Abstract
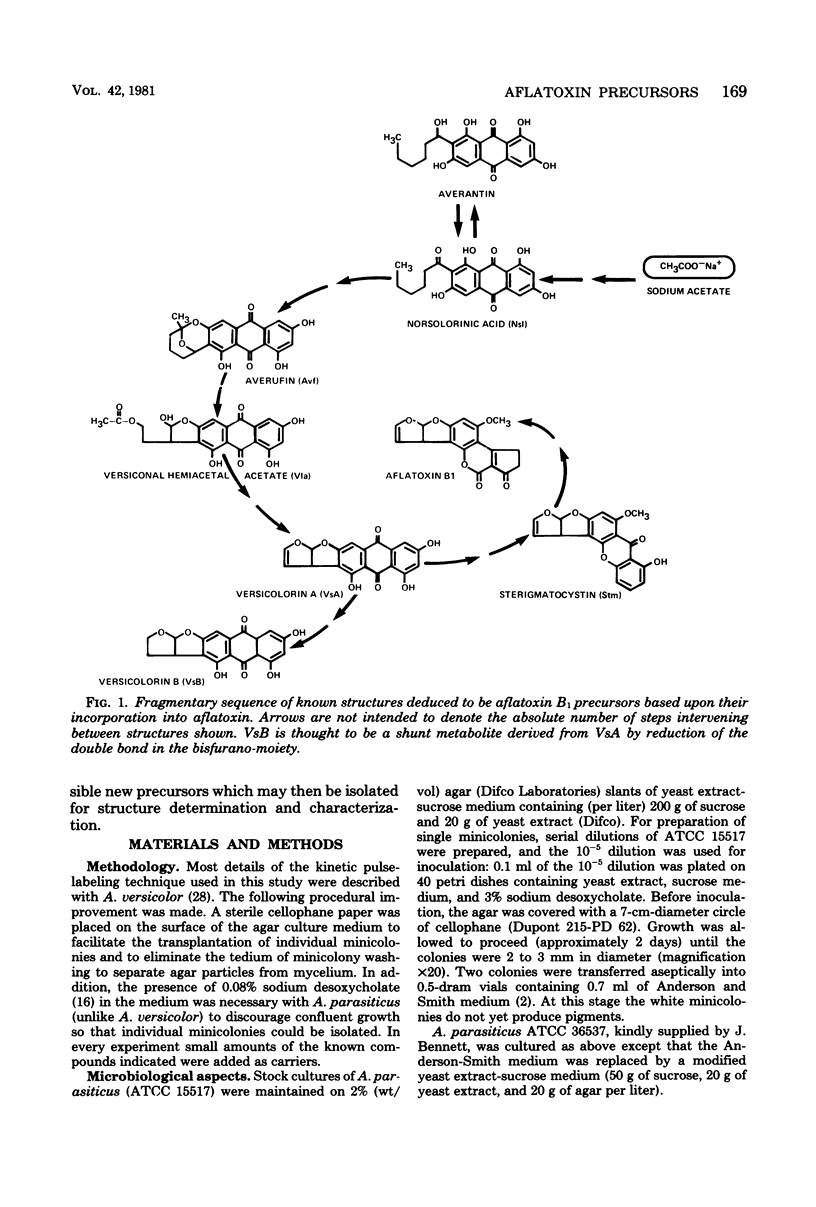
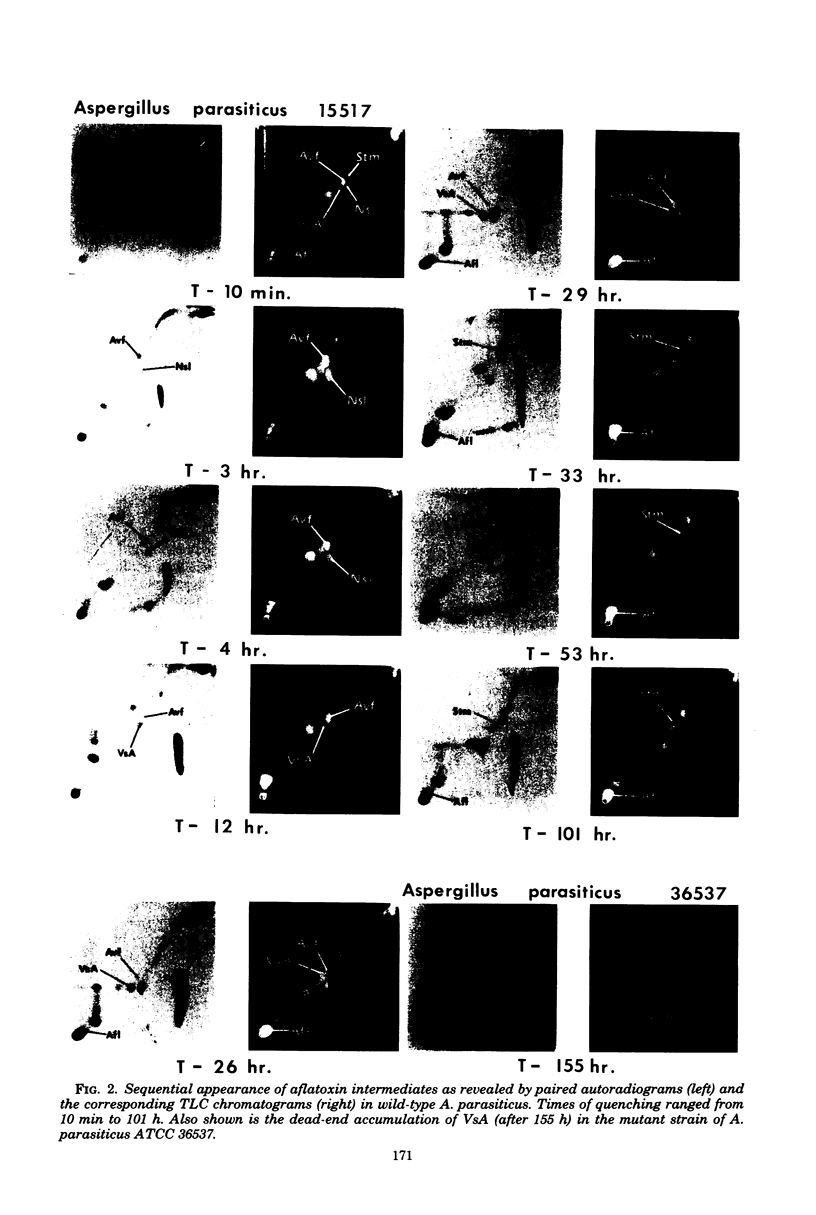
Kinetic pulse-labeling of aflatoxin pathway compounds was carried out in Aspergillus parasiticus, beginning with radioactive acetate. Norsolorinic acid, averufin, versicolorin A, and sterigmatocystin (all known as compounds which can be incorporated into the aflatoxin molecule) were radiotraced to follow their order of appearance. Aflatoxin species B1, B2, G1, and G2 were included. Norsolorinic acid and averufin appeared as early transient intermediates followed in order by versicolorin A, aflatoxins, and sterigmatocystin. To date, a mutually confirming array of results has been obtained with established precursors in wild-type strains of A. parasiticus and A. versicolor (as well as with an aflatoxin pathway mutant of A. parasiticus), which together establish a practical methodology for recognition of new pathway intermediates. The kinetic of pulse-labeling for sterigmatocystin in relation to aflatoxins suggests that duel branchlets may exist to flatoxins; i.e., sterigmatocystin may not be an obligatory aflatoxin precursor.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson J. G., Smith J. E. The production of conidiophores and conidia by newly germinated conidia of Aspergillus niger (microcycle conidiation). J Gen Microbiol. 1971 Dec;69(2):185–197. doi: 10.1099/00221287-69-2-185. [DOI] [PubMed] [Google Scholar]
