Abstract
Growth hormone (GH) is widely used as a performance-enhancing drug. One of its best-characterized effects is increasing levels of circulating insulin-like growth factor I (IGF-I), which is primarily of hepatic origin. It also induces synthesis of IGF-I in most non-hepatic tissues. The effects of GH in promoting postnatal body growth are IGF-I dependent, but IGF-I-independent functions are beginning to be elucidated. Although benefits of GH administration have been reported for those who suffer from GH deficiency, there is currently very little evidence to support an anabolic role for supraphysiological levels of systemic GH or IGF-I in skeletal muscle of healthy individuals. There may be other performance-enhancing effects of GH. In contrast, the hypertrophic effects of muscle-specific IGF-I infusion are well documented in animal models and muscle cell culture systems. Studies examining the molecular responses to hypertrophic stimuli in animals and humans frequently cite upregulation of IGF-I messenger RNA or immunoreactivity. The circulatory/systemic (endocrine) and local (autocrine/paracrine) effects of GH and IGF-I may have distinct effects on muscle mass regulation.
Keywords: growth hormone, IGF-I, skeletal muscle, performance, muscle mass
Introduction
Skeletal muscle comprises about 40% of the body mass in humans. An adequate muscle mass is critical for health as muscle has several important functions: locomotion, breathing, thermogenesis, protection of internal organs, glucose and fat metabolism. The regulation of muscle mass is of interest to a diverse group of people. There are those, such as power athletes and body builders, who are primarily interested in increasing their muscle mass. Others are concerned with preventing muscle loss. This is critical for the frail elderly, those with myopathies, cancer, sepsis, HIV/AIDS and other diseases, those suffering from reduced mobility as a result of injury, and astronauts. The mechanisms regulating muscle mass maintenance are widely studied due to the importance of this tissue for health.
Muscle is a highly plastic tissue able to adapt to changing functional demands. Increased load on muscle results in an increase in its mass or hypertrophy, whereas unloading or disuse leads to a decrease in mass or atrophy. Exercise is a key regulator of muscle mass, as is nutrition (Rennie et al., 2004).
Hormonal factors are also important. It is evident that men have a greater muscle mass than women. This is primarily due to the anabolic effects of testosterone. Indeed, anabolic steroids have long been used by body builders due to their dramatic effects on muscle bulk. Growth hormone (GH) and insulin-like growth factor I (IGF-I) have a key role in the regulation of body size in growing animals but their role in adults is less clear. IGF-I clearly has anabolic activity but its mechanism of action as an endocrine, circulating hormone may be distinct from its activity as an autocrine/paracrine growth factor.
This review begins with a basic introduction to the GH/IGF-I axis and the mechanisms of muscle mass regulation. The evidence for an effect of these molecules on muscle mass in human, animal and cell culture models is examined followed by a discussion of their use as performance-enhancing drugs.
The GH/IGF-I axis
GH or somatotropin is a peptide hormone produced and secreted mainly by the somatotroph cells of the anterior hypophysis (pituitary gland). GH secretion occurs in a pulsatile pattern and is regulated by hypothalamic hormones. GH-releasing hormone induces GH secretion, whereas somatostatin (somatotropin release inhibiting factor hormone) inhibits its secretion (Goldenberg and Barkan, 2007). In addition, the peptide ghrelin is a potent GH secretagogue that is synthesized in the hypothalamus, the pituitary and the stomach (Kojima et al., 1999). Various stimuli affect the frequency and magnitude of the GH pulses: gender, age, adiposity, sleep, diet and exercise. Consequently, serum GH levels vary greatly throughout the day.
The GH receptor (GHR) is ubiquitously expressed (Frick et al., 1998) and GH has direct effects on most tissues, including skeletal muscle (d'Ercole et al., 1984; Gostelli-Peter et al., 1994; Jorgensen et al., 2006). GH binding results in dimerization of two GH receptors and intracellular signalling involves the Janus kinase and the signal transducers and activators of transcription (Stat) pathway (Smit et al., 1996).
GH stimulates the synthesis of IGF-I in most tissues (Figure 1; d'Ercole et al., 1984; Gostelli-Peter et al., 1994). The liver is the organ chiefly responsible for the production of serum IGF-I. GH administration causes rapid upregulation of IGF-I mRNA and protein in the liver (Mathews et al., 1986) and animals with liver-specific IGF-I deletions show only 10–25% of serum IGF-I levels compared with controls (Sjögren et al., 1999; Yakar et al., 1999). Unlike GH, serum IGF-I levels are quite stable in healthy humans and show little day-to-day intraindividual variability. Serum IGF-I levels above or below the normal age-corrected range are a good indicator of GH dysfunction (Buckway et al., 2001), although care must be taken to consider other factors such as malnutrition and liver problems that affect serum IGF-I. GH secretion is regulated by a negative feedback system in that elevated serum IGF-I inhibits GH secretion (Figure 1; Berelowitz et al., 1981; Bermann et al., 1994).
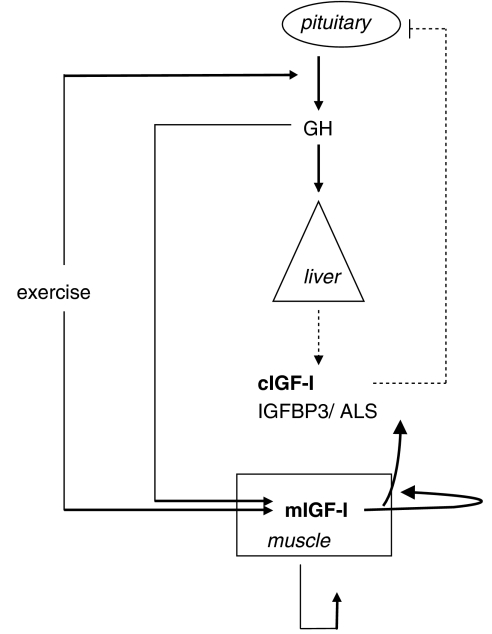
Figure 1.
Schematic representation of the growth hormone (GH)/insulin-like growth factor I (IGF-I) axis. GH is synthesized in the pituitary and induces synthesis of IGF-I in most tissues (liver and muscle). The liver is the main source of circulating IGF-I (cIGF-I) although some cIGF-I comes from other tissues, including muscle. cIGF-I is part of a negative feedback loop regulating GH release. IGF-I synthesized in muscle (mIGF-I) in response to exercise or GH acts in an autocrine/paracrine way to stimulate hypertrophy.
The effects of IGF-I are mediated mainly by the type 1 IGF receptor (IGFR1), which has tyrosine kinase activity and signals through the phosphatidylinositol 3 kinase (PI3K)/AKT pathway (Figure 2). IGF-I also binds to the insulin receptor (IR) but with much lower (about 100-fold lower) affinity than to the IGF1R (Pandini et al., 2002). The IR and IGF1R are dimeric transmembrane receptors and can form functional hybrids. The roles of hybrid receptors in cellular responses remains unclear.
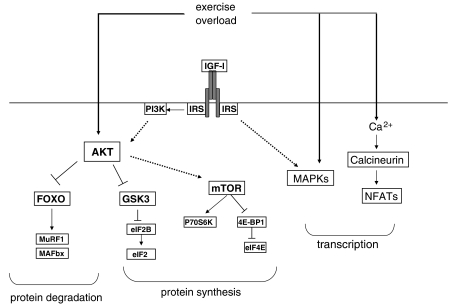
Figure 2.
Signalling pathways regulated by insulin-like growth factor I (IGF-I) and/or exercise. Exercise has been shown to activate several different pathways in muscle. They include AKT, MAPK (ERK1, ERK2) and calcineurin. Exercise also induces synthesis of IGF-I in muscle. IGF-IR signals through many of the same pathways as exercise. Signalling through phosphatidylinositol 3 kinase (PI3K)/AKT is of particular importance as this increases protein synthesis and inhibits protein degradation via inactivation of FOXO transcription factors. Interestingly, although exercise activates AKT and induces increased protein synthesis, it also increases protein degradation (not illustrated), probably as a result of increased protein remodelling. If net protein synthesis results, this will lead to muscle hypertrophy. Thus, exercise and IGF-I have overlapping but distinct effects on muscle.
There are six IGF-binding proteins (IGFBPs). The IGFBPs were initially isolated from serum and are proteins of about 30 kDa able to bind IGF-I and IGF-II but not insulin (Bach et al., 1993). Most serum IGF-I is found in a tripartite complex with IGFBP3 and the acid labile subunit (ALS). IGF-IGFBP complexes can leave the circulation and access tissue unless they are bound to the ALS. In serum, they increase the circulating half-life and delivery of IGF-I to tissues. In tissues, they modulate IGF action as they have higher affinity for IGFs than the receptors. IGFs are released by proteolysis of IGFBPs or binding of the IGFBPs to the extracellular matrix (Parker et al., 1998). IGFBPs can also be phosphorylated and this affects their affinity for IGFs (Kajantie et al., 2002). IGF-independent actions have been described for most IGFBPs and can involve intracellular localization (Xu et al., 2004) or integrin binding (Jones et al., 1993).
Mechanisms of muscle mass regulation
There are two main mechanisms by which muscle mass may be increased: hypertrophy or an increase in myofibre size and hyperplasia or an increase in myofibre number. It is generally accepted that the number of fibres within a muscle is fixed during the perinatal period (Stickland, 1981). It has been suggested, however, that myofibre splitting occurs if a myofibre becomes too large (Antonio and Gonyea, 1993), but this has not been reported in humans. New myofibres may also form as a result of fusion of satellite cells (see below) and small myotubes and myofibres expressing myogenic markers can be found in human muscle after training (Kadi and Thornell, 1999). Nevertheless, the consensus is that an increase in muscle cross-sectional area (CSA) is primarily due to an increase in myofibre CSA rather than myofibre number.
The balance between protein synthesis and degradation is a critical determinant of muscle CSA (Gibson et al., 1988; reviewed in Baar et al., 2006). Net protein synthesis results in greater myofibrillar content which is accommodated in a larger myofibres. Significant myofibre hypertrophy also requires an increase in the number of myonuclei so that a constant myonuclear domain (volume of cytoplasm supported by a single nucleus) is maintained. In a muscle, the ratio of DNA/protein is fairly constant (Roy et al., 1999).
