours after injection, even after high doses, while men have only low levels of GH. (Giannoulis et al., 2005). There is quicker clearance of GH in women, probably reflecting their extra body fat that contains a high density of GH receptors (Vahl et al., 1997). Longer-acting GH preparations are currently being developed.
The pharmacokinetics of IGF-I is complicated by the presence of a family of highly specific binding proteins (IGFBPs) that coordinate and regulate the biological functions of IGF-I. Less than 5% of serum IGF-I is free and most is bound in a ternary complex of IGF-I, IGFBP-3 and an acid-labile subunit (Figure 1). The half-life of free IGF-I is only a few minutes, whereas the half-lives of IGF-I bound in a binary and ternary complex are 20–30 min and 12–15 h, respectively (Guler et al., 1989). When subcutaneous IGF-I is administered to healthy volunteers, the maximum concentration is achieved at about 7 h and half life is 20 h (Grahnen et al., 1993). The half-life is prolonged when IGF-I is administered as a complex with IGFBP-3. In patients with severe GH insensitivity syndrome, IGF-I concentrations peaked between 15 and 19 h after the injection of the complex, and a single injection was effective in increasing IGF-I concentrations in these patients for a 24 h period (Camacho-Hubner et al., 2006).
The half-life of intravenous insulin is only 4 min, but apart from the treatment of diabetic emergencies and possibly the replenishment of glycogen using an insulin clamp (Sönksen and Sönksen, 2000), pharmacologically administered insulin is through subcutaneous injection (Matthews et al., 1985). Insulin manufacturers have developed many preparations of insulin including insulin analogues in the attempt to provide insulin replacement to people with diabetes in the most physiological way (Peterson, 2006). Consequently, the shortest-acting insulin analogues appear in the circulation within 5–10 min of injection and cleared within 4–6 h, whereas longer-acting insulins are present for over 24 h.
Potential adverse effects of GH administration
The side effects of GH administration to adults with GH deficiency are well documented, and any athlete receiving GH will potentially be at risk of these side effects (Powrie et al., 1995). It is believed, however, that many athletes are using doses that are up to 10 times higher than those used therapeutically. The effects of chronically administering this dose of GH are unknown, but it would be reasonable to expect that athletes may develop some of the features of acromegaly with prolonged use (Table 2).
Sodium and fluid retention
Growth hormone causes fluid retention through its action on the kidney to promote sodium reabsorption (Powrie et al., 1995; Moller et al., 1999). This may be manifest as ankle swelling, hypertension and headache.
Diabetes
Patients with acromegaly develop insulin resistance and hyperinsulinaemia, and up to 40% become diabetic (Sönksen et al., 1967; Ezzat et al., 1994; Colao et al., 2000).
Cardiomyopathy
Cardiovascular complications are a major cause of morbidity and mortality in patients with acromegaly (Colao et al., 2001). The excess of GH and IGF-I causes a specific derangement of cardiomyocytes, leading to abnormalities in cardiac muscle structure and function, inducing a specific cardiomyopathy. In the early phase, there is a hyperkinetic syndrome, characterized by increased heart rate and systolic output. Two-thirds of patients have concentric cardiac hypertrophy and this is commonly associated with diastolic dysfunction and eventually with impaired systolic function leading to heart failure, if the acromegaly is left untreated. In addition, abnormalities of cardiac rhythm and those of heart valves have also been described. The coexistence of arterial hypertension and diabetes may further aggravate acromegalic cardiomyopathy. It is alleged that the American sprinter, Florence Griffith-Joyner (Flo Jo), purchased GH from fellow sprinter Darrell Robinson. When Flo Jo died at the age of 38 years, her heart was enlarged consistent with cardiomyopathy (Sullivan, 1998).
Cancer
Although controversial, the consensus statement of the Growth Hormone Research Society (2001) was that there is no increased risk of cancer when GH is given at physiological replacement doses (2001). There is evidence to suggest that acromegaly, where GH levels have been much higher than physiological doses for many years, may be associated with increased rates of colorectal, thyroid, breast and prostate cancers (Jenkins et al., 2006).
Creutzfeld–Jakob disease
Initially, the only source of GH came from extracts of human pituitary glands, and tragically, this was discovered to be a source for the prion-induced Creutzfelt–Jacob disease (Brown et al., 1985). As a result, pituitary-derived GH was withdrawal from the market place in 1985 and was replaced with recombinant human GH in 1987. Despite the dangers, supplies of pituitary-derived GH continue to be available on the black market to this day, and athletes continue to use this and thus a case of Creutzfelt–Jacob disease may emerge in an elite athlete at some time in the future.
Potential adverse effects of IGF-I and insulin administration
We have only limited experience with the use of exogenous IGF-I, and so most of the known side effects relate to short-term usage only. It seems reasonable to hypothesize, however, that many of the longer-term effects of GH administration would also occur with IGF-I, as the anabolic effects of GH are closely related to the production of IGF-I in different tissues.
In the clinical trials, the commonest short-term side effects are oedema, headache, arthralgia, jaw pain and hypoglycaemia (Kemp et al., 2006; Kemp, 2007). These appear to be more marked when IGF-I is used alone as the recombinant human IGFBP-3 seems to buffer the acute effects of IGF-I.
The side effects of insulin are well documented from our experience in treating people with diabetes. The most commonly experienced side effect is hypoglycaemia. Weight gain is also a problem in people with diabetes, but this is probably less of an issue for athletes whose diet and training regimens are closely monitored.
Detection of GH abuse
Two complementary approaches have been investigated to detect GH abuse: the first is based on the detection of different pituitary GH isoforms, whereas the second relies on measurement of GH-dependent markers. The very different approaches are viewed as a major strength, as their different properties mean that they are useful in different situations.
The isoform or differential immunoassay method
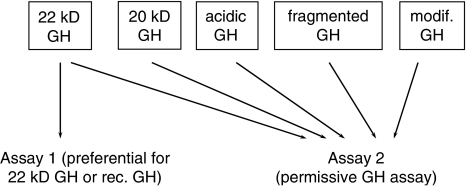
Growth hormone exists as multiple isoforms; 70% of circulating GH is in the form of a 22-kilo Dalton (kDa) polypeptide, whereas 5–10% occurs as a 20 kDa isoform, from mRNA splicing. Dimers and oligomers of GH exist as do acidic, desaminated, acylated and fragmented forms (Baumann, 1999). The differential immunoassay approach is based on the principle that endogenous GH occurs as a number of isoforms, whereas rhGH contains only the 22 kDa isoform (Figure 3). When rhGH is administered in sufficiently high doses, there is a suppression of endogenous GH secretion through negative feedback to the pituitary, and therefore the ratio between 22 kDa GH and non-22 kDa GH is increased (Bidlingmaier et al., 2003). This change in ratio can be detected with specific immunoassays that distinguish the different isoforms.
Figure 3.
Principle of the isoform method. rhGH contains 22 kDa and this is specifically recognized by assay 1. Pituitary GH contains multiple isoforms and these are recognized by assay 2. When rhGH is administered, endogenous production of pituitary GH decreases, and therefore the ratio between assay 1 and assay 2 increases after rhGH administration.
The isoform method was first established by Christian Strasburger and Martin Bidlingmaier in Germany by employing one assay that specifically measured 22 kDa GH and another permissive assay that measured all GH isoforms (Figure 3). A slightly different approach has been adopted by an Australian Japanese Consortium that developed assays that specifically measure either 22 or 20 kDa GH (Momomura et al., 2000).