- Anderson M. S., Dutton M. F. Biosynthesis of versicolorin A. Appl Environ Microbiol. 1980 Oct;40(4):706–709. doi: 10.1128/aem.40.4.706-709.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BATTAILE J., LOOMIS W. D. Biosynthesis of terpenes. II. The site and sequence of terpene formation in peppermint. Biochim Biophys Acta. 1961 Aug 19;51:545–552. doi: 10.1016/0006-3002(61)90612-6. [DOI] [PubMed] [Google Scholar]
- Bennett J. W., Lee L. S., Shoss S. M., Boudreaux G. H. Identification of averantin as an aflatoxin B1 precursor: placement in the biosynthetic pathway. Appl Environ Microbiol. 1980 Apr;39(4):835–839. doi: 10.1128/aem.39.4.835-839.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox R. H., Churchill F., Cole R. J., Dormer J. W. Carbon-13 nuclear magnetic resonance studies of the structure and biosynthesis of versiconal acetate. J Am Chem Soc. 1977 Apr 27;99(9):3159–3161. doi: 10.1021/ja00451a049. [DOI] [PubMed] [Google Scholar]
- Donkersloot J. A., Mateles R. I., Yang S. S. Isolation of averufin from a mutant of Aspergillus parasiticus impaired in aflatoxin biosynthesis. Biochem Biophys Res Commun. 1972 Jun 9;47(5):1051–1055. doi: 10.1016/0006-291x(72)90939-4. [DOI] [PubMed] [Google Scholar]
- Hsieh D. P., Lin M. T., Yao R. C. Conversion of sterigmatocystin to aflatoxin B 1 by Aspergillus parasiticus. Biochem Biophys Res Commun. 1973 Jun 8;52(3):992–997. doi: 10.1016/0006-291x(73)91035-8. [DOI] [PubMed] [Google Scholar]