Myofibres are post-mitotic cells, and their nuclei do not proliferate. New myonuclei are provided by a population called satellite cells (reviewed in Zammit et al., 2006). These cells lie just under the basal lamina of myofibres, and are normally found in a quiescent state. Once activated by exercise or muscle damage, satellite cells proliferate and fuse with existing muscle fibres, thus providing new nuclei for hypertrophy and repair. The absence of a satellite cell proliferative response following γ-irradiation of the muscle limits hypertrophic gains (Rosenblatt and Parry, 1992).
There are many signalling pathways involved in regulating muscle mass (Figure 2; Glass, 2005; Shavlakadze and Grounds, 2006). A central player appears be the PI3K/AKT pathway as it activates protein synthesis and inhibits protein degradation. Crucially, the PI3K/AKT pathway is activated by exercise and lies downstream of the IGF-I and insulin receptors. Both insulin and IGF-I can stimulate protein synthesis in skeletal muscle (Bolster et al., 2004).
There are three AKT isoforms (AKT1, AKT2 and AKT3) and it appears that AKT1 is important for growth regulation, whereas AKT2 is involved in metabolism (Cho et al., 2001). Downstream of AKT is mTOR. It forms two complexes with other molecules, mTORC1 and mTORC2. The first is involved in regulating protein synthesis and is sensitive to rapamycin. Hence the name mTOR: mammalian target of rapamycin. The second is involved in controlling the actin cytoskeleton and is not sensitive to rapamycin. The complex goes on to activate the translational regulators 4E-BP1 and p70S6-K. Phosphorylation of 4E-BP1 results in release of eIF4E, which is required for binding of mRNA to the ribosome. This initiates translation. Phosphorylation of p70S6-K increases translation of ribosomal and other mRNAs containing a 5′ tract of oligopyrimidines. Therefore, mTORC1 may regulate both ribosomal biogenesis and translation initiation. It can also be activated directly by essential amino acids such as leucine (Kimball et al., 1999).
Protein synthesis also depends on the energy status in the muscle as it is an ATP-dependent process, and therefore is also regulated by the AMP-dependent kinase AMPK (Bolster et al., 2002). Treatment of rats with an AMPK-activating drug leads to a reduction in protein synthesis accompanied by a decrease in activation of mTOR, p70S6K and 4E-BP1.
Protein degradation resulting from disease or disuse can be inhibited by AKT activation. This occurs because AKT phosphorylates and thereby prevents nuclear translocation of the FOXO family of transcription factors. FOXO1 and FOXO3 regulate the expression of two ubiquitin protein ligases in muscle: muscle atrophy F-box or atrogen-1 (MAFbx) and muscle RING finger 1 (MuRF1; Stitt et al., 2004). Ubiquitin ligases link ubiquitin to proteins thereby targeting them for degradation by the ubiquitin–proteasome, an ATP-dependent proteolysis complex.
Another pathway of protein degradation in skeletal muscle is autophagy, the bulk degradation of proteins and organelles by lysosomal enzymes. The mechanisms responsible for the induction and regulation of the autophagy programme are poorly understood but appear to involve FOXO transcription factors as well, in particular FOXO3. Autophagy can be inhibited by AKT, but not rapamycin. Thus, FOXO3 controls the two major systems of protein breakdown in skeletal muscle, the ubiquitin–proteasomal and autophagic/lysosomal pathways (Mammucari et al., 2007).
GH and IGF-I in the regulation of body and muscle growth
GH regulates postnatal body growth. In both mice and humans, GH deficiency or GH insensitivity (Laron's syndrome caused by inactivating mutations of the GH receptor gene) minimally affect birth size, but lead to reduced growth during childhood and adolescence resulting in diminished stature (Savage et al., 1993; Zhou et al., 1997; Efstratiadis, 1998). Supraphysiological GH in the young leads to pituitary gigantism, whereas adult-onset GH tumours result in a condition called acromegaly characterized by overgrowth of bony tissue (brow and lower jaw protrusion, enlargement of the extremities), osteoarthritis, carpal tunnel syndrome, headaches, cardiomyopathies, hyperglycaemia, hypertension and diabetes mellitus (Ayuk and Sheppard, 2006). In mice, enlargement of the heart and general increase in organ size are features of systemic GH overproduction or administration (Kopchick et al., 1999).
The growth-promoting effects of GH are mainly mediated by IGF-I (Le Roith et al., 2001; Mauras and Haymond, 2005; Walenkamp and Wit, 2007). IGF-I infusion into hypophysectomized rats promotes growth in the absence of GH (Behringer et al., 1990). However, the IGF-I knockout mouse is less growth retarded than the IGF-I and GHR double knockout, suggesting that GH also has IGF-I-independent effects (Lupu et al., 2001). There are effects that cannot be mimicked by infusion of IGF-I. It has been demonstrated that GH administration increases skeletal muscle IGF-I mRNA production in hypophysectomized rats 20-fold, whereas the increase observed after IGF-I treatment is only 2.5-fold (Gostelli-Peter et al., 1994). This may be relevant to skeletal muscle mass regulation as the autocrine/paracrine levels of IGF-I appear to be more important than the systemic/circulating levels of IGF-I as discussed later.
IGF-I, unlike GH, is critical for intrauterine growth (Baker et al., 1993; Liu et al., 1993; Powell-Braxton et al., 1993). The IGF-I and IGFIR knockout mice have birth weights of 60 and 45% of normal, respectively (Liu et al., 1993), whereas mice with severe GH deficiency or GH insensitivity have normal birth weight. Disruptions in the IGF-I signalling pathway also result in reduced intrauterine growth as observed in mice deficient in Akt1 (Cho et al., 2001), insulin receptor substrate-1 (IRS-1) and IRS-2 (Araki et al., 1994; Tamemoto et al., 1994). Critically, high levels of circulating IGF-I do not seem to be required for intrauterine growth as liver-specific IGF-I knockouts show similar body weight to controls at birth and up to 3 months of age (Sjögren et al., 1999; Yakar et al., 1999). The liver-specific knockout has some postnatal growth reduction compared with wild types, but it is not as severe as in the total IGF knockout (Baker et al., 1993). This implies that locally produced, autocrine/paracrine IGF-I plays an important role in pre- and postnatal growth. Another explanation, however, is also plausible. The free, bioavailable IGF-I levels in the liver knockouts may be similar to those in the wild-type animals. If this is the case, the change in circulating IGF-I might not be expected to have any effect.
Insulin also binds to the IGF-IR and this may explain some of its growth promoting effects (Kjeldsen et al., 1991). Conversely, IGF-I binds to the insulin receptor and shares its hypoglycaemic effect (Schoenle et al., 1991; Clemmons et al., 1992). It is more likely, however, that the shared intracellular signalling pathways or the activities of hybrid receptors are responsible for the common activities of IGF-I and insulin.
In addition to regulating growth during development, activation of the IGFR1 affects cell proliferation and differentiation (Liu et al., 1993). The importance of IGF-I activity during development is reflected in the fact that the IGF-IR knockouts are embryonic lethal and die of respiratory failure (Liu et al., 1993). Depending on the genetic background of the animals, up to 15% of IGF-I knockouts survive to adulthood but are infertile and growth retarded (Baker et al., 1993).
Muscle mass increases in proportion to body size during the growth phase. The growth-promoting effects of GH and IGF-I in young animals and humans are well documented, but increases in muscle mass are usually in proportion to the increase in body size. Rodents reach sexual maturity around 6 months, but this does not necessarily coincide with a cessation of body growth. Many studies use animals that are 6–12 weeks of age making it difficult to separate the effects of GH and IGF-I on body growth versus muscle mass.
It is therefore noteworthy that in the GH receptor knockout mouse, the absolute muscle weight as well as the ratio of muscle to body weight is reduced as compared with wild-type animals (Sotiropoulos et al., 2006). Myofibre CSA, but not number, was also reduced suggesting that systemic GH does have a role to play in regulating muscle mass in this model. As these mice have a reduction in circulating IGF-I and tissue IGF-I expression is at least in part GH dependent, it is difficult to separate the effects of the two hormones. It has been shown that mice lacking IGF1R specifically in muscle have smaller muscles than their wild-type counterparts as well as reduced myofibre CSA (Kim et al., 2005a). GH administration leads to increased muscle weight and myofibre CSA in wild-type animals, but not in the muscle IGF1R knockouts. Thus, the effects of GH on muscle in animals are likely to be mediated by IGF-I.
GH, IGF-I, exercise and muscle mass in humans
Care must be taken in extrapolating animal studies to humans, as species differences do exist, but the data seem to indicate similar roles for GH and IGF-I in the regulation of postnatal growth. In humans, GH deficiency results in a similar growth pattern to primary IGF-I deficiency, underlining the role of GH-dependent IGF-I production (Walenkamp and Wit, 2007). It is also interesting to note that the growth response following treatment of GH-deficient patients with GH is better than that observed after treating patients suffering from GH insensitivity with IGF-I (Savage et al., 2006; Walenkamp and Wit, 2007). This may be due to the IGF-I-independent effects of GH as discussed in the previous section. Alternatively, the low levels of IGFBP3 found in GH-insensitive patients may result in shorter IGF-I half-life and thus the diminished effects of the infused protein.
People with GH deficiency (GHD) tend to have increased body fat and decreased fat-free mass in comparison to control subjects. They also have decreased muscle strength and exercise tolerance (Ayuk and Sheppard, 2006; Molitch et al., 2006; Woodhouse et al., 2006). Strength has been shown to increase in GHD patients following 6 months of GH administration (Cuneo et al., 1991), but changes in myofibre CSA were not observed (Cuneo et al., 1992). This suggests that GH does not affect muscle mass in adult humans.
Observations in people with acromegaly suggest that chronic high levels of circulating GH and IGF-I may actually be detrimental to muscle function. Although people with acromegaly have large muscles, they have less specific force (force per CSA) than expected and they often present histological signs of myopathy (Nagulesparen et al., 1976; Woodhouse et al., 2006). Furthermore, people with acromegaly tend to have smaller type II than type I fibre CSAs. This is in contrast to the general population. As type II fibres are important for power generation, this could account for their relative weakness. Overexpression of human GH in transgenic mice has been reported to increase the percentage of type I fibres (Dudley and Portanova, 1987) and in GH receptor knockout mice there are fewer type I fibres and more type II fibres relative to the wild-type animals (Sotiropoulos et al., 2006). Taken together, these observations argue for a negative rather than positive effect of long-term supraphysiological circulating GH (or IGF-I) on power output and strength. It is important to remember, however, that people with acromegaly have had the disease for many years and often present other hormonal abnormalities. Therefore, they are not the best model for assessing the effects of supraphysiological GH in otherwise healthy people.
Controlled trials have examined the effects of GH or placebo administration in combination with resistance exercise, a known anabolic stimulus, in healthy young and elderly human subjects. Studying healthy elderly subjects is of interest because they represent a model of GH deficiency in that circulating levels of GH and IGF-I decline with age. Although a cause and effect relationship remains to be demonstrated, significant loss of muscle mass is also associated with increased age (sarcopaenia). The landmark studies of Rudman et al. (1990, 1991) showed that GH administration results in increased lean body mass and decreased fat/muscle ratio in elderly subjects. The question is whether this is due to an increased muscle mass.
Taaffe et al. (1994, 1996) showed that in healthy elderly men (mean age 70.3 years) myofibre CSA and strength gains were not different between those following a resistance training programme in combination with recombinant human GH (rhGH) or placebo. In two other studies, both on 31 elderly males (mean age >70 years), GH plus exercise had no effect over placebo plus exercise on strength, power or hypertrophy gains following 12 weeks (Lange et al., 2002) or 6 months (Hennessey et al., 2001) administration and training.
Measuring protein synthesis and breakdown rates is a much more sensitive technique for assessing the anabolic effects of GH administration on muscle, and has the advantage that acute and short-term studies can be performed. The rate of muscle protein turnover is slow and changes in muscle mass over periods of less than 3 months are difficult to detect (Rennie, 2003). It must be noted that exercise results in both increased protein synthesis and breakdown and that it is net protein synthesis which is the important outcome for hypertrophy (Rennie et al., 2004).
Welle et al. (1996) reported differences in strength following 12 weeks GH administration in older men (mean age 66 years), but did not observe differences between GH and placebo groups in terms of mean fractional rates of myofibrillar protein breakdown or mean postabsorptive fractional rate of myofibrillar protein synthesis. Superior increases in whole body protein synthesis have been observed in both young (mean age 23 years; Yarasheski et al., 1992) and old (mean age 67 years; Yarasheski et al., 1995) untrained subjects undertaking resistance exercise in combination with GH relative to those on placebo, but interestingly, this effect was not mirrored in quadriceps protein synthesis rates, suggesting that the GH effects are not on muscle tissue. Yarasheski et al. (1993) also demonstrated that there was no effect of 2 weeks GH administration on quadriceps protein synthesis rates or whole body protein breakdown in young experienced weight lifters (mean age 23 years). The data suggest that there is no beneficial effect of administering GH in combination with an exercise programme for muscle mass gains.
Increased muscle protein synthesis and net whole body protein synthesis have, however, been reported in elderly subjects with low serum GH following 1 month GH or IGF-I treatment (Butterfield et al., 1997). No measurements of muscle size or function were undertaken. Increased muscle protein synthesis was also observed in young, healthy men following GH administration at rest and after an overnight fast (Fryburg et al., 1991; Fryburg and Barrett, 1993). As an exercise bout can affect protein synthesis for up to 72 h (Rennie et al., 2004), it is possible that in studies employing exercise protocols the exercise stimulus masks any effects of GH administration.
Despite the observations of an effect of GH and IGF-I on protein synthesis, the fact remains that gains in muscle mass are not observed in healthy subjects after long-term GH administration so any benefits are unlikely to be due to muscle mass gains. In the GH plus exercise groups, circulating IGF-I levels and fat-free mass were consistently increased in comparison to placebo groups. Thus, it is possible to extrapolate that increasing circulating IGF-I would also be without consequence for muscle mass in healthy humans. Administration of IGF-I acutely activates muscle protein synthesis (Fryburg et al., 1995), but similarly to GH a 1-year administration did not result in increased lean body mass (Friedlander et al., 2001). The effects of GH on fat-free mass may be due to water retention, which is a known side effect of GH administration, or to an increase in soft tissue due to the stimulatory effects of GH on collagen synthesis.
Despite the lack of evidence for anabolic activity of GH in healthy humans, there is evidence for anticatabolic activity of GH as well as IGF-I. In a study comparing infusion of IGF-I with GH, it was demonstrated that both agents reduce negative nitrogen balance during calorific restriction in humans. A single dose of GH was administered during 24-h period, whereas IGF-I was infused continuously for 16 h each day. Serum IGF-I concentrations were threefold higher in the IGF-I-treated subjects compared with those on GH, but the treatments were equally effective at reducing the negative nitrogen balance. This suggests that the GH treatment was more potent (Clemmons et al., 1992), which is in line with GH having both IGF-I-mediated and direct effects. Alternatively, it may be due to the negative feedback inhibition of endogenous GH release or on autocrine/paracrine actions of the tissue IGF-I in the IGF-I treatment group. It is noteworthy that neither GH nor IGF-I resulted in positive nitrogen balance.
A subsequent study was performed to compare IGF-I treatment alone and in combination with GH (Kupfer et al., 1993). The protocols were identical as were the effects of IGF-I on its own, but the combination of IGF-I and GH was much more effective resulting in a positive nitrogen balance within 2 days of initiation of treatment. Muscle protein synthesis was not measured, so it remains to be established whether these effects apply to muscle or other tissues. The effects of GH in combination with IGF-I on protein synthesis and the applicability for the treatment of diseases associated with catabolism, particularly those for which GH treatment on its own has proven ineffective, remain to be determined.
Combined IGF-I/IGFBP3 injection also seems to be very effective in increasing net protein balance across the leg in burn patients who are catabolic but was not effective in non-catabolic patients (Herndon et al., 1999). It has been shown in endurance-trained athletes, that 1 and 4 weeks GH administration reduced leucine oxidation during exercise by 50%, and increased the rate of non-oxidative leucine disposal at rest (a measure of protein synthesis) and during exercise (Healy et al., 2003). This may be of benefit for protein sparing in endurance athletes, but a muscle-specific effect was not determined.
In summary, normal GH/IGF-I function does have a role in the development and maintenance of muscle mass, as gathered from evidence in GH-deficient patients, burn patients, hypophysectomized animals, and animal models in which GH receptor and IGF-IR activity are lacking. GH or IGF-I administration have, however, no proven benefits for muscle mass in healthy subjects in whom GH function is normal (Figure 3). In most animal studies, GH is administered while the animals are still growing and this may confound the results in comparison to administration in fully grown animals. In addition, the species differences between rodents and humans in the functioning of the GH/IGF-I axis must be taken into account. Studies with transgenic and knockout animals are also complicated by the fact that the embryonic development of the tissue can be affected and this can have different consequences to altering gene expression once the animal has reached maturity.
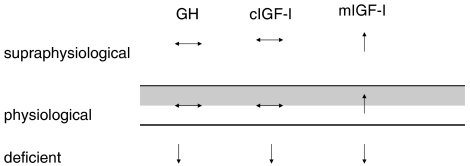
Figure 3.
Overview of the effects of different levels of growth hormone (GH), circulating IGF-I (cIGF-I) and IGF-I synthesized in muscle (mIGF-I) on muscle mass and/or performance. In healthy subjects, supraphysiological GH and cIGF-I have no effect on muscle mass. In contrast, supraphysiological levels of mIGF-I increase muscle mass and may play a role in the hypertrophic adaptation to exercise. Deficiency in GH or IGF-I results in reduction in muscle mass.
Autocrine and paracrine effects of IGF-I
Direct infusion of either GH or IGF-I into rat muscle does result in increased mass providing evidence that it is the local autocrine/paracrine rather than systemic endocrine effects that are important for hypertrophy (Adams and McCue, 1998). GH infusion also leads to increased IGF-I protein in the infused muscle, so it is likely that the hypertrophic effects of GH are mediated by locally produced IGF-I.
In support of this, there is strong evidence that the effects of GH in bone are mediated by locally produced IGF-I. GH stimulates longitudinal bone growth when injected directly into the proximal tibial epiphyseal growth plates of hypophysectomized rats, but only on the injected side (Isaksson et al., 1982). Coinfusion of anti-IGF-I antiserum together with GH into the arterial supply of the hindlimb completely abolishes the effect of GH (Schlechter et al., 1986). GH treatment also increases the number of IGF-immunoreactive cells and IGF-I messenger RNA expression in the proliferative zone of the growth plate.
Resistance exercise is an anabolic stimulus to which there is almost always hypertrophic adaptation. In animal models, compensatory hypertrophy of an exercised muscle is accompanied by increases in IGF-I mRNA and IGF-I peptide production specifically in the exercised muscle, and this precedes increases in muscle DNA and protein content (Adams and Haddad, 1996). IGF-I upregulation is also observed in muscle after stretch-induced myofibre hypertrophy (Czerwinski et al., 1994) and during muscle regeneration following injury (Levinovitz et al., 1992). Coleman et al. (1995) first reported that expression of IGF-I under control of a muscle-specific promoter in transgenic mice leads to significant hypertrophy confined to muscle tissue without affecting circulating levels of IGF-I or body size. Other muscle-specific IGF-I transgenics exist, and muscle-specific IGF-I overexpression has been shown to prevent age-related atrophy and to ameliorate myopathic phenotypes (Musaro et al., 2001, Shavlakadze et al., 2005).
Local upregulation of IGF-I also seems to augment the resistance training response even in animals with normal GH status. In rats, direct injection into muscle of an adenoviral vector encoding IGF-I in combination with resistance training increased both mass and force production over and above either treatment alone (Lee et al., 2004). Injection of IGF-I vectors also prevented loss of muscle mass following a detraining period. Resistance exercise must, however, have had additional effects to upregulating local IGF-I production as it was more effective than IGF-I at increasing muscle mass. Indeed a recent paper showed that mechanical stimulation can induce hypertrophy in MKR mice, which overexpress a dominant-negative insulin receptor specifically in skeletal muscle that inhibits both IR or IGF1R signalling (Spangenburg et al., 2008). This is surprising but not altogether unexpected as there are other pathways that are regulated by activity in muscle and can result in AKT signalling. AKT phosphorylation was also not impaired in the MKR mouse in response to overload.
Histological observations suggest that exercise and IGF-I have different modes of action (Lee et al., 2004). IGF-I stimulates the fusion of satellite cells with existing fibres, as determined by an increase in the number of myofibres with centrally versus peripherally located nuclei. Resistance exercise on its own does not appreciably increase the number of centrally located nuclei. Centrally located nuclei are considered to be an index of newly fused nuclei or new myofibre formation. As the myofibre matures, the nuclei move to the periphery of the cell. Thus, it is possible that exercise did not induce appreciable satellite cell activation and fusion or that exercise is important for maturation of fibres and peripheral localization/maturation of myofibres. Alternatively, persistent increased IGF-I may actually delay myofibre maturation.
Comparable studies in humans have understandably not been performed. There are however several reports of IGF-I mRNA upregulation following resistance exercise (Bamman et al., 2001; Hameed et al., 2003, 2004; Kim et al., 2005b). In one study, increased IGF-I immunoreactivity was also observed, but the antibody used cross reacts with IGF-II, so the results may be not be specific (Fiatarone-Singh et al., 1999). Net release of IGF-I by muscle during endurance exercise has been observed in humans by measuring arterial venous differences in hormone concentrations (Brahm et al., 1997).
In line with the autocrine–paracrine theory of IGF-I action, the endocrinological status of animals and humans does not seem to affect the ability of muscles to hypertrophy following exercise. Hypophysectomized rats that have decreased circulating GH and IGF-I are able to hypertrophy to the same extent as controls (DeVol et al., 1990). Humans with GH deficiency or the very elderly with low GH and IGF-I also adapt to resistance exercise by increasing muscle mass and strength.
Likewise, systemic GH status does not seem to affect exercise-induced upregulation of muscle IGF-I mRNA in rats (DeVol et al., 1990). In elderly humans, GH administration neither causes an increase in muscle mass nor induces IGF-mRNA upregulation in muscle despite increasing serum IGF-I (Taaffe et al., 1996). GH administration alone has, however, been shown to affect the expression of a 3′ splice variant of IGF-I (IGF-IEa) in the muscles of elderly men (Hameed et al., 2004). The discrepancy between the results of Hameed et al. and Taaffe et al. may be explained by the fact that not all IGF-I mRNA measured by the latter was IGF-IEa. Three IGF-I 3′ splice variants have been identified in human muscle. These splice variants share common sequences, which were the ones measured by Taaffe et al. Thus, if fluctuations in one isoform are compensated by decreases in another, unchanged total IGF-I transcript levels will be observed if common regions are used for quantification. The structure and putative functions of the IGF-I splice variants in muscle are discussed elsewhere (Shavlakadze et al., 2005; Barton, 2006).
Interplay between systemic and locally produced IGF-I
The interactions between circulating and local IGF-I expression may also play a role in regulating muscle mass. In contrast to transgenic mice overexpressing IGF-I under control of muscle-specific promoters, transgenics overexpressing IGF-I more ubiquitously, under control of the metallothionein promoter, have increased concentration of IGF-I in serum (Mathews et al., 1988). They also exhibit increased body weight and organomegaly, but only modest improvement in muscle mass. In another model where IGF-I expression is controlled by a muscle-specific promoter, but the construct contains a somatostatin signal peptide to ensure secretion, increased circulating and muscle IGF-I levels are observed, but muscle hypertrophy is not (Shavlakadze et al., 2006). This contrasts sharply with animals in which IGF-I is expressed in muscle without leading to concomitant increases in serum IGF-I (Coleman et al., 1995; Musaro et al., 2001). It is possible that increased circulating IGF-I affects GH and thus has consequences for the synthesis of autocrine/paracrine levels of IGF-I. Alternatively, increased circulating IGF-I may directly inhibit either muscle IGF-I synthesis or the effects of locally produced IGF-I. Indeed, it has been reported that IGF-I mRNA is downregulated in cultured muscle cells following IGF-I treatment (Frost et al., 2002).
Recently, we observed that GH administration does not upregulate IGF-IEa in skeletal muscles of young men (M Aperghis et al., 2004) whereas in older men, GH resulted in increased skeletal muscle IGF-IEa (Hameed et al., 2004). In the young men, GH administration led to serum IGF-I levels that were supraphysiological, whereas in older men serum IGF-I levels after treatment were equivalent to the pretreatment levels observed in young men. The effects of supraphysiological versus normal circulating IGF-I on muscle hypertrophy and local IGF-I production remain to be investigated in detail.
Cell culture studies
A simple and useful system to study signalling pathways in skeletal muscle is cultured muscle cells or myoblasts derived from muscle explants. They can be induced to proliferate and differentiate in vitro. Differentiation involves irreversible cell cycle arrest and fusion into multinucleated myotubes that express muscle-specific markers such as myogenin, sarcomeric myosin heavy chain and muscle creatine kinase. Unlike myotubes formed during embryonic development, cultured myotubes do not mature into myofibres. This in vitro model is nevertheless useful to distinguish between the effects of IGF-I on proliferation, differentiation and hypertrophy as IGF-I can be added or signalling can be modulated at different stages of the differentiation process.
Treatment of proliferating C2C12 myoblasts (a rodent cell line) with IGF-I leads to increased proliferation, but once the cells have stopped proliferating, treatment leads to increased fusion and hypertrophy of resulting myotubes (Rommel et al., 2001). Hypertrophy is also observed following IGF-I treatment of primary avian (Vandenburgh et al., 1991) and human muscle cells (Jacquemin et al., 2004, 2007).
IGF-I increases the size of human myotubes whether treatment begins while myoblasts are still proliferating or after proliferation has ceased (Jacquemin et al., 2004, 2007). IGF-I appears to regulate human myotube size by activating protein synthesis, inhibiting protein degradation and inducing fusion of reserve cells. During differentiation in culture, the majority of cells exit the cell cycle and fuse, but there is always a small number of so-called reserve cells that remain mononucleated. Fusion of a greater proportion of reserve cells increases the number of nuclei found within myotubes (fusion index) and this will result in larger myotubes. Inhibition of several pathways (p42MAPK, calcineurin, AKT) reduces the fusion index and protein synthesis responses of human myoblasts to IGF-I treatment. On the other hand, inhibition of GSK3, a negative regulator of protein synthesis, mimics these responses in the absence of IGF-I. Inhibition of the p38 MAPK pathway has no effect, which is consistent with a role for this in myoblast proliferation rather than differentiation.
The effect of IGF-I on reserve cell recruitment appears to be indirect and to result from increased production of the cytokine interleukin-13 by treated myotubes. It remains to be demonstrated whether induction of satellite cells fusion is induced by interleukin-13 in vivo and whether expression on this cytokine in muscle is regulated by IGF-I. It is also unclear whether fusion of nuclei is a cause or consequence of activation of protein synthesis and cell size increase. The latter seems more likely as the phenotype of cells treated with rapamycin is much more dramatic than those treated with other inhibitors.
Treatment of mouse primary muscle cells with IGF-I or GH increases myotube size to the same extent (Sotiropoulos et al., 2006). In agreement with the studies on human myoblasts using IGF-I, the GH treatment experiments resulted in larger myotubes with more nuclei and seemed to involve the signalling via the transcription factor NFATc2 (Figure 4). This would suggest that in culture the effects of GH on muscle size are also mediated by IGF-I as demonstrated in vivo (Kim et al., 2005a). Combined GH and IGF-I treatment was, however, more effective in increasing myotube size than either hormone on its own. Furthermore, hypertrophy of GHR−/− myotubes following IGF-I treatment was inferior to that of wild-type myotubes. These observations suggest that GH also has IGF-I-independent effects. This is supported by a comparison of the phenotypes of GHR−/− and IGF-I−/− knockout animals (Lupu et al., 2001).
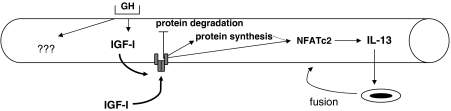
Figure 4.
Effects of growth hormone (GH) and circulating IGF-I (IGF-I) on cultured myotubes. Both GH and IGF-I induce myotube hypertrophy. IGF-I increases protein synthesis and inhibits protein degradation. In addition, it induces fusion of myoblasts by upregulating synthesis of interleukin (IL)-13. It is possible that the IL-13 response is secondary to protein synthesis, with new nuclei being recruited only when required to maximize growth. Indeed treatment of cultures with rapamycin, inhibitor of mTOR, inhibits both hypertrophy and increase in fusion index of IGF-I-treated myotubes (Jacquemin et al., 2007). Cotreatment of cultures with GH and IGF-I induces greater hypertrophic gains than either treatment alone. Thus, the hormones have distinct and overlapping effects on cells.
Other effects of GH and IGF-I
In adult humans, GH administration is lipolytic and causes increase in serum-free fatty acids. In turn, this inhibits glucose uptake to the heart, adipose tissue and muscle and may underlie the hyperglycaemia and insulin resistance associated with acromegaly. GH also causes an increase in water absorption by the gut and sodium retention leading to extracellular fluid accumulation, carpal tunnel syndrome and hypertension. For a more in depth discussion of these effects of GH, the reader is referred to two comprehensive and recent reviews (Woodhouse et al., 2006; Gibney et al., 2007).
GH and IGF-I have opposite metabolic effects. In the study of Clemmons et al. (1992) GH administration caused hyperglycaemia, whereas IGF-I administration caused hypoglycaemia. Combination of the two hormones attenuated the hypoglycaemic and hypoinsulinaemic effects of IGF-I and conversely the hyperglycaemic, hyperinsulinemic effects of GH (Kupfer et al., 1993). The hormones had opposite effects on IGBP3 with IGF-I causing a reduction of this molecule in serum, whereas coadministration with GH resulted in increased IGFBP3. Given the effects of combined administration, it seems plausible that the side effects of administering either protein on its own negate the benefits, whereas the combination of the two hormones annuls the side effects and potentiates the benefits. Administration of IGF-I with IGFBP3 also seems to ameliorate some of the side effects (Herndon et al., 1999).
GH as a performance-enhancing drug
Resistance and endurance exercise induce GH release. The higher the exercise intensity, the greater the magnitude of the peak GH pulse (Gibney et al., 2007). IGF-I also increases slightly after acute exercise. Chronic exercise leads to increased levels of both IGF-I and GH in serum, and athletes tend to have higher values than the general population, suggesting a role for GH and IGF-I in exercise adaptation (Healy et al., 2005). These observations along with the role of GH on postnatal body growth led to the suggestion that treatment with GH is anabolic for muscle. The use of GH and IGF-I is banned by the World Anti Doping Agency not only because of the principle of fair play in competitive sports, but also because of the adverse effects of supraphysiological doses on health. In addition, although rhGH has been available since 1985, GH extracted from the pituitary glands of human cadavers is still available in some countries. Administration from this source carries a significant risk of infection of transmissible brain diseases such as Creutzfeldt–Jakob Disease.
The use of GH in amateur and professional sports seems to be widespread, although the evidence is quite strong that supraphysiological GH administration does not potentiate the effects of exercise on muscle mass and strength in healthy individuals. IGF-I use is probably more limited as it is less readily available than GH.
The attraction of GH abuse may be due to several reasons (Rennie, 2003; Rigamonti et al., 2005; Saugy et al., 2006; Gibney et al., 2007). First, GH is lipolytic although this benefit may not always be evident in well-trained athletes with low body fat (Deyssig et al., 1993). Second, GH has known effects on collagen and bone turnover and it has been suggested that its supraphysiological administration may strengthen connective tissue thereby paralleling increase in strength brought about by exercise (or other measures such as anabolic steroids), thus decreasing risk of injury to these tissues. Third, GH has anectodal side effects such as improving skin tone, eyesight and recovery time from injury, all of which may be considered beneficial to the athlete undergoing strenuous training. Fourth, athletes often take performance-enhancing substances in combination, a practice known as stacking. Appropriate, placebo controlled trials using GH in combination with other substances are few. Fifth, at the higher doses reportedly used by athletes, GH may be more effective than at the doses approved for research studies, which are limited due to complications associated with GH administration. The fluid retention which occurs with GH is usually well tolerated and most subjects are happy to remain on GH. Athralgia, carpal tunnel syndrome, oedema and atrial fibrillation are reported in studies using GH administration sometimes leading subjects to withdraw.
Exercise performance (maximal oxygen uptake, ventilatory threshold and muscle strength) is lower than predicted for age, gender and height in GHD patients and these improve with GH replacement (Woodhouse et al., 2006). These improvements are in proportion to increased lean body mass. This suggests that body composition and metabolic adaptations rather than increased muscle mass may be responsible for performance gains. The effects on fuel metabolism, VO2max and ventilatory threshold may be of consequence to athletes seeking to improve endurance (Woodhouse et al., 2006; Gibney et al., 2007). A combination of testosterone and GH led to improved body composition and VO2max in elderly men, suggesting that GH does have a performance-enhancing effect (Giannoulis et al., 2006). GH also improves aerobic performance in those who have a history of androgenic anabolic steroid use (Graham et al., 2007).
Conclusion
The mechanisms that lead to muscle adaptation to overload are not completely understood. Neither are those that regulate muscle mass development and maintenance. GH and IGF-I clearly play a role in muscle development pre- and postnatally. In GHD adults, there is evidence that serum GH affects muscle mass maintenance, but in healthy adults neither GH nor IGF-I has or enhances the hypertrophic effects of exercise. In contrast, much evidence supports the hypertrophic effect of autocrine/paracrine IGF-I in animals and suggests that it may play a role in adaptation to overload in both animals and humans. Increased muscle expression of IGF-I also enhances the effects of training in animals. Local injection of GH or IGF-I protein or plasmids is effective in animal models and may eventually be used with therapeutic ends. There is evidence for an effect of GH on other performance parameters that is related to increased lean body mass as opposed to increased skeletal muscle mass.
Conflict of interest
The author states no conflict of interest.
References
- Adams GR, Haddad F. The relationships among IGF-1, DNA content, and protein accumulation during skeletal muscle hypertrophy. J Appl Physiol. 1996;81:2509–2516. doi: 10.1152/jappl.1996.81.6.2509. [DOI] [PubMed] [Google Scholar]
- Adams GR, McCue SA. Localized infusion of IGF-I results in skeletal muscle hypertrophy in rats. J Appl Physiol. 1998;84:1716–1722. doi: 10.1152/jappl.1998.84.5.1716. [DOI] [PubMed] [Google Scholar]
- Antonio J, Gonyea WJ. Progressive stretch overload of skeletal muscle results in hypertrophy before hyperplasia. J Appl Physiol. 1993;75:1263–1271. doi: 10.1152/jappl.1993.75.3.1263. [DOI] [PubMed] [Google Scholar]
- Aperghis M, Hameed M, Bouloux P, Goldspink G, Harridge S. Two weeks of GH administration does not increase the expression of insulin-like growth factor-I mRNA splice variants in the skeletal muscle of young men. J Physiol. 2004;558P:C4. [Google Scholar]
- Araki E, Lipes MA, Patti ME, Bruning JC, Haag B, III, Johnson RS, et al. Alternative pathway of insulin signalling in mice with targeted disruption of the IRS-1 gene. Nature. 1994;372:186–190. doi: 10.1038/372186a0. [DOI] [PubMed] [Google Scholar]
- Ayuk J, Sheppard MC. Growth hormone and its disorders. Postgrad Med J. 2006;82:24–30. doi: 10.1136/pgmj.2005.036087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baar K, Nader G, Bodine S. Resistance exercise, muscle loading/unloading and the control of muscle mass. Essays Biochem. 2006;42:61–74. doi: 10.1042/bse0420061. [DOI] [PubMed] [Google Scholar]
- Bach LA, Hsieh S, Sakano K, Fujiwara H, Perdue JF, Rechler MM. Binding of mutants of human insulin-like growth factor II to insulin-like growth factor binding proteins 1–6. J Biol Chem. 1993;268:9246–9254. [PubMed] [Google Scholar]
- Baker J, Liu JP, Robertson EJ, Efstratiadis A. Role of insulin-like growth factors in embryonic and postnatal growth. Cell. 1993;75:73–82. [PubMed] [Google Scholar]
- Bamman MM, Shipp JR, Jiang J, Gower B, Hunter GR, Goodman A, et al. Mechanical load increases muscle IGF-I and androgen receptor mRNA concentrations in humans. Am J Physiol Endocrinol Metab. 2001;280:E383–E390. doi: 10.1152/ajpendo.2001.280.3.E383. [DOI] [PubMed] [Google Scholar]
- Barton ER. The ABCs of IGF-I isoforms: impact on muscle hypertrophy and implications for repair. Appl Physiol Nutr Metab. 2006;31:791–797. doi: 10.1139/h06-054. [DOI] [PubMed] [Google Scholar]
- Behringer RR, Lewin TM, Quaife CJ, Palmiter RD, Brinster RL, D'Ercole AJ. Expression of insulin-like growth factor I stimulates normal somatic growth in growth hormone-deficient transgenic mice. Endocrinology. 1990;127:1033–1040. doi: 10.1210/endo-127-3-1033. [DOI] [PubMed] [Google Scholar]
- Berelowitz M, Szabo M, Frohman LA, Firestone S, Chu L, Hintz RL. Somatomedin-C mediates growth hormone negative feedback by effects on both the hypothalamus and the pituitary. Science. 1981;212:1279–1281. doi: 10.1126/science.6262917. [DOI] [PubMed] [Google Scholar]
- Bermann M, Jaffe CA, Tsai W, DeMott-Friberg R, Barkan AL. Negative feedback regulation of pulsatile growth hormone secretion by insulin-like growth factor I. Involvement of hypothalamic somatostatin. J Clin Invest. 1994;94:138–145. doi: 10.1172/JCI117299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolster DR, Crozier SJ, Kimball SR, Jefferson LS. AMP-activated protein kinase suppresses protein synthesis in rat skeletal muscle through down-regulated mammalian target of rapamycin (mTOR) signaling. J Biol Chem. 2002;277:23977–23980. doi: 10.1074/jbc.C200171200. [DOI] [PubMed] [Google Scholar]
- Bolster DR, Jefferson LS, Kimball SR. Regulation of protein synthesis associated with skeletal muscle hypertrophy by insulin-, amino acid- and exercise-induced signalling. Proc Nutr Soc. 2004;63:351–356. doi: 10.1079/PNS2004355. [DOI] [PubMed] [Google Scholar]
- Brahm H, Piehl-Aulin K, Saltin B, Ljunghall S. Net fluxes over working thigh of hormones, growth factors and biomarkers of bone metabolism during short lasting dynamic exercise. Calcif Tissue Int. 1997;60:175–180. doi: 10.1007/s002239900210. [DOI] [PubMed] [Google Scholar]
- Buckway CK, Guevara-Aguirre J, Pratt KL, Burren CP, Rosenfeld RG. The IGF-I generation test revisited: a marker of GH sensitivity. J Clin Endocrinol Metab. 2001;86:5176–5183. doi: 10.1210/jcem.86.11.8019. [DOI] [PubMed] [Google Scholar]
- Butterfield GE, Thompson J, Rennie MJ, Marcus R, Hintz RL, Hoffman AR. Effect of rhGH and rhIGF-I treatment on protein utilization in elderly women. Am J Physiol. 1997;272:E94–E99. doi: 10.1152/ajpendo.1997.272.1.E94. [DOI] [PubMed] [Google Scholar]
- Cho H, Thorvaldsen JL, Chu Q, Feng F, Birnbaum MJ. Akt1/PKBalpha is required for normal growth but dispensable for maintenance of glucose homeostasis in mice. J Biol Chem. 2001;276:38349–38352. doi: 10.1074/jbc.C100462200. [DOI] [PubMed] [Google Scholar]
- Clemmons DR, Smith-Banks A, Underwood LE. Reversal of diet-induced catabolism by infusion of recombinant insulin-like growth factor-I in humans. J Clin Endocrinol Metab. 1992;75:234–238. doi: 10.1210/jcem.75.1.1619015. [DOI] [PubMed] [Google Scholar]
- Coleman ME, DeMayo F, Yin KC, Lee HM, Geske R, Montgomery C, et al. Myogenic vector expression of insulin-like growth factor I stimulates muscle cell differentiation and myofiber hypertrophy in transgenic mice. J Biol Chem. 1995;270:12109–12116. doi: 10.1074/jbc.270.20.12109. [DOI] [PubMed] [Google Scholar]
- Cuneo RC, Salomon F, Wiles CM, Hesp R, Sonksen PH. Growth hormone treatment in growth hormone-deficient adults. I. Effects on muscle mass and strength. J Appl Physiol. 1991;70:688–694. doi: 10.1152/jappl.1991.70.2.688. [DOI] [PubMed] [Google Scholar]
- Cuneo RC, Salomon F, Wiles CM, Round JM, Jones D, Hesp R, et al. Histology of skeletal muscle in adults with GH deficiency: comparison with normal muscle and response to GH treatment. Horm Res. 1992;37:23–28. doi: 10.1159/000182276. [DOI] [PubMed] [Google Scholar]
- Czerwinski SM, Martin JM, Bechtel PJ. Modulation of IGF mRNA abundance during stretch-induced skeletal muscle hypertrophy and regression. J Appl Physiol. 1994;76:2026–2030. doi: 10.1152/jappl.1994.76.5.2026. [DOI] [PubMed] [Google Scholar]
- D'Ercole J, Stiles AD, Underwood LE. Tissue concentrations of somatomedin C: further evidence for multiple sites of synthesis and paracrine or autocrine mechanisms of action. Proc Natl Acad Sci USA. 1984;81:935–939. doi: 10.1073/pnas.81.3.935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeVol DL, Rotwein P, Sadow JL, Novakofski J, Bechtel PJ. Activation of insulin-like growth factor gene expression during work-induced skeletal muscle growth. Am J Physiol. 1990;259:E89–E95. doi: 10.1152/ajpendo.1990.259.1.E89. [DOI] [PubMed] [Google Scholar]
- Deyssig R, Frisch H, Blum WF, Waldhor T. Effect of growth hormone treatment on hormonal parameters, body composition and strength in athletes. Acta Endocrinol (Copenh) 1993;128:313–318. doi: 10.1530/acta.0.1280313. [DOI] [PubMed] [Google Scholar]
- Dudley GA, Portanova R. Histochemical characteristics of soleus muscle in hGH transgenic mice. Proc Soc Exp Biol Med. 1987;185:403–408. doi: 10.3181/00379727-185-42561. [DOI] [PubMed] [Google Scholar]
- Efstratiadis A. Genetics of mouse growth. Int J Dev Biol. 1998;42:955–976. [PubMed] [Google Scholar]
- Fiatarone-Singh MA, Wenjing D, Manfredi TJ, Solares GS, O'Neill EF, Clements KM, et al. Insulin-like growth factor I in skeletal muscle after weight-lifting exercise in frail elders. Am J Physiol Endocrinol Metab. 1999;277:E135–E143. doi: 10.1152/ajpendo.1999.277.1.E135. [DOI] [PubMed] [Google Scholar]
- Frick GP, Tai LR, Baumbach WR, Goodman HM. Tissue distribution, turnover, and glycosylation of the long and short growth hormone receptor isoforms in rat tissues. Endocrinology. 1998;139:2824–2830. doi: 10.1210/endo.139.6.6047. [DOI] [PubMed] [Google Scholar]
- Friedlander AL, Butterfield GE, Moynihan S, Grillo J, Pollack M, Holloway L, et al. One year of insulin-like growth factor I treatment does not affect bone density, body composition, or psychological measures in postmenopausal women. J Clin Endocrinol Metab. 2001;86:1496–1503. doi: 10.1210/jcem.86.4.7377. [DOI] [PubMed] [Google Scholar]
- Frost RA, Nystrom GJ, Lang CH. Regulation of IGF-I mRNA and signal transducers and activators of transcription-3 and -5 (Stat-3 and -5) by GH in C2C12 myoblasts. Endocrinology. 2002;143:492–503. doi: 10.1210/endo.143.2.8641. [DOI] [PubMed] [Google Scholar]
- Fryburg DA, Barrett EJ. Growth hormone acutely stimulates skeletal muscle but not whole-body protein synthesis in humans. Metabolism. 1993;42:1223–1227. doi: 10.1016/0026-0495(93)90285-v. [DOI] [PubMed] [Google Scholar]
- Fryburg DA, Gelfand RA, Barrett EJ. Growth hormone acutely stimulates forearm muscle protein synthesis in normal humans. Am J Physiol. 1991;260:E499–E504. doi: 10.1152/ajpendo.1991.260.3.E499. [DOI] [PubMed] [Google Scholar]
- Fryburg DA, Jahn LA, Hill SA, Oliveras DM, Barrett EJ. Insulin and insulin-like growth factor-I enhance human skeletal muscle protein anabolism during hyperaminoacidemia by different mechanisms. J Clin Invest. 1995;96:1722–1729. doi: 10.1172/JCI118217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giannoulis MG, Sonksen PH, Umpleby M, Breen L, Pentecost C, Whyte M, et al. The effects of growth hormone and/or testosterone in healthy elderly men: a randomized controlled trial. J Clin Endocrinol Metab. 2006;91:477–484. doi: 10.1210/jc.2005-0957. [DOI] [PubMed] [Google Scholar]
- Gibney J, Healy ML, Sonksen PH. The growth hormone/insulin-like growth factor-I axis in exercise and sport. Endocr Rev. 2007;28:603–624. doi: 10.1210/er.2006-0052. [DOI] [PubMed] [Google Scholar]
- Gibson JN, Smith K, Rennie MJ. Prevention of disuse muscle atrophy by means of electrical stimulation: maintenance of protein synthesis. Lancet. 1988;2:767–770. doi: 10.1016/s0140-6736(88)92417-8. [DOI] [PubMed] [Google Scholar]
- Glass DJ. Skeletal muscle hypertrophy and atrophy signaling pathways. Int J Biochem Cell Biol. 2005;37:1974–1984. doi: 10.1016/j.biocel.2005.04.018. [DOI] [PubMed] [Google Scholar]
- Goldenberg N, Barkan A. Factors regulating growth hormone secretion in humans. Endocrinol Metab Clin North Am. 2007;36:37–55. doi: 10.1016/j.ecl.2006.11.003. [DOI] [PubMed] [Google Scholar]
- Gostelli-Peter M, Winterhalter KH, Schmid C, Froesch ER, Zapf J. Expression and regulation of insulin like growth factor I (IGF-I) and IGF binding protein messenger ribonucleic acid levels in tissues of hypophysectomized rats infused with IGF-I and growth hormone. Endocrinology. 1994;135:2558–2567. doi: 10.1210/endo.135.6.7527334. [DOI] [PubMed] [Google Scholar]
- Graham MR, Baker JS, Evans P, Kicman A, Cowan D, Hullin D, et al. Short-term recombinant human growth hormone administration improves respiratory function in abstinent anabolic-androgenic steroid users. Growth Horm IGF Res. 2007;17:328–335. doi: 10.1016/j.ghir.2007.04.003. [DOI] [PubMed] [Google Scholar]
- Hameed M, Lange KH, Andersen JL, Schjerling P, Kjaer M, Harridge SD, et al. The effect of recombinant human growth hormone and resistance training on IGF-I mRNA expression in the muscles of elderly men. J Physiol. 2004;555:231–240. doi: 10.1113/jphysiol.2003.051722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hameed M, Orrell RW, Cobbold M, Goldspink G, Harridge SD. Expression of IGF-I splice variants in young and old human skeletal muscle after high resistance exercise. J Physiol. 2003;547:247–254. doi: 10.1113/jphysiol.2002.032136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Healy ML, Dall R, Gibney J, Bassett E, Ehrnborg C, Pentecost C, et al. Toward the development of a test for growth hormone (GH) abuse: a study of extreme physiological ranges of GH-dependent markers in 813 elite athletes in the postcompetition setting. J Clin Endocrinol Metab. 2005;90:641–649. doi: 10.1210/jc.2004-0386. [DOI] [PubMed] [Google Scholar]
- Healy ML, Gibney J, Russell-Jones DL, Pentecost C, Croos P, Sönksen PH, et al. High dose growth hormone exerts an anabolic effect at rest and during exercise in endurance-trained athletes. J Clin Endocrinol Metab. 2003;88:5221–5226. doi: 10.1210/jc.2002-021872. [DOI] [PubMed] [Google Scholar]
- Hennessey JV, Chromiak JA, DellaVentura S, Reinert SE, Puhl J, Kiel DP, et al. Growth hormone administration and exercise effects on muscle fiber type and diameter in moderately frail older people. J Am Geriatr Soc. 2001;49:852–858. doi: 10.1046/j.1532-5415.2001.49173.x. [DOI] [PubMed] [Google Scholar]
- Herndon DN, Ramzy PI, DebRoy MA, Zheng M, Ferrando AA, Chinkes DL, et al. Muscle protein catabolism after severe burn: effects of IGF-1/IGFBP-3 treatment. Ann Surg. 1999;229:713–720. doi: 10.1097/00000658-199905000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaksson OG, Jansson JO, Gause IA. Growth hormone stimulates longitudinal bone growth directly. Science. 1982;216:1237–1239. doi: 10.1126/science.7079756. [DOI] [PubMed] [Google Scholar]
- Jacquemin V, Butler-Browne GS, Furling D, Mouly V. IL-13 mediates the recruitment of reserve cells for fusion during IGF-1-induced hypertrophy of human myotubes. J Cell Sci. 2007;120:670–681. doi: 10.1242/jcs.03371. [DOI] [PubMed] [Google Scholar]
- Jacquemin V, Furling D, Bigot A, Butler-Browne GS, Mouly V. IGF-1 induces human myotube hypertrophy by increasing cell recruitment. Exp Cell Res. 2004;299:148–158. doi: 10.1016/j.yexcr.2004.05.023. [DOI] [PubMed] [Google Scholar]
- Jones JI, Gockerman A, Busby WH, Jr, Wright G, Clemmons DR. Insulin-like growth factor binding protein 1 stimulates cell migration and binds to the alpha 5 beta 1 integrin by means of its Arg-Gly-Asp sequence. Proc Natl Acad Sci USA. 1993;90:10553–10557. doi: 10.1073/pnas.90.22.10553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorgensen JO, Jessen N, Pedersen SB, Vestergaard E, Gormsen L, Lund SA, et al. GH receptor signalling in skeletal muscle and adipose tissue in human subjects following exposure to an intravenous GH bolus. Am J Physiol Endocrinol Metab. 2006;291:899–905. doi: 10.1152/ajpendo.00024.2006. [DOI] [PubMed] [Google Scholar]
- Kadi F, Thornell LE. Training affects myosin heavy chain phenotype in the trapezius muscle of women. Histochem Cell Biol. 1999;112:73–78. doi: 10.1007/s004180050393. [DOI] [PubMed] [Google Scholar]
- Kajantie E, Dunkel L, Rutanen EM, Seppälä M, Koistinen R, Sarnesto A, et al. IGF-I, IGF binding protein (IGFBP)-3, phosphoisoforms of IGFBP-1, and postnatal growth in very low birth weight infants. J Clin Endocrinol Metab. 2002;87:2171–2179. doi: 10.1210/jcem.87.5.8457. [DOI] [PubMed] [Google Scholar]
- Kim H, Barton E, Muja N, Yakar S, Pennisi P, Leroith D. Intact insulin and insulin-like growth factor-I receptor signaling is required for growth hormone effects on skeletal muscle growth and function in vivo. Endocrinology. 2005a;146:1772–1779. doi: 10.1210/en.2004-0906. [DOI] [PubMed] [Google Scholar]
- Kim JS, Kosek DJ, Petrella JK, Cross JM, Bamman MM. Resting and load-induced levels of myogenic gene transcripts differ between older adults with demonstrable sarcopenia and young men and women. J Appl Physiol. 2005b;9:2149–2158. doi: 10.1152/japplphysiol.00513.2005. [DOI] [PubMed] [Google Scholar]
- Kimball SR, Shantz LM, Horetsky RL, Jefferson LS. Leucine regulates translation of specific mRNAs in L6 myoblasts through mTOR-mediated changes in availability of eIF4E and phosphorylation of ribosomal protein S6. J Biol Chem. 1999;274:11647–11652. doi: 10.1074/jbc.274.17.11647. [DOI] [PubMed] [Google Scholar]
- Kjeldsen T, Andersen AS, Wiberg FC, Rasmussen JS, Schaffer L, Balschmidt P, et al. The ligand specificities of the insulin receptor and the insulin-like growth factor I receptor reside in different regions of a common binding site. Proc Natl Acad Sci USA. 1991;88:4404–4408. doi: 10.1073/pnas.88.10.4404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojima M, Hosoda H, Date Y, Nakazato M, Matsuo H, Kangawa K. Ghrelin is a growth hormone releasing acylated peptide from the stomach. Nature. 1999;402:656–660. doi: 10.1038/45230. [DOI] [PubMed] [Google Scholar]
- Kopchick JJ, Bellush LL, Coschigano KT. Transgenic models of growth hormone action. Annu Rev Nutr. 1999;19:437–461. doi: 10.1146/annurev.nutr.19.1.437. [DOI] [PubMed] [Google Scholar]
- Kupfer SR, Underwood LE, Baxter RC, Clemmons DR. Enhancement of the anabolic effects of growth hormone and insulin-like growth factor I by use of both agents simultaneously. J Clin Invest. 1993;91:391–396. doi: 10.1172/JCI116212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lange KH, Andersen JL, Beyer N, Isaksson F, Larsson B, Rasmussen MH, et al. GH administration changes myosin heavy chain isoforms in skeletal muscle but does not augment muscle strength or hypertrophy, either alone or combined with resistance exercise training in healthy elderly men. J Clin Endocrinol Metab. 2002;87:513–523. doi: 10.1210/jcem.87.2.8206. [DOI] [PubMed] [Google Scholar]
- Lee S, Barton ER, Sweeney HL, Farrar RP. Viral expression of insulin-like growth factor-I enhances muscle hypertrophy in resistance-trained rats. J Appl Physiol. 2004;96:1097–1104. doi: 10.1152/japplphysiol.00479.2003. [DOI] [PubMed] [Google Scholar]
- Le Roith D, Bondy C, Yakar S, Liu JL, Butler A. The somatomedin hypothesis: 2001. Endocr Rev. 2001;22:53–74. doi: 10.1210/edrv.22.1.0419. [DOI] [PubMed] [Google Scholar]
- Levinovitz A, Jennische E, Oldfors A, Edwall D, Norstedt G. Activation of insulin-like growth factor II expression during skeletal muscle regeneration in the rat: correlation with myotube formation. Mol Endocrinol. 1992;6:1227–1234. doi: 10.1210/mend.6.8.1406701. [DOI] [PubMed] [Google Scholar]
- Liu JP, Baker J, Perkins AS, Robertson EJ, Efstratiadis A. Mice carrying null mutations of the genes encoding insulin-like growth factor I (IGF-1) and type 1 IGF receptor (IGF1R) Cell. 1993;75:59–72. [PubMed] [Google Scholar]
- Lupu F, Terwilliger JD, Lee K, Segre GV, Efstratiadis A. Roles of growth hormone and insulin-like growth factor 1 in mouse postnatal growth. Dev Biol. 2001;229:141–162. doi: 10.1006/dbio.2000.9975. [DOI] [PubMed] [Google Scholar]
- Mammucari C, Milan G, Romanello V, Masiero E, Rudolf R, Del Piccolo P, et al. FoxO3 controls autophagy in skeletal muscle in vivo. Cell Metab. 2007;6:458–471. doi: 10.1016/j.cmet.2007.11.001. [DOI] [PubMed] [Google Scholar]
- Mathews LS, Hammer RE, Behringer RR, D'Ercole AJ, Bell GI, Brinster RL, et al. Growth enhancement of transgenic mice expressing human insulin-like growth factor I. Endocrinology. 1988;123:2827–2833. doi: 10.1210/endo-123-6-2827. [DOI] [PubMed] [Google Scholar]
- Mathews LS, Norstedt G, Palmiter RD. Regulation of insulin-like growth factor I gene expression by growth hormone. Proc Natl Acad Sci USA. 1986;83:9343–9347. doi: 10.1073/pnas.83.24.9343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauras N, Haymond MW. Are the metabolic effects of GH and IGF-I separable. Growth Horm IGF Res. 2005;15:19–27. doi: 10.1016/j.ghir.2004.12.003. [DOI] [PubMed] [Google Scholar]
- Molitch ME, Clemmons DR, Malozowski S, Merriam GR, Shalet SM, Vance ML. Evaluation and treatment of adult growth hormone deficiency: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab. 2006;91:1621–1634. doi: 10.1210/jc.2005-2227. [DOI] [PubMed] [Google Scholar]
- Musaro A, McCullagh K, Paul A, Houghton L, Dobrowolny G, Molinaro M, et al. Localized Igf-1 transgene expression sustains hypertrophy and regeneration in senescent skeletal muscle. Nat Genet. 2001;27:195–200. doi: 10.1038/84839. [DOI] [PubMed] [Google Scholar]
- Nagulesparen M, Trickey R, Davies MJ, Jenkins JS. Muscle changes in acromegaly. Br Med J. 1976;2:914–915. doi: 10.1136/bmj.2.6041.914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandini G, Frasca F, Mineo R, Sciacca L, Vigneri R, Belfiore A. Insulin/insulin-like growth factor I hybrid receptors have different biological characteristics depending on the insulin receptor isoform involved. J Biol Chem. 2002;277:39684–39695. doi: 10.1074/jbc.M202766200. [DOI] [PubMed] [Google Scholar]
- Parker A, Rees C, Clarke J, Busby WH, Jr, Clemmons DR. Binding of insulin-like growth factor (IGF)-binding protein-5 to smooth-muscle cell extracellular matrix is a major determinant of the cellular response to IGF-I. Mol Biol Cell. 1998;9:2383–2392. doi: 10.1091/mbc.9.9.2383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell-Braxton L, Hollingshead P, Warburton C, Dowd M, Pitts-Meek S, Dalton D, et al. IGF-I is required for normal embryonic growth in mice. Genes Dev. 1993;7:2609–2617. doi: 10.1101/gad.7.12b.2609. [DOI] [PubMed] [Google Scholar]
- Rennie MJ. Claims for the anabolic effects of growth hormone: a case of the emperor's new clothes. Br J Sports Med. 2003;37:100–105. doi: 10.1136/bjsm.37.2.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rennie MJ, Wackerhage H, Spangenburg EE, Booth FW. Control of the size of the human muscle mass. Annu Rev Physiol. 2004;66:799–828. doi: 10.1146/annurev.physiol.66.052102.134444. [DOI] [PubMed] [Google Scholar]
- Rigamonti AE, Cella SG, Marazzi N, Di Luigi L, Sartorio A, Muller EE. Growth hormone abuse: methods of detection. Trends Endocrinol Metab. 2005;16:160–166. doi: 10.1016/j.tem.2005.03.009. [DOI] [PubMed] [Google Scholar]
- Rommel C, Bodine SC, Clarke BA, Rossman R, Nunez L, Stitt TN, et al. Mediation of IGF-1-induced skeletal myotube hypertrophy by PI(3)K/Akt/mTOR and PI(3)K/Akt/GSK3 pathways. Nat Cell Biol. 2001;3:1009–1013. doi: 10.1038/ncb1101-1009. [DOI] [PubMed] [Google Scholar]
- Rosenblatt JD, Parry DJ. Gamma irradiation prevents compensatory hypertrophy of overloaded mouse extensor digitorum longus muscle. J Appl Physiol. 1992;73:2538–2543. doi: 10.1152/jappl.1992.73.6.2538. [DOI] [PubMed] [Google Scholar]
- Roy RR, Monke SR, Allen DL, Edgerton VR. Modulation of myonuclear number in functionally overloaded and exercised rat plantaris fibers. J Appl Physiol. 1999;87:634–642. doi: 10.1152/jappl.1999.87.2.634. [DOI] [PubMed] [Google Scholar]
- Rudman D, Feller AG, Cohn L, Shetty KR, Rudman IW, Draper MW. Effects of human growth hormone on body composition in elderly men. Horm Res Suppl. 1991;1:73–81. doi: 10.1159/000182193. [DOI] [PubMed] [Google Scholar]
- Rudman D, Feller AG, Nagraj HS, Gergans GA, Lalitha PY, Goldberg AF, et al. Effects of human growth hormone in men over 60 years old. N Engl J Med. 1990;323:1–6. doi: 10.1056/NEJM199007053230101. [DOI] [PubMed] [Google Scholar]
- Saugy M, Robinson N, Saudan C, Baume N, Avois L, Mangin P. Human growth hormone doping in sport. Br J Sports Med. 2006;40 Suppl 1:i35–i39. doi: 10.1136/bjsm.2006.027573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savage MO, Attie KM, David A, Metherell LA, Clark AJ, Camacho-Hübner C. Endocrine assessment, molecular characterization and treatment of growth hormone insensitivity disorders. Nat Clin Pract Endocrinol Metab. 2006;2:395–407. doi: 10.1038/ncpendmet0195. [DOI] [PubMed] [Google Scholar]
- Savage MO, Blum WF, Ranke MB, Postel-Vinay MC, Cotterill AM, Hall K, et al. Clinical features and endocrine status in patients with growth hormone insensitivity (Laron syndrome) J Clin Endocrinol Metab. 1993;77:1465–1471. doi: 10.1210/jcem.77.6.7505286. [DOI] [PubMed] [Google Scholar]
- Schlechter NL, Russell SM, Spencer EM, Nicoll CS. Evidence suggesting that the direct growth-promoting effect of growth hormone on cartilage in vivo is mediated by local production of somatomedin. Proc Natl Acad Sci USA. 1986;83:7932–7934. doi: 10.1073/pnas.83.20.7932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenle EJ, Zenobi PD, Torresani T, Werder EA, Zachmann M, Froesch ER. Recombinant human insulin-like growth factor I (rhIGF I) reduces hyperglycaemia in patients with extreme insulin resistance. Diabetologia. 1991;34:675–679. doi: 10.1007/BF00400998. [DOI] [PubMed] [Google Scholar]
- Shavlakadze T, Boswell JM, Burt DW, Asante EA, Tomas FM, Davies MJ, et al. Rskalpha-actin/hIGF-1 transgenic mice with increased IGF-I in skeletal muscle and blood: impact on regeneration, denervation and muscular dystrophy. Growth Horm IGF Res. 2006;16:157–173. doi: 10.1016/j.ghir.2005.11.003. [DOI] [PubMed] [Google Scholar]
- Shavlakadze T, Grounds M. Of bears, frogs, meat, mice and men: complexity of factors affecting skeletal muscle mass and fat. Bioessays. 2006;28:994–1009. doi: 10.1002/bies.20479. [DOI] [PubMed] [Google Scholar]
- Shavlakadze T, Winn N, Rosenthal N, Grounds MD. Reconciling data from transgenic mice that overexpress IGF-I specifically in skeletal muscle. Growth Horm IGF Res. 2005;15:4–18. doi: 10.1016/j.ghir.2004.11.001. [DOI] [PubMed] [Google Scholar]
- Sjögren K, Liu JL, Blad K, Skrtic S, Vidal O, Wallenius V, et al. Liver-derived insulin-like growth factor I (IGF-I) is the principal source of IGF-I in blood but is not required for postnatal body growth in mice. Proc Natl Acad Sci USA. 1999;96:7088–7092. doi: 10.1073/pnas.96.12.7088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smit LS, Meyer DJ, Billestrup N, Norstedt G, Schwartz J, Carter-Su C. The role of the growth hormone (GH) receptor and JAK1 and JAK2 kinases in the activation of Stats 1, 3, and 5 by GH. Mol Endocrinol. 1996;10:519–533. doi: 10.1210/mend.10.5.8732683. [DOI] [PubMed] [Google Scholar]
- Sotiropoulos A, Ohanna M, Kedzia C, Menon RK, Kopchick JJ, Kelly PA, et al. Growth hormone promotes skeletal muscle cell fusion independent of insulin-like growth factor 1 up-regulation. Proc Natl Acad Sci USA. 2006;103:7315–7320. doi: 10.1073/pnas.0510033103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spangenburg EE, Le Roith D, Ward CW, Bodine SC. A functional insulin-like growth factor receptor is not necessary for load-induced skeletal muscle hypertrophy. J Physiol. 2008;586:283–291. doi: 10.1113/jphysiol.2007.141507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stickland NC. Muscle development in the human fetus as exemplified by m sartorius: a quantitative study. J Anat. 1981;132:557–579. [PMC free article] [PubMed] [Google Scholar]
- Stitt TN, Drujan D, Clarke BA, Panaro F, Timofeyva Y, Kline WO, et al. The IGF-1/PI3K/Akt pathway prevents expression of muscle atrophy-induced ubiquitin ligases by inhibiting FOXO transcription factors. Mol Cell. 2004;14:395–403. doi: 10.1016/s1097-2765(04)00211-4. [DOI] [PubMed] [Google Scholar]
- Taaffe DR, Jin IH, Vu TH, Hoffman AR, Marcus R. Lack of effect of recombinant human growth hormone (GH) on muscle morphology and GH-insulin-like growth factor expression in resistance-trained elderly men. J Clin Endocrinol Metab. 1996;81:421–425. doi: 10.1210/jcem.81.1.8550787. [DOI] [PubMed] [Google Scholar]
- Taaffe DR, Pruitt L, Reim J, Hintz RL, Butterfield G, Hoffman AR, et al. Effect of recombinant human growth hormone on the muscle strength response to resistance exercise in elderly men. J Clin Endocrinol Metab. 1994;79:1361–1366. doi: 10.1210/jcem.79.5.7525633. [DOI] [PubMed] [Google Scholar]
- Tamemoto H, Kadowaki T, Tobe K, Yagi T, Sakura H, Hayakawa T, et al. Insulin resistance and growth retardation in mice lacking insulin receptor substrate-1. Nature. 1994;372:182–186. doi: 10.1038/372182a0. [DOI] [PubMed] [Google Scholar]
- Vandenburgh HH, Karlisch P, Shansky J, Feldstein R. Insulin and IGF-I induce pronounced hypertrophy of skeletal myofibers in tissue culture. Am J Physiol. 1991;260:C475–C484. doi: 10.1152/ajpcell.1991.260.3.C475. [DOI] [PubMed] [Google Scholar]
- Walenkamp MJ, Wit JM. Genetic disorders in the GH IGF-I axis in mouse and man. Eur J Endocrinol. 2007;157:S15–S26. doi: 10.1530/EJE-07-0148. [DOI] [PubMed] [Google Scholar]
- Welle S, Thornton C, Statt M, McHenry B. Growth hormone increases muscle mass and strength but does not rejuvenate myofibrillar protein synthesis in healthy subjects over 60 years old. J Clin Endocrinol Metab. 1996;81:3239–3243. doi: 10.1210/jcem.81.9.8784075. [DOI] [PubMed] [Google Scholar]
- Woodhouse LJ, Mukherjee A, Shalet SM, Ezzat S. The influence of growth hormone status on physical impairments, functional limitations, and health-related quality of life in adults. Endocr Rev. 2006;27:287–317. doi: 10.1210/er.2004-0022. [DOI] [PubMed] [Google Scholar]
- Xu Q, Li S, Zhao Y, Maures TJ, Yin P, Duan C. Evidence that IGF binding protein-5 functions as a ligand-independent transcriptional regulator in vascular smooth muscle cells. Circ Res. 2004;94:E46–E54. doi: 10.1161/01.RES.0000124761.62846.DF. [DOI] [PubMed] [Google Scholar]
- Yakar S, Liu JL, Stannard B, Butler A, Accili D, Sauer B, et al. Normal growth and development in the absence of hepatic insulin-like growth factor I. Proc Natl Acad Sci USA. 1999;96:7324–7329. doi: 10.1073/pnas.96.13.7324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarasheski KE, Campbell JA, Smith K, Rennie MJ, Holloszy JO, Bier DM. Effect of growth hormone and resistance exercise on muscle growth in young men. Am J Physiol. 1992;262:E261–E267. doi: 10.1152/ajpendo.1992.262.3.E261. [DOI] [PubMed] [Google Scholar]
- Yarasheski KE, Zachweija JJ, Angelopoulos TJ, Bier DM. Short-term growth hormone treatment does not increase muscle protein synthesis in experienced weight lifters. J Appl Physiol. 1993;74:3073–3076. doi: 10.1152/jappl.1993.74.6.3073. [DOI] [PubMed] [Google Scholar]
- Yarasheski KE, Zachwieja JJ, Campbell JA, Bier DM. Effect of growth hormone and resistance exercise on muscle growth and strength in older men. Am J Physiol. 1995;268:E268–E276. doi: 10.1152/ajpendo.1995.268.2.E268. [DOI] [PubMed] [Google Scholar]
- Zammit PS, Partridge TA, Yablonka-Reuveni Z. The skeletal muscle satellite cell: the stem cell that came in from the cold. J Histochem Cytochem. 2006;54:1177–1191. doi: 10.1369/jhc.6R6995.2006. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Xu BC, Maheshwari HG, He L, Reed M, Lozykowski M, et al. A mammalian model for Laron syndrome produced by targeted disruption of the mouse growth hormone receptor/binding protein gene (the Laron mouse) Proc Natl Acad Sci USA. 1997;94:13215–13220. doi: 10.1073/pnas.94.24.13215. [DOI] [PMC free article] [PubMed] [Google Scholar]