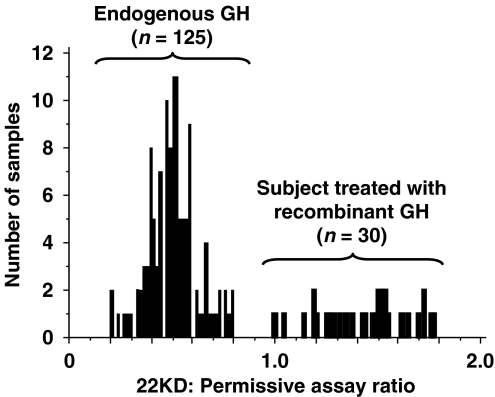
When the German method is applied to a normal population, the ratio between 22 kDa and total GH is less than 1 with a normal distribution of values, whereas individuals receiving GH have values that are greater than one (Figure 4) (Wu et al., 1999). Age, sex, sporting discipline, ethnicity and pathological states do not seem to affect the relative proportions of GH isoforms (Holt, 2007), but exercise causes a transient relative increase in the 22 kDa isoform, thereby lowering the sensitivity of the test if samples are taken immediately after competition (Wallace et al., 2001a, 2001b).
Figure 4.
Ratio between 22 kDa-hGH assay and total hGH assay in serum samples obtained from 125 controls and 30 individuals treated with rhGH. Mean values are 1.43±0.21 for treated individuals and 0.50±0.12 for controls (P<0.0001). Adapted from Wu et al. (1999). Figure reproduced with the permission of Christian Strasburger.
The short half-life and rapid clearance of rhGH, even when injected subcutaneously, means that the ‘window of opportunity' for detection of GH doping with this test is less than 36 h (Keller et al., 2007). As GH is usually administered in the evening, GH is frequently undetectable in a blood sample taken the following day (Giannoulis et al., 2005). The 20 kDa GH remains suppressed for 14–30 h in women depending on the dose used, whereas in men, 20 kDa GH remains undetectable for up to 36 h (Keller et al., 2007). Spontaneous GH secretion returns 48 h after the last dose of rhGH treatment (Wu et al., 1990). Consequently, any athlete who stops administering GH several days before the competition will not be detected. Thus the isoform method is unlikely to catch a GH abuser in the classical ‘post competition' dope testing scenario, and the optimal use of this method must be in unannounced ‘out of competition' testing. This method does not detect individuals receiving cadaveric GH, IGF-I or GH secretagogues.
This test was introduced at the Athens and Turin Olympic Games and no positives were detected in samples taken ‘post competition'. One of the World Anti-Doping Agency rules for the use of immunoassays is that two antibodies recognizing different epitopes are needed for each analyte. The German group had carefully characterized their assays and antibodies, and this may have been an overriding factor in the decision to implement the test.
The GH-dependent marker method
Growth hormone administration leads to the alteration of the concentrations or ratios of several serum proteins, and this change may be used as a means of detecting exogenous GH. An ideal marker or combination of markers would have well-defined reference ranges, would change in response to GH administration and would remain altered after GH has been discontinued (Holt, 2007). The marker should be largely unaffected by other regulators of GH secretion such as exercise or injury and should be validated across populations.
This approach was pioneered by the large multi-centre GH-2000 project coordinated by Peter Sönksen with funding from the European Union under their BIOMED 2 initiative, with additional funding from the International Olympic Committee and the rhGH manufacturers Novo Nordisk and Pharmacia. The aim was to develop a test in time for the Sydney Olympic Games. It had three main components, the first of which was a cross-sectional study of elite athletes at National or International events to establish a reference range of selected markers of GH action (Healy et al., 2005). Blood samples taken within 2 h of competitions showed that the markers were dependent on age as is the case in the general population, but in contrast, sporting discipline, gender and body shape had little effect. The findings of this study were subsequently confirmed by the Australian Japanese consortium led by Ken Ho. In a study of 1103 elite athletes sampled out of competition, less than 10% of the total variance of the markers was explained by gender, sporting discipline, ethnicity and body mass index, whereas age contributed to between 20 and 35% of the variance (Nelson et al., 2006).
The second component of the GH-2000 project was the ‘wash-out' study (Wallace et al., 1999, 2000). When the project was conceived, 25 potential markers of GH action were considered. The aim of the ‘wash-out' study was to narrow this number down to the most suitable markers for a more in-depth analysis. rhGH was administered to recreational male athletes for 1 week, with blood samples collected during and after the GH administration. Subjects also undertook exercise tests to assess the potential effect of ‘competition' on the markers. Nine markers, either members of the IGF–IGF-binding protein axis or markers of bone and soft tissue turnover, were then selected for analysis in the third component of the GH-2000 project. This was a 28-day GH administration study involving self-administered rhGH at two doses to 102 recreational athletes under double-blind, placebo-controlled conditions to evaluate the potential markers for their ability to discriminate active drug from placebo and to assess the ‘window of opportunity' when the test remained positive after rhGH was stopped (Dall et al., 2000; Longobardi et al., 2000).
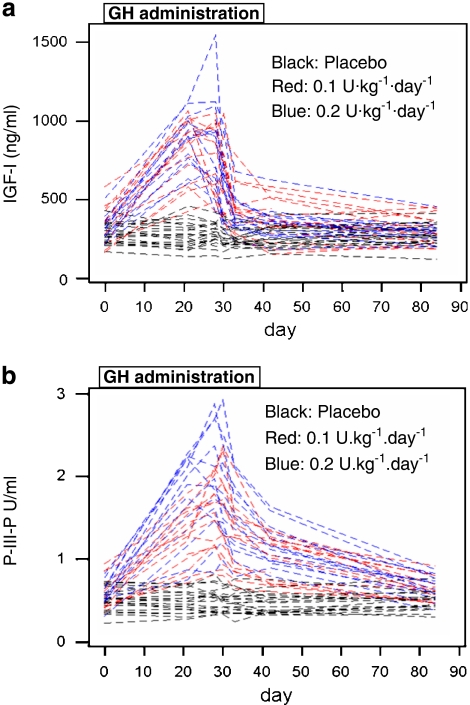
From these studies, the GH-2000 project proposed a test based on IGF-I and type 3 pro-collagen (P-III-P) (Powrie et al., 2007) (Figure 5). These markers were chosen because they provided the best discrimination between individuals receiving GH or placebo during the randomized controlled trial. They exhibit little diurnal or day-to-day variation and are largely unaffected by exercise or gender (McHugh et al., 2005). In the wash-out study, IGF-I and P-III-P increased 20 and 10.2%, respectively, following exercise, but this increase was small in comparison with the larger 300% increase in the markers with GH (Wallace et al., 1999, 2000). Although discrimination was the prime reason for the selection, it is important to note that these proteins are produced by different tissues, thereby reducing the number of pathological conditions that could lead to an elevation in both markers and potential false-positives.
Figure 5.
Change in IGF-I (a) and P-III-P (b) following the administration of GH or placebo for 28 days to 50 healthy male volunteers.
It is known that there is sexual dimorphism in the GH–IGF axis. There are small differences in IGF-I and P-III-P concentrations in elite male and female athletes (Healy et al., 2005), although gender explained around 1% of the overall variance in these markers (Nelson et al., 2006). Women are known to be inherently more resistant to the actions of GH and so the increase in markers is less pronounced in women than in men (Dall et al., 2000; Longobardi et al., 2000). This potential disadvantage may be offset because women may need to receive higher doses to obtain a performance-enhancing benefit.
Although a single marker could be used, by combining markers in conjunction with gender-specific equations, ‘discriminant functions', the sensitivity and specificity of the ability to detect GH abuse can be improved compared with single-marker analysis (Powrie et al., 2007).
The procedure used to generate the discriminant functions involves splitting the available data into two: a ‘training' set of data is used to calculate the discriminant function and a ‘confirmatory' set is then used to validate the sensitivity and specificity of the discriminant function. The confirmatory set required to ensure the model is applicable to the general population and not just the ‘training' set.