- Hsieh D. P., Lin M. T., Yao R. C., Singh R. Biosynthesis of aflatoxin. Conversion of norsolorinic acid and other hypothetical intermediates into aflatoxin B1. J Agric Food Chem. 1976 Nov-Dec;24(6):1170–1174. doi: 10.1021/jf60208a018. [DOI] [PubMed] [Google Scholar]
- LARGE P. J., PEEL D., QUAYLE J. R. Microbial growth on C1 compounds. II. Synthesis of cell constituents by methanol- and formate-grown Pseudomonas AM 1, and methanol-grown Hyphomicrobium vulgare. Biochem J. 1961 Dec;81:470–480. doi: 10.1042/bj0810470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee L. S., Bennett J. W., Cucullu A. F., Ory R. L. Biosynthesis of aflatoxin B1. Conversion of versicolorin A to aflatoxin B1 by Aspergillus parasiticus. J Agric Food Chem. 1976 Nov-Dec;24(6):1167–1170. doi: 10.1021/jf60208a017. [DOI] [PubMed] [Google Scholar]
- Lee L. S., Bennett J. W., Cucullu A. F., Stanley J. B. Synthesis of versicolorin A by a mutant strain of Aspergillus parasiticus deficient in aflatoxin production. J Agric Food Chem. 1975 Nov-Dec;23(6):1132–1134. doi: 10.1021/jf60202a011. [DOI] [PubMed] [Google Scholar]
- Lin M. T., Hsieh D. P. Averufin in the biosynthesis of aflatoxin B. J Am Chem Soc. 1973 Mar 7;95(5):1668–1669. doi: 10.1021/ja00786a056. [DOI] [PubMed] [Google Scholar]
- Litt G. J., Johl R. G. A simple apparatus for circular thin-layer chromatography. J Chromatogr. 1965 Dec;20(3):605–606. doi: 10.1016/s0021-9673(01)97470-9. [DOI] [PubMed] [Google Scholar]
- QUAYLE J. R., KEECH D. B. Carbon assