Abstract
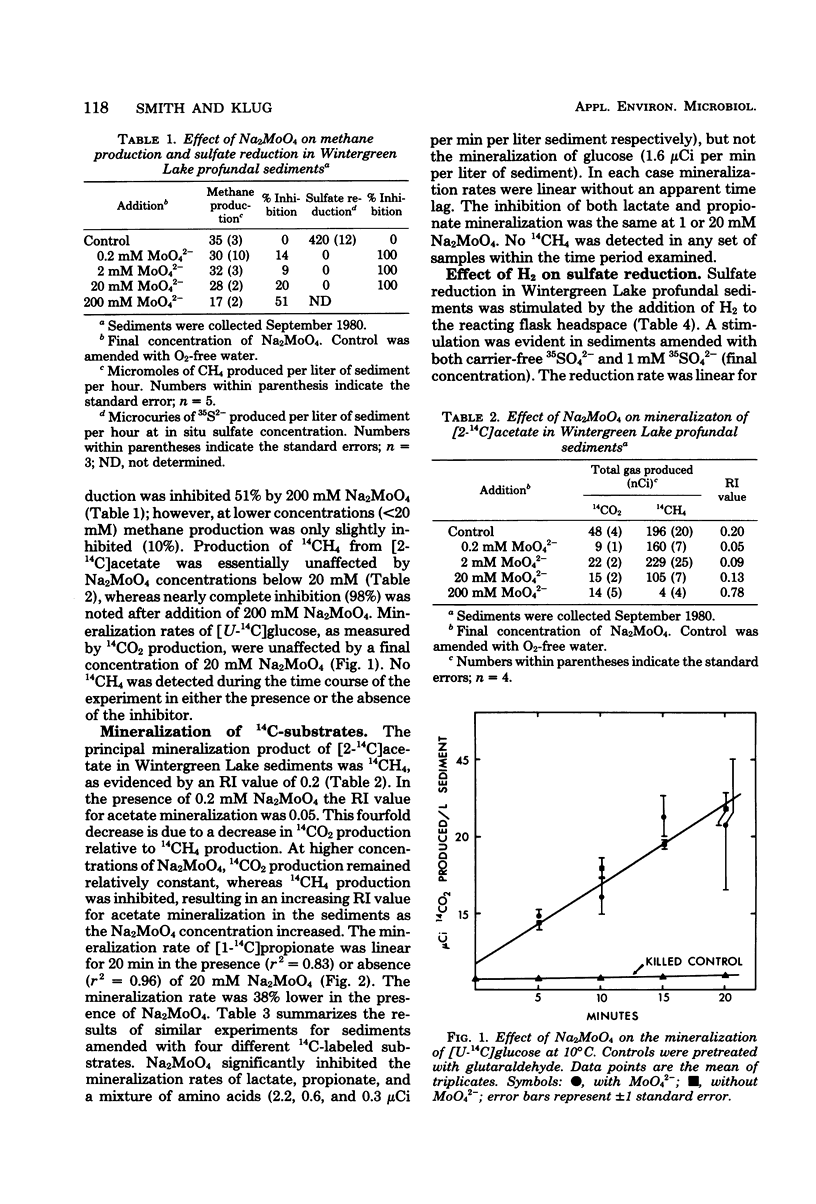
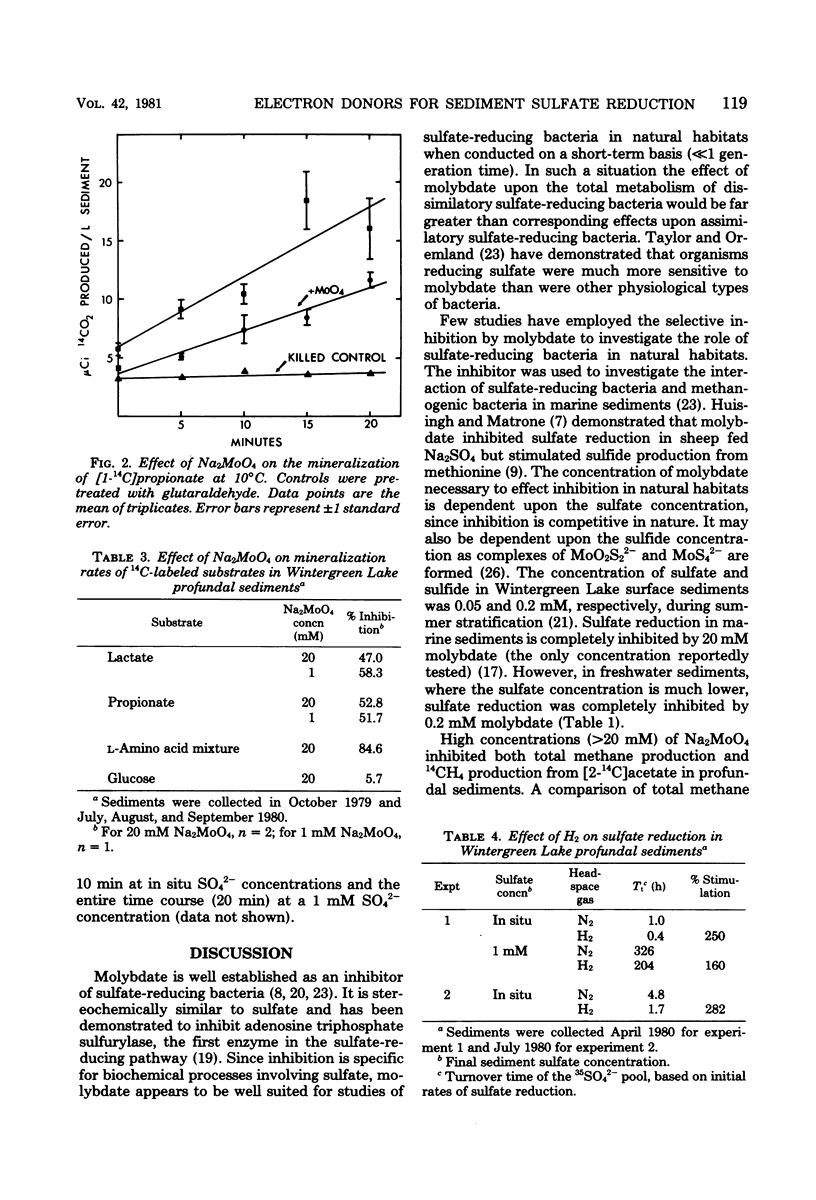
Mineralization rates of 14C-labeled substrates were determined in the presence and absence of Na2MoO4, an inhibitor of sulfate reduction, in the profundal sediments of a shallow eutrophic lake. Sulfate reduction was inhibited by Na2MoO4 at all concentrations tested (0.2 to 200 mM), whereas methane production was inhibited at Na2MoO4 concentrations greater than 20 mM. Initial mineralization rates of glucose were unaffected by Na2MoO4; however, Na2MoO4 decreased the mineralization rates of lactate (58%), propionate (52%), an amino acid mixture (85%), and acetate (14%). These decreases in the rates of mineralization were attributed to inhibition of sulfate reduction. Hydrogen stimulated the reduction of 35SO42− 2.5- to 2.8-fold, demonstrating potential hydrogen oxidation by sulfate-reducing bacteria. These results indicate that sulfate reducers utilize an array of substrates as electron donors and are of potential significance to the in situ mineralization of lactate, propionate, and free amino acids in these sediments.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abram J. W., Nedwell D. B. Hydrogen as a substrate for methanogenesis and sulphate reduction in anaerobic saltmarsh sediment. Arch Microbiol. 1978 Apr 27;117(1):93–97. doi: 10.1007/BF00689357. [DOI] [PubMed] [Google Scholar]
- Abram J. W., Nedwell D. B. Inhibition of methanogenesis by sulphate reducing bacteria competing for transferred hydrogen. Arch Microbiol. 1978 Apr 27;117(1):89–92. doi: 10.1007/BF00689356. [DOI] [PubMed] [Google Scholar]
- Badziong W., Thauer R. K., Zeikus J. G. Isolation and characterization of Desulfovibrio growing on hydrogen plus sulfate as the sole energy source. Arch Microbiol. 1978 Jan 23;116(1):41–49. doi: 10.1007/BF00408732. [DOI] [PubMed] [Google Scholar]