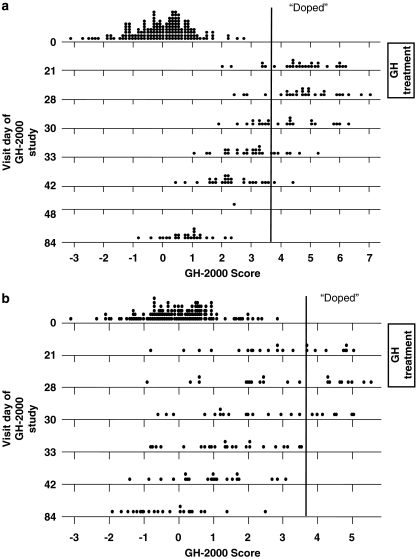
The sensitivity of any test is dependent on the specificity. Standard medical practice accepts as ‘normal' values those being within two standard deviations of the mean, but by definition, 5% of the population lie outside the ‘normal range'. This creates an unacceptably high false-positive rate if applied to athletes. The specificity to be used has not been determined by the anti-doping authorities, but nevertheless the GH-2000 formulae show reasonable sensitivity even up to false-positive rates of 1 in 10 000 and beyond (Figure 6). The formula has been modified more recently to take into account the effect of age to prevent younger athletes from being placed at a disadvantage (Powrie et al., 2007).
Figure 6.
Change in GH-2000 score in men (a) and women (b) following 28 days of GH administration. Dotplot of the standardized scores for each visit day of the studies by group and data set. The mean of a normal population is 0 and the standard deviation is 1. Using a pre-defined sensitivity of 1 in 10 000, samples with a score of >3.7 are identified correctly as receiving GH (labelled as ‘doped'). Note none of the baseline or placebo values are above 3.7.
The results of the GH-2000 project were presented at an International Olympic Committee workshop in Rome in March 1999 to review critically and assure the quality of the results. The conclusion of the workshop was strong support for the methodology, but it was felt that several issues needed to be addressed before the test could be fully implemented at an Olympic games. The biggest issue was related to potential ethnic effects of GH, as the vast majority of volunteers in the GH-2000 study were white Europeans. It was felt that injury could confound the test and further work was needed to develop immunoassays owned by International Olympic Committee and subsequently World Anti-Doping Agency to prevent arbitrary changes being made to commercially owned immunoassays.
Apart from the assay development, these issues have largely been addressed by the GH-2004 study. A further cross-sectional study of elite athletes has shown that although there are small differences in the mean values between ethnic groups—for example, the IGF-I concentrations in Afro-Caribbean men are approximately 8.2% lower than white European men—nearly all the values lie within the 99% prediction intervals for white European athletes, regardless of ethnic background. A further double-blind GH administration study suggests that the response to GH in other ethnic groups is similar to white European amateur athletes. The effect of injury was systemically examined by the GH-2004 team who followed 143 men and 40 women following a sporting injury. There was no change in IGF-I over the 12-week follow-up, but P-III-P increased by approximately 20%, reaching a peak 2–3 weeks after injury. This, however, did not cause any false-positive readings in the proposed test combining IGF-I with P-III-P.
Several other groups have also examined the use of GH-dependent markers, the first of which pre-dated the GH-2000 study. In this first study, the ratio of IGFBP-2 to IGFBP-3 was found to discriminate between those taking GH or placebo (Kicman et al., 1997). These findings were not supported by the GH-2000 study, although several other groups have confirmed the utility of IGF-I and P-III-P. The Institut für Dopinganalytik und Sportbiochemie in Kreischa, Germany, undertook a 14-day GH administration study in amateur male athletes and derived a discriminant function based on IGF-I, P-III-P and IGFBP-3 (Kniess et al., 2003). Most recently, the Australian Japanese Consortium presented the results of an 8-week GH administration study at the American Endocrine Society meeting in Toronto in June 2007. This also confirmed the value of IGF-I and P-III-P, although this suggested that an alternative bone marker (carboxyterminal cross-linked telopeptide of type I collagen) might provide better discrimination during the wash-out phase. This study also examined the effect of co-administration of anabolic steroids in males and showed that there were additive effects on P-III-P.
These confirmatory studies are important because it is unknown how well these GH-2000 formulae will perform in ‘real life', where the patterns and doses of GH abused by athletes are unclear. When the male GH-2000 formula was applied to an independent data set obtained from the Institut für Dopinganalytik und Sportbiochemie Kreischa, 90% of the individuals who had received GH were correctly identified and there were no false-positives, findings that were identical to those of the GH-2000 data when the formula was used (Erotokritou-Mulligan et al., 2007).
Although this methodology has been rigorously tested, the development of World Anti-Doping Agency-owned immunoassays has lagged behind the science underpinning the method, despite the International Olympic Committee having been made aware of the need for these assays before the worldwide introduction of this test.
Future technologies to detect GH
Surface plasmon resonance
Surface plasmon technology is a non-labelled optical methodology that measures the refractive index of small quantities of a material absorbed onto a metal surface allowing measurement of mass. This technology is being applied for the detection of GH and dependent markers by the Barcelona anti-doping laboratory, but at present does not yield the same sensitivity as conventional immunoassays.
Mass spectrometry
Surface-enhanced laser desorption/ionization–time-of-flight mass spectrometry is a proteomic technique in which proteins are bound to proprietary protein chips with different types of adsorptive surfaces. It can be used to analyse peptide and protein expression patterns in a variety of clinical and biological samples, and biomarker discovery can be achieved by comparing the protein profiles obtained from control and patient groups to elucidate differences in protein expression. This technique has been applied to the detection of GH abuse to find potential new markers of GH abuse, such as the haemoglobin α-chain (Chung et al., 2006), but the sensitivity is insufficient to deal with analyses of IGF-I and P-III-P.
The detection of IGF-I
At present, there are no technologies to detect IGF-I abuse, but it is reasonable to adopt a similar approach as the GH-dependent marker test, and this is currently being evaluated by the GH-2004 team. Similarly, this approach should detect athletes abusing GH secretagogues.
The detection of insulin
The challenges of detecting insulin are in many ways similar to GH in that insulin is a naturally occurring pulsatile peptide hormone. At present, there are no methods to detect endogenous insulin abuse but urinary mass spectroscopy may be useful to detect the presence of analogue insulin. This technique involves concentration of the urine and followed by isolation by immunoaffinity chromatography. The eluate may then be analysed using microbore liquid chromatography/tandem mass spectrometry that produces characteristic product spectra obtained from the analogues that are distinguishable from human insulin (Thevis et al., 2006; Thomas et al., 2007). Some insulin analogues are handled differently from insulin and excreted in much higher quantities, which may facilitate this approach (Tompkins et al., 1981).
Future challenges
A major challenge for the future is the use of gene doping, where DNA is incorporated into target tissues, such as skeletal muscle, with or without the aid of a vector, such as an adenovirus. Expression of the gene may then lead to enhanced local production of an anabolic substance such as IGF-I.
Proof-of-concept experiments have been undertaken in animals in which injection of a recombinant adeno-associated virus genetically manipulated to induce myocyte overexpression of IGF-I in young mice induced a 15% increase in muscle mass and a 14% increase in muscle strength without inducing a systemic increase in IGF-I (Barton-Davis et al., 1998).
It is unclear whether this technology is being used by athletes; certainly, it has not been used in clinical practice, where it is highly needed despite significant investment. Anecdotal evidence, however, suggests that athletes are considering its use and this would provide new challenges to the anti-doping community. Traditional blood and urine testing may be of no benefit if gene doping causes no change in serum concentrations of the relevant proteins. Although the detection of vectors may be possible and changes in blood markers may occur, new technologies will be needed to catch this new form of doping.
Conclusion
Doping with GH and its related protein IGF-I remains a major challenge for those working in anti-doping. Anecdotal evidence suggests that abuse with GH and insulin is common, whereas the abuse of IGF-I is set to increase. There are compelling physiological reasons to explain why GH may have performance benefits. The athletes are risking long-term harm by using these drugs. Over the last decade, there have been major advances in methodologies to detect GH, and this should mean that once World Anti-Doping Agency has established suitable assays for IGF-I and P-III-P in its worldwide network of laboratories, athletes will no longer be able to cheat by taking GH without being caught.
Acknowledgments
The GH-2004 project is funded by the United States Anti-Doping Agency and the World Anti-Doping Agency. Our thanks go to the rest of the GH-2004 team: Eryl Bassett, Ioulietta Erotokritou-Mulligan, David Cowan, Christiaan Bartlett and Cathy McHugh. The GH-2004 study was undertaken in the Wellcome Trust Clinical Research Facility (WT-CRF) at Southampton General Hospital and we acknowledge the support of the WT-CRF nurses and Southampton medical students who have supported the study. We also pay tribute to our scientific collaborators, Astrid Kniess, Ken Ho, Anne Nelson, Christian Strasburger and Martin Bidlingmaier.
Abbreviations
- FFA
free fatty acid
- GH
growth hormone
- IGF-I
insulin-like growth factor-I
- IGFBP
IGF binding protein
- P-III-P
type 3 pro-collagen
- rhGH
recombinant human growth hormone
Conflict of interest
RIGH and PHS have received research funding from the World Anti-Doping Agency and US Anti-Doping Agency.
References
- Argente J, Caballo N, Barrios V, Munoz MT, Pozo J, Chowen JA, et al. Multiple endocrine abnormalities of the growth hormone and insulin-like growth factor axis in patients with anorexia nervosa: effect of short- and long-term weight recuperation. J Clin Endocrinol Metab. 1997;82:2084–2092. doi: 10.1210/jcem.82.7.4090. [DOI] [PubMed] [Google Scholar]
- Bak JF, Moller N, Schmitz O. Effects of growth hormone on fuel utilization and muscle glycogen synthase activity in normal humans. Am J Physiol. 1991;260:736–742. doi: 10.1152/ajpendo.1991.260.5.E736. [DOI] [PubMed] [Google Scholar]
- Barton-Davis ER, Shoturma DI, Musaro A, Rosenthal N, Sweeney HL. Viral mediated expression of insulin-like growth factor I blocks the aging-related loss of skeletal muscle function. Proc Natl Acad Sci USA. 1998;95:15603–15607. doi: 10.1073/pnas.95.26.15603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann G. Growth hormone heterogeneity in human pituitary and plasma. Horm Res. 1999;51 Suppl 1:2–6. doi: 10.1159/000053128. [DOI] [PubMed] [Google Scholar]
- Bidlingmaier M, Wu Z, Strasburger CJ. Problems with GH doping in sports. J Endocrinol Invest. 2003;26:924–931. doi: 10.1007/BF03345245. [DOI] [PubMed] [Google Scholar]
- Boulware SD, Tamborlane WV, Rennert NJ, Gesundheit N, Sherwin RS. Comparison of the metabolic effects of recombinant human insulin-like growth factor-I and insulin Dose–response relationships in healthy young and middle-aged adults. J Clin Invest. 1994;93:1131–1139. doi: 10.1172/JCI117065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown P, Gajdusek DC, Gibbs CJ, Jr, Asher DM. Potential epidemic of Creutzfeldt–Jakob disease from human growth hormone therapy. N Engl J Med. 1985;313:728–731. doi: 10.1056/NEJM198509193131205. [DOI] [PubMed] [Google Scholar]
- Burstein S, Chen IW, Tsang RC. Effects of growth hormone replacement therapy on 1 25-dihydroxyvitamin D and calcium metabolism. J Clin Endocrinol Metab. 1983;56:1246–1251. doi: 10.1210/jcem-56-6-1246. [DOI] [PubMed] [Google Scholar]
- Camacho-Hubner C, Rose S, Preece MA, Sleevi M, Storr HL, Miraki-Moud F, et al. Pharmacokinetic studies of recombinant human insulin-like growth factor I (rhIGF-I)/rhIGF-binding protein-3 complex administered to patients with growth hormone insensitivity syndrome. J Clin Endocrinol Metab. 2006;91:1246–1253. doi: 10.1210/jc.2005-1017. [DOI] [PubMed] [Google Scholar]
- Carroll PV, Christ ER, Sönksen PH. Growth hormone replacement in adults with growth hormone deficiency: assessment of current knowledge. Trends Endocrinol Metab. 2000;11:231–238. doi: 10.1016/s1043-2760(00)00268-x. [DOI] [PubMed] [Google Scholar]
- Carroll PV, Umpleby M, Ward GS, Imuere S, Alexander E, Dunger D, et al. rhIGF-I administration reduces insulin requirements decreases growth hormone secretion and improves the lipid profile in adults with IDDM. Diabetes. 1997;46:1453–1458. doi: 10.2337/diab.46.9.1453. [DOI] [PubMed] [Google Scholar]
- Charlton M, Nair KS. Protein metabolism in insulin-dependent diabetes mellitus. J Nutr. 1998;128:323S–327S. doi: 10.1093/jn/128.2.323S. [DOI] [PubMed] [Google Scholar]
- Chipman JJ, Zerwekh J, Nicar M, Marks J, Pak CY. Effect of growth hormone administration: reciprocal changes in serum 1 alpha 25-dihydroxyvitamin D and intestinal calcium absorption. J Clin Endocrinol Metab. 1980;51:321–324. doi: 10.1210/jcem-51-2-321. [DOI] [PubMed] [Google Scholar]
- Chung L, Clifford D, Buckley M, Baxter RC. Novel biomarkers of human growth hormone action from serum proteomic profiling using protein chip mass spectrometry. J Clin Endocrinol Metab. 2006;91:671–677. doi: 10.1210/jc.2005-1137. [DOI] [PubMed] [Google Scholar]
- Colao A, Baldelli R, Marzullo P, Ferretti E, Ferone D, Gargiulo P, et al. Systemic hypertension and impaired glucose tolerance are independently correlated to the severity of the acromegalic cardiomyopathy. J Clin Endocrinol Metab. 2000;85:193–199. doi: 10.1210/jcem.85.1.6318. [DOI] [PubMed] [Google Scholar]
- Colao A, Marzullo P, DiSomma C, Lombardi G. Growth hormone and the heart. Clin Endocrinol. 2001;54:137–154. doi: 10.1046/j.1365-2265.2001.01218.x. [DOI] [PubMed] [Google Scholar]
- Cuneo RC, Salomon F, McGauley GA, Sönksen PH. The growth hormone deficiency syndrome in adults. Clin Endocrinol. 1992;37:387–397. doi: 10.1111/j.1365-2265.1992.tb02347.x. [DOI] [PubMed] [Google Scholar]
- Cuneo RC, Salomon F, Wiles CM, Hesp R, Sönksen PH. Growth hormone treatment in growth hormone-deficient adults I Effects on muscle mass and strength. J Appl Physiol. 1991a;70:688–694. doi: 10.1152/jappl.1991.70.2.688. [DOI] [PubMed] [Google Scholar]
- Cuneo RC, Salomon F, Wiles CM, Hesp R, Sönksen PH. Growth hormone treatment in growth hormone-deficient adults II Effects on exercise performance. J Appl Physiol. 1991b;70:695–700. doi: 10.1152/jappl.1991.70.2.695. [DOI] [PubMed] [Google Scholar]
- Dall R, Longobardi S, Ehrnborg C, Keay N, Rosen T, Jorgensen JO, et al. The effect of four weeks of supraphysiological growth hormone administration on the insulin-like growth factor axis in women and men. GH-2000 Study Group. J Clin Endocrinol Metab. 2000;85:4193–4200. doi: 10.1210/jcem.85.11.6964. [DOI] [PubMed] [Google Scholar]
- Duchaine D. Underground Steroid Handbook. HLR Technical Books: Venice, California, USA; 1982. [Google Scholar]
- Erotokritou-Mulligan I, Bassett EE, Kniess A, Sönksen PH, Holt RIG. Validation of the growth hormone (GH)-dependent marker method of detecting GH abuse in sport through the use of independent data sets. Growth Horm IGF Res. 2007;17:416–423. doi: 10.1016/j.ghir.2007.04.013. [DOI] [PubMed] [Google Scholar]
- Ezzat S, Forster MJ, Berchtold P, Redelmeier DA, Boerlin V, Harris AG. Acromegaly clinical and biochemical features in 500 patients. Medicine. 1994;73:233–240. [PubMed] [Google Scholar]
- Fainaru-Wada M, Williams L. Game of Shadows: Barry Bonds, Balco, and the Steroids Scandal That Rocked Professional Sports. Gotham Books pp1-332. ISBN: 9781592401994. 2006.