- Boone D. R., Bryant M. P. Propionate-Degrading Bacterium, Syntrophobacter wolinii sp. nov. gen. nov., from Methanogenic Ecosystems. Appl Environ Microbiol. 1980 Sep;40(3):626–632. doi: 10.1128/aem.40.3.626-632.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cappenberg T. E., Prins R. A. Interrelations between sulfate-reducing and methane-producing bacteria in bottom deposits of a fresh-water lake. 3. Experiments with 14C-labeled substrates. Antonie Van Leeuwenhoek. 1974;40(3):457–469. doi: 10.1007/BF00399358. [DOI] [PubMed] [Google Scholar]
- Huisingh J., Matrone G. Copper-molybdenum interactions with the sulfate-reducing system in rumen microorganisms. Proc Soc Exp Biol Med. 1972 Feb;139(2):518–521. doi: 10.3181/00379727-139-36177. [DOI] [PubMed] [Google Scholar]
- Huisingh J., McNeill J. J., Matrone G. Sulfate reduction by a Desulfovibrio species isolated from sheep rumen. Appl Microbiol. 1974 Sep;28(3):489–497. doi: 10.1128/am.28.3.489-497.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huisingh J., Milholland D. C., Matrone G. Effect of molybdate on sulfide production from methionine and sulfate by ruminal microorganisms of sheep. J Nutr. 1975 Sep;105(9):1199–1205. doi: 10.1093/jn/105.9.1199. [DOI] [PubMed] [Google Scholar]
- Laanbroek H. J., Pfennig N. Oxidation of short-chain fatty acids by sulfate-reducing bacteria in freshwater and in marine sediments. Arch Microbiol. 1981 Jan;128(3):330–335. doi: 10.1007/BF00422540. [DOI] [PubMed] [Google Scholar]