- Fowelin J, Attvall S, Lager I, Bengtsson BA. Effects of treatment with recombinant human growth hormone on insulin sensitivity and glucose metabolism in adults with growth hormone deficiency. Metab Clin Exp. 1993;42:1443–1447. doi: 10.1016/0026-0495(93)90197-v. [DOI] [PubMed] [Google Scholar]
- Fowelin J, Attvall S, von Schenck H, Smith U, Lager I. Characterization of the insulin-antagonistic effect of growth hormone in man. Diabetologia. 1991;34:500–506. doi: 10.1007/BF00403286. [DOI] [PubMed] [Google Scholar]
- Fowelin J, Attvall S, von Schenck H, Smith U, Lager I. Characterization of the insulin-antagonistic effect of growth hormone in insulin-dependent diabetes mellitus. Diabet Med. 1995;12:990–996. doi: 10.1111/j.1464-5491.1995.tb00411.x. [DOI] [PubMed] [Google Scholar]
- Franke WW, Berendonk B. Hormonal doping and androgenization of athletes: a secret program of the German Democratic Republic government. Clin Chem. 1997;43:1262–1279. [PubMed] [Google Scholar]
- Fryburg DA. Insulin-like growth factor I exerts growth hormone- and insulin-like actions on human muscle protein metabolism. Am J Physiol. 1994;267:331–336. doi: 10.1152/ajpendo.1994.267.2.E331. [DOI] [PubMed] [Google Scholar]
- Fryburg DA, Gelfand RA, Barrett EJ. Growth hormone acutely stimulates forearm muscle protein synthesis in normal humans. Am J Physiol. 1991;260:499–504. doi: 10.1152/ajpendo.1991.260.3.E499. [DOI] [PubMed] [Google Scholar]
- Giannoulis MG, Boroujerdi MA, Powrie J, Dall R, Napoli R, Ehrnborg C, et al. Gender differences in growth hormone response to exercise before and after rhGH administration and the effect of rhGH on the hormone profile of fit normal adults. Clin Endocrinol. 2005;62:315–322. doi: 10.1111/j.1365-2265.2005.02216.x. [DOI] [PubMed] [Google Scholar]
- Giannoulis MG, Sönksen PH, Umpleby M, Breen L, Pentecost C, Whyte M, et al. The effects of growth hormone and/or testosterone in healthy elderly men: a randomized controlled trial. J Clin Endocrinol Metab. 2006;91:477–484. doi: 10.1210/jc.2005-0957. [DOI] [PubMed] [Google Scholar]
- Gomez JM, Gomez N, Fiter J, Soler J. Effects of long-term treatment with GH in the bone mineral density of adults with hypopituitarism and GH deficiency and after discontinuation of GH replacement. Horm Metab Res. 2000;32:66–70. doi: 10.1055/s-2007-978591. [DOI] [PubMed] [Google Scholar]
- Graham MR, Baker JS, Evans P, Kicman A, Cowan DA, Davies B.Physical effects of short term GH administration in abstinent Steroid dependency Horm Res 2008(in press) [DOI] [PubMed]
- Grahnen A, Kastrup K, Heinrich U, Gourmelen M, Preece MA, Vaccarello MA, et al. Pharmacokinetics of recombinant human insulin-like growth factor I given subcutaneously to healthy volunteers and to patients with growth hormone receptor deficiency. Acta Paediatr Suppl. 1993;82 Suppl 391:9–13. doi: 10.1111/j.1651-2227.1993.tb12918.x. [DOI] [PubMed] [Google Scholar]
- Growth Hormone Research Society Critical evaluation of the safety of recombinant human growth hormone administration: statement from the Growth Hormone Research Society. J Clin Endocrinol Metab. 2001;86:1868–1870. doi: 10.1210/jcem.86.5.7471. [DOI] [PubMed] [Google Scholar]
- Guler HP, Zapf J, Schmid C, Froesch ER. Insulin-like growth factors I and II in healthy man estimations of half-lives and production rates. Acta Endocrinol. 1989;121:753–758. doi: 10.1530/acta.0.1210753. [DOI] [PubMed] [Google Scholar]
- Haffner D, Schaefer F, Girard J, Ritz E, Mehls O. Metabolic clearance of recombinant human growth hormone in health and chronic renal failure. J Clin Invest. 1994;93:1163–1171. doi: 10.1172/JCI117069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halloran BP, Spencer EM. Dietary phosphorus and 1 25-dihydroxyvitamin D metabolism: influence of insulin-like growth factor I. Endocrinol. 1988;123:1225–1229. doi: 10.1210/endo-123-3-1225. [DOI] [PubMed] [Google Scholar]
- Hansen TK. Pharmacokinetics and acute lipolytic actions of growth hormone impact of age body composition binding proteins and other hormones. Growth Horm IGF Res. 2002;12:342–358. doi: 10.1016/s1096-6374(02)00061-8. [DOI] [PubMed] [Google Scholar]
- Healy ML, Dall R, Gibney J, Bassett E, Ehrnborg C, Pentecost C, et al. Toward the development of a test for growth hormone (GH). Abuse: a study of extreme physiological ranges of GH-dependent markers in 813 elite athletes in the postcompetition setting. J Clin Endocrinol Metab. 2005;90:641–649. doi: 10.1210/jc.2004-0386. [DOI] [PubMed] [Google Scholar]
- Ho KY, Veldhuis JD, Johnson ML, Furlanetto R, Evans WS, Alberti KG, et al. Fasting enhances growth hormone secretion and amplifies the complex rhythms of growth hormone secretion in man. J Clin Invest. 1988;81:968–975. doi: 10.1172/JCI113450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt RIG. The metabolic effects of growth hormone. CME Bull Endocrinol Diabet. 2004;5:11–17. [Google Scholar]
- Holt RIG. Beyond reasonable doubt: catching the growth hormone cheats. Pediatr Endocrinol Rev. 2007;4:228–232. [PubMed] [Google Scholar]
- Holt RIG, Simpson HL, Sonksen PH. The role of the growth hormone-insulin-like growth factor axis in glucose homeostasis. Diabet Med. 2003;20:3–15. doi: 10.1046/j.1464-5491.2003.00827.x. [DOI] [PubMed] [Google Scholar]
- Holt RIG, Webb E, Pentecost C, Sönksen PH. Aging and physical fitness are more important than obesity in determining exercise-induced generation of GH. J Clin Endocrinol Metab. 2001;86:5715–5720. doi: 10.1210/jcem.86.12.8092. [DOI] [PubMed] [Google Scholar]
- Houssay BA, Biasotti A. La diabetes pancreatica de los perros hipofiseoprivos. Rev Soc Argent Biol. 1930;6:251–296. [Google Scholar]
- Jacob R, Barrett E, Plewe G, Fagin KD, Sherwin RS. Acute effects of insulin-like growth factor I on glucose and amino acid metabolism in the awake fasted rat comparison with insulin. J Clin Invest. 1989;83:1717–1723. doi: 10.1172/JCI114072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob R, Hu X, Niederstock D, Hasan S, McNulty PH, Sherwin RS, et al. IGF-I stimulation of muscle protein synthesis in the awake rat: permissive role of insulin and amino acids. Am J Physiol. 1996;270:60–66. doi: 10.1152/ajpendo.1996.270.1.E60. [DOI] [PubMed] [Google Scholar]
- Janssen YJ, Frolich M, Roelfsema F. The absorption profile and availability of a physiological subcutaneously administered dose of recombinant human growth hormone (GH). In adults with GH deficiency. Br J Clin Pharmacol. 1999;47:273–278. doi: 10.1046/j.1365-2125.1999.00892.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins PJ, Mukherjee A, Shalet SM. Does growth hormone cause cancer. Clin Endocrinol. 2006;64:115–121. doi: 10.1111/j.1365-2265.2005.02404.x. [DOI] [PubMed] [Google Scholar]
- Jorgensen JO, Pedersen SA, Thuesen L, Jorgensen J, Ingemann-Hansen T, Skakkebaek NE, et al. Beneficial effects of growth hormone treatment in GH-deficient adults. Lancet. 1989;1:1221–1225. doi: 10.1016/s0140-6736(89)92328-3. [DOI] [PubMed] [Google Scholar]
- Jorgensen JO, Thuesen L, Muller J, Ovesen P, Skakkebaek NE, Christiansen JS. Three years of growth hormone treatment in growth hormone-deficient adults: near normalization of body composition and physical performance. Eur J Endocrinol. 1994;130:224–228. doi: 10.1530/eje.0.1300224. [DOI] [PubMed] [Google Scholar]
- Kearns GL, Kemp SF, Frindik JP. Single and multiple dose pharmacokinetics of methionyl growth hormone in children with idiopathic growth hormone deficiency. J Clin Endocrinol Metab. 1991;72:1148–1156. doi: 10.1210/jcem-72-5-1148. [DOI] [PubMed] [Google Scholar]
- Keller A, Wu Z, Kratzsch J, Keller E, Blum WF, Kniess A, et al. Pharmacokinetics and pharmacodynamics of GH: dependence on route and dosage of administration. Eur J Endocrinol. 2007;156:647–653. doi: 10.1530/EJE-07-0057. [DOI] [PubMed] [Google Scholar]
- Kemp SF. Mecasermin rinfabate. Drugs Today. 2007;43:149–155. doi: 10.1358/dot.2007.43.3.1079876. [DOI] [PubMed] [Google Scholar]
- Kemp SF, Fowlkes JL, Thrailkill KM. Efficacy and safety of mecasermin rinfabate. Expert Opin Biol Ther. 2006;6:533–538. doi: 10.1517/14712598.6.5.533. [DOI] [PubMed] [Google Scholar]
- Kicman AT, Miell JP, Teale JD, Powrie J, Wood PJ, Laidler P, et al. Serum IGF-I and IGF binding proteins 2 and 3 as potential markers of doping with human GH. Clin Endocrinol. 1997;47:43–50. doi: 10.1046/j.1365-2265.1997.2111036.x. [DOI] [PubMed] [Google Scholar]
- Kniess A, Ziegler E, Kratzsch J, Thieme D, Muller RK. Potential parameters for the detection of hGH doping. Anal Bioanal Chem. 2003;376:696–700. doi: 10.1007/s00216-003-1926-x. [DOI] [PubMed] [Google Scholar]
- Lanning NJ, Carter-Su C. Recent advances in growth hormone signaling. Rev Endocr Metab Disord. 2006;7:225–235. doi: 10.1007/s11154-007-9025-5. [DOI] [PubMed] [Google Scholar]
- Le Roith D, Bondy C, Yakar S, Liu JL, Butler A. The somatomedin hypothesis: 2001. Endocr Rev. 2001;22:53–74. doi: 10.1210/edrv.22.1.0419. [DOI] [PubMed] [Google Scholar]
- Li CH, Evans HM, Simpson ME. Isolation and properties of the anterior hypophyseal growth hormone. J Biol Chem. 1945;159:353–366. [Google Scholar]
- Liu Z, Barrett EJ. Human protein metabolism: its measurement and regulation. Am J Physiol Endocrinol Metab. 2002;283:1105–1112. doi: 10.1152/ajpendo.00337.2002. [DOI] [PubMed] [Google Scholar]
- Longobardi S, Keay N, Ehrnborg C, Cittadini A, Rosen T, Dall R, et al. Growth hormone (GH). Effects on bone and collagen turnover in healthy adults and its potential as a marker of GH abuse in sports: a double blind placebo-controlled study. The GH-2000 Study Group. J Clin Endocrinol Metab. 2000;85:1505–1512. doi: 10.1210/jcem.85.4.6551. [DOI] [PubMed] [Google Scholar]
- Louard RJ, Fryburg DA, Gelfand RA, Barrett EJ. Insulin sensitivity of protein and glucose metabolism in human forearm skeletal muscle. J Clin Invest. 1992;90:2348–2354. doi: 10.1172/JCI116124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund B, Eskildsen PC, Norman AW, Sorensen OH. Calcium and vitamin D metabolism in acromegaly. Acta Endocrinol. 1981;96:444–450. doi: 10.1530/acta.0.0960444. [DOI] [PubMed] [Google Scholar]
- Marcus C, Bolme P, Micha-Johansson G, Margery V, Bronnegard M. Growth hormone increases the lipolytic sensitivity for catecholamines in adipocytes from healthy adults. Life Sci. 1994;54:1335–1341. doi: 10.1016/0024-3205(94)00512-5. [DOI] [PubMed] [Google Scholar]
- Matthews DR, Rudenski AS, Burnett MA, Darling P, Turner RC. The half-life of endogenous insulin and C-peptide in man assessed by somatostatin suppression. Clin Endocrinol. 1985;23:71–79. doi: 10.1111/j.1365-2265.1985.tb00185.x. [DOI] [PubMed] [Google Scholar]
- McGauley GA, Cuneo RC, Salomon F, Sönksen PH. Psychological well-being before and after growth hormone treatment in adults with growth hormone deficiency. Horm Res. 1990;33 Suppl 4:52–54. doi: 10.1159/000181584. [DOI] [PubMed] [Google Scholar]
- McHugh CM, Park RT, Sönksen PH, Holt RIG. Challenges in detecting the abuse of growth hormone in sport. Clin Chem. 2005;51:1587–1593. doi: 10.1373/clinchem.2005.047845. [DOI] [PubMed] [Google Scholar]
- McNab TL, Khandwala HM. Acromegaly as an endocrine form of myopathy: case report and review of literature. Endocr Pract. 2005;11:18–22. doi: 10.4158/EP.11.1.18. [DOI] [PubMed] [Google Scholar]
- Moller J, Nielsen S, Hansen TK. Growth hormone and fluid retention. Horm Res. 1999;51 Suppl 3:116–120. doi: 10.1159/000053173. [DOI] [PubMed] [Google Scholar]
- Moller N, Gjedsted J, Gormsen L, Fuglsang J, Djurhuus C. Effects of growth hormone on lipid metabolism in humans. Growth Horm IGF Res. 2003;13 Suppl A:S18–S21. doi: 10.1016/s1096-6374(03)00048-0. [DOI] [PubMed] [Google Scholar]
- Moller N, Jorgensen JO, Moller J, Orskov L, Ovesen P, Schmitz O, et al. Metabolic effects of growth hormone in humans. Metab Clin Exp. 1995;44:33–36. doi: 10.1016/0026-0495(95)90218-x. [DOI] [PubMed] [Google Scholar]
- Momomura S, Hashimoto Y, Shimazaki Y, Irie M. Detection of exogenous growth hormone (GH). Administration by monitoring ratio of 20- and 22 kDa-GH in serum and urine. Endocr J. 2000;47:97–101. doi: 10.1507/endocrj.47.97. [DOI] [PubMed] [Google Scholar]
- Moreira-Andres MN, Canizo FJ, Hawkins F. Is there a place for urinary growth hormone measurement. Acta Endocrinol. 1993;128:197–201. doi: 10.1530/acta.0.1280197. [DOI] [PubMed] [Google Scholar]
- Nelson AE, Howe CJ, Nguyen TV, Leung KC, Trout GJ, Seibel MJ, et al. Influence of demographic factors and sport type on growth hormone-responsive markers in elite athletes. J Clin Endocrinol Metab. 2006;91:4424–4432. doi: 10.1210/jc.2006-0612. [DOI] [PubMed] [Google Scholar]
- Patel MB, Arden NK, Masterson LM, Phillips DI, Swaminathan R, Syddall HE, et al. Investigating the role of the growth hormone-insulin-like growth factor (GH-IGF). Axis as a determinant of male bone mineral density (BMD) Bone. 2005;37:833–841. doi: 10.1016/j.bone.2005.06.016. [DOI] [PubMed] [Google Scholar]
- Peterson GE. Intermediate and long-acting insulins: a review of NPH insulin insulin glargine and insulin detemir. Curr Med Res Opin. 2006;22:2613–2619. doi: 10.1185/030079906X154178. [DOI] [PubMed] [Google Scholar]
- Pombo M, Pombo CM, Astorga R, Cordido F, Popovic V, Garcia Mayor RV, et al. Regulation of growth hormone secretion by signals produced by the adipose tissue. J Endocrinol Invest. 1999;22:22–26. [PubMed] [Google Scholar]
- Powrie J, Weissberger A, Sönksen P. Growth hormone replacement therapy for growth hormone-deficient adults. Drugs. 1995;49:656–663. doi: 10.2165/00003495-199549050-00002. [DOI] [PubMed] [Google Scholar]
- Powrie JK, Bassett EE, Rosen T, Jorgensen JO, Napoli R, Sacca L, et al. Detection of growth hormone abuse in sport. Growth Horm IGF Res. 2007;17:220–226. doi: 10.1016/j.ghir.2007.01.011. [DOI] [PubMed] [Google Scholar]
- Prinz PN, Weitzman ED, Cunningham GR, Karacan I. Plasma growth hormone during sleep in young and aged men. J Gerontol. 1983;38:519–524. doi: 10.1093/geronj/38.5.519. [DOI] [PubMed] [Google Scholar]
- Refetoff S, Sönksen PH. Disappearance rate of endogenous and exogenous human growth hormone in man. J Clin Endocrinol Metab. 1970;30:386–392. doi: 10.1210/jcem-30-3-386. [DOI] [PubMed] [Google Scholar]
- Rennie MJ. Claims for the anabolic effects of growth hormone: a case of the emperor's new clothes. Br J Sports Med. 2003;37:100–105. doi: 10.1136/bjsm.37.2.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rickert VI, Pawlak-Morello C, Sheppard V, Jay MS. Human growth hormone: a new substance of abuse among adolescents. Clin Pediatr. 1992;31:723–726. doi: 10.1177/000992289203101206. [DOI] [PubMed] [Google Scholar]
- Russell Jones DL, Weissberger AJ, Bowes SB, Kelly JM, Thomason M, Umpleby AM, et al. The effects of growth hormone on protein metabolism in adult growth hormone deficient patients. Clin Endocrinol. 1993;38:427–431. doi: 10.1111/j.1365-2265.1993.tb00525.x. [DOI] [PubMed] [Google Scholar]
- Russell Jones DL, Bates AT, Umpleby AM, Hennessy TR, Bowes SB, Hopkins KD, et al. A comparison of the effects of IGF-I and insulin on glucose metabolism fat metabolism and the cardiovascular system in normal human volunteers. Eur J Clin Invest. 1995;25:403–411. doi: 10.1111/j.1365-2362.1995.tb01721.x. [DOI] [PubMed] [Google Scholar]
- Russell Jones DL, Umpleby AM, Hennessy TR, Bowes SB, Shojaee Moradie F, Hopkins KD, et al. Use of a leucine clamp to demonstrate that IGF-I actively stimulates protein synthesis in normal humans. Am J Physiol. 1994;267:591–598. doi: 10.1152/ajpendo.1994.267.4.E591. [DOI] [PubMed] [Google Scholar]
- Salomon F, Cuneo RC, Hesp R, Sönksen PH. The effects of treatment with recombinant human growth hormone on body composition and metabolism in adults with growth hormone deficiency. N Eng J Med. 1989;321:1797–1803. doi: 10.1056/NEJM198912283212605. [DOI] [PubMed] [Google Scholar]
- Salomon F, Cuneo RC, Umpleby AM, Sönksen PH. Interactions of body fat and muscle mass with substrate concentrations and fasting insulin levels in adults with growth hormone deficiency. Clin Sci. 1994;87:201–206. doi: 10.1042/cs0870201. [DOI] [PubMed] [Google Scholar]
- Savine R, Sönksen P. Growth hormone—hormone replacement for the somatopause. Horm Res. 2000;53 Suppl 3:37–41. doi: 10.1159/000023531. [DOI] [PubMed] [Google Scholar]
- Shojaee Moradie F, Umpleby AM, Thomason MJ, Jackson NC, Boroujerdi MA, Sönksen PH, et al. A comparison of the effects of insulin-like growth factor-I insulin and combined infusions of insulin and insulin-like growth factor-I on glucose metabolism in dogs. Eur J Clin Invest. 1995;25:920–928. doi: 10.1111/j.1365-2362.1995.tb01968.x. [DOI] [PubMed] [Google Scholar]
- Sjogren K, Liu JL, Blad K, Skrtic S, Vidal O, Wallenius V, et al. Liver-derived insulin-like growth factor I (IGF-I) is the principal source of IGF-I in blood but is not required for postnatal body growth in mice. Proc Natl Acad Sci USA. 1999;96:7088–7092. doi: 10.1073/pnas.96.12.7088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjogren K, Wallenius K, Liu JL, Bohlooly YM, Pacini G, Svensson L, et al. Liver-derived IGF-I is of importance for normal carbohydrate and lipid metabolism. Diabetes. 2001;50:1539–1545. doi: 10.2337/diabetes.50.7.1539. [DOI] [PubMed] [Google Scholar]
- Sohmiya M, Kato Y. Renal clearance metabolic clearance rate and half-life of human growth hormone in young and aged subjects. J Clin Endocrinol Metab. 1992;75:1487–1490. doi: 10.1210/jcem.75.6.1464653. [DOI] [PubMed] [Google Scholar]
- Sönksen P, Sönksen J. Insulin: understanding its action in health and disease. Br J Anaesth. 2000;85:69–79. doi: 10.1093/bja/85.1.69. [DOI] [PubMed] [Google Scholar]
- Sönksen PH. Insulin growth hormone and sport. J Endocrinol. 2001;170:13–25. doi: 10.1677/joe.0.1700013. [DOI] [PubMed] [Google Scholar]
- Sönksen PH, Greenwood FC, Ellis JP, Lowy C, Rutherford A, Nabarro JD. Changes of carbohydrate tolerance in acromegaly with progress of the disease and in response to treatment. J Clin Endocrinol Metab. 1967;27:1418–1430. doi: 10.1210/jcem-27-10-1418. [DOI] [PubMed] [Google Scholar]
- Stefano JT, Correa-Giannella ML, Ribeiro CM, Alves VA, Massarollo PC, Machado MC, et al. Increased hepatic expression of insulin-like growth factor-I receptor in chronic hepatitis. World J Gastroenterol. 2006;12:3821–3828. doi: 10.3748/wjg.v12.i24.3821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan T.Drug questions cloud memory career of FloJo The Cincinnati Enquirer 1998. accessed 14 March 2008
- Takahashi Y, Kipnis DM, Daughaday WH. Growth hormone secretion during sleep. J Clin Invest. 1968;47:2079–2090. doi: 10.1172/JCI105893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamborlane WV, Sherwin RS, Koivisto V, Hendler R, Genel M, Felig P. Normalization of the growth hormone and catecholamine response to exercise in juvenile-onset diabetic subjects treated with a portable insulin infusion pump. Diabetes. 1979;28:785–788. doi: 10.2337/diab.28.8.785. [DOI] [PubMed] [Google Scholar]
- Tessari P, Trevisan R, Inchiostro S, Biolo G, Nosadini R, De Kreutzenberg SV, et al. Dose-response curves of effects of insulin on leucine kinetics in humans. Am J Physiol. 1986;251:334–342. doi: 10.1152/ajpendo.1986.251.3.E334. [DOI] [PubMed] [Google Scholar]
- Thevis M, Thomas A, Delahaut P, Bosseloir A, Schanzer W. Doping control analysis of intact rapid-acting insulin analogues in human urine by liquid chromatography-tandem mass spectrometry. Anal Chem. 2006;78:1897–1903. doi: 10.1021/ac052095z. [DOI] [PubMed] [Google Scholar]
- Thomas A, Thevis M, Delahaut P, Bosseloir A, Schanzer W. Mass spectrometric identification of degradation products of insulin and its long-acting analogues in human urine for doping control purposes. Anal Chem. 2007;79:2518–2524. doi: 10.1021/ac062037t. [DOI] [PubMed] [Google Scholar]
- Tompkins CV, Brandenburg D, Jones RH, Sönksen PH. Mechanism of action of insulin and insulin analogues A comparison of the hepatic and peripheral effects on glucose turnover of insulin proinsulin and three insulin analogues modified at positions A1 and B29. Diabetologia. 1981;20:94–101. doi: 10.1007/BF00262008. [DOI] [PubMed] [Google Scholar]
- Toogood AA. Growth hormone (GH). Status and body composition in normal ageing and in elderly adults with GH deficiency. Horm Res. 2003;60:105–111. doi: 10.1159/000071234. [DOI] [PubMed] [Google Scholar]
- Umpleby AM, Sönksen PH.The chalonic action of insulin in man Substrate and Energy Metabolism in Man 1985John Libby and Co: London, UK; 169–178.In: Garrow JS, Halliday D (eds). [Google Scholar]
- Vahl N, Moller N, Lauritzen T, Christiansen JS, Jorgensen JO. Metabolic effects and pharmacokinetics of a growth hormone pulse in healthy adults: relation to age sex and body composition. J Clin Endocrinol Metab. 1997;82:3612–3618. doi: 10.1210/jcem.82.11.4388. [DOI] [PubMed] [Google Scholar]
- van den Berg G, Veldhuis JD, Frolich M, Roelfsema F. An amplitude-specific divergence in the pulsatile mode of growth hormone (GH). Secretion underlies the gender difference in mean GH concentrations in men and premenopausal women. J Clin Endocrinol Metab. 1996;81:2460–2467. doi: 10.1210/jcem.81.7.8675561. [DOI] [PubMed] [Google Scholar]
- van der Lely AJ, Tschop M, Heiman ML, Ghigo E. Biological physiological pathophysiological and pharmacological aspects of ghrelin. Endocr Rev. 2004;25:426–457. doi: 10.1210/er.2002-0029. [DOI] [PubMed] [Google Scholar]
- Veldhuis JD. A tripeptidyl ensemble perspective of interactive control of growth hormone secretion. Horm Res. 2003;60:86–101. doi: 10.1159/000071232. [DOI] [PubMed] [Google Scholar]
- Veldhuis JD, Iranmanesh A, Ho KK, Waters MJ, Johnson ML, Lizarralde G. Dual defects in pulsatile growth hormone secretion and clearance subserve the hyposomatotropism of obesity in man. J Clin Endocrinol Metab. 1991;72:51–59. doi: 10.1210/jcem-72-1-51. [DOI] [PubMed] [Google Scholar]
- Wallace JD, Cuneo RC, Baxter R, Orskov H, Keay N, Pentecost C, et al. Responses of the growth hormone (GH). And insulin-like growth factor axis to exercise GH administration and GH withdrawal in trained adult males: a potential test for GH abuse in sport. J Clin Endocrinol Metab. 1999;84:3591–3601. doi: 10.1210/jcem.84.10.6037. [DOI] [PubMed] [Google Scholar]
- Wallace JD, Cuneo RC, Bidlingmaier M, Lundberg PA, Carlsson L, Boguszewski CL, et al. Changes in non-22-kilodalton (kDa). Isoforms of growth hormone (GH). After administration of 22-kDa recombinant human GH in trained adult males. J Clin Endocrinol Metab. 2001a;86:1731–1737. doi: 10.1210/jcem.86.4.7379. [DOI] [PubMed] [Google Scholar]
- Wallace JD, Cuneo RC, Bidlingmaier M, Lundberg PA, Carlsson L, Boguszewski CL, et al. The response of molecular isoforms of growth hormone to acute exercise in trained adult males. J Clin Endocrinol Metab. 2001b;86:200–206. doi: 10.1210/jcem.86.1.7129. [DOI] [PubMed] [Google Scholar]
- Wallace JD, Cuneo RC, Lundberg PA, Rosen T, Jorgensen JO, Longobardi S, et al. Responses of markers of bone and collagen turnover to exercise growth hormone (GH). Administration and GH withdrawal in trained adult males. J Clin Endocrinol Metab. 2000;85:124–133. doi: 10.1210/jcem.85.1.6262. [DOI] [PubMed] [Google Scholar]
- Wu RH, St Louis Y, Martino-Nardi J, Wesoly S, Sobel EH, Sherman B, et al. Preservation of physiological growth hormone (GH). Secretion in idiopathic short stature after recombinant GH therapy. J Clin Endocrinol Metab. 1990;70:1612–1615. doi: 10.1210/jcem-70-6-1612. [DOI] [PubMed] [Google Scholar]
- Wu Z, Bidlingmaier M, Dall R, Strasburger CJ. Detection of doping with human growth hormone. Lancet. 1999;353:895. doi: 10.1016/S0140-6736(99)00775-8. [DOI] [PubMed] [Google Scholar]
- Yakar S, Liu JL, Fernandez AM, Wu Y, Schally AV, Frystyk J, et al. Liver-specific igf-1 gene deletion leads to muscle insulin insensitivity. Diabetes. 2001;50:1110–1118. doi: 10.2337/diabetes.50.5.1110. [DOI] [PubMed] [Google Scholar]
- Yakar S, Liu JL, Stannard B, Butler A, Accili D, Sauer B, et al. Normal growth and development in the absence of hepatic insulin-like growth factor I. Proc Natl Acad Sci USA. 1999;96:7324–7329. doi: 10.1073/pnas.96.13.7324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zenobi PD, Graf S, Ursprung H, Froesch ER. Effects of insulin-like growth factor-I on glucose tolerance insulin levels and insulin secretion. J Clin Invest. 1992;89:1908–1913. doi: 10.1172/JCI115796. [DOI] [PMC free article] [PubMed] [Google Scholar]