- Mountfort D. O., Asher R. A., Mays E. L., Tiedje J. M. Carbon and electron flow in mud and sandflat intertidal sediments at delaware inlet, nelson, new zealand. Appl Environ Microbiol. 1980 Apr;39(4):686–694. doi: 10.1128/aem.39.4.686-694.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PECK H. D., Jr Symposium on metabolism of inorganic compounds. V. Comparative metabolism of inorganic sulfur compounds in microorganisms. Bacteriol Rev. 1962 Mar;26:67–94. doi: 10.1128/br.26.1.67-94.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peck H. D. THE ATP-DEPENDENT REDUCTION OF SULFATE WITH HYDROGEN IN EXTRACTS OF DESULFOVIBRIO DESULFURICANS. Proc Natl Acad Sci U S A. 1959 May;45(5):701–708. doi: 10.1073/pnas.45.5.701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith R. L., Klug M. J. Reduction of sulfur compounds in the sediments of a eutrophic lake basin. Appl Environ Microbiol. 1981 May;41(5):1230–1237. doi: 10.1128/aem.41.5.1230-1237.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strayer R. F., Tiedje J. M. Kinetic parameters of the conversion of methane precursors to methane in a hypereutrophic lake sediment. Appl Environ Microbiol. 1978 Aug;36(2):330–340. doi: 10.1128/aem.36.2.330-340.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winfrey M. R., Zeikus J. G. Effect of sulfate on carbon and electron flow during microbial methanogenesis in freshwater sediments. Appl Environ Microbiol. 1977 Feb;33(2):275–281. doi: 10.1128/aem.33.2.275-281.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winfrey M. R., Zeikus J. G. Microbial methanogenesis and acetate metabolism in a meromictic lake. Appl Environ Microbiol. 1979 Feb;37(2):213–221. doi: 10.1128/aem.37.2.213-221.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolin M. J., Miller T. L. Molybdate and sulfide inhibit H2 and increase formate production from glucose by Ruminococcus albus. Arch Microbiol. 1980 Feb;124(2-3):137–142. doi: 10.1007/BF00427718. [DOI] [PubMed] [Google Scholar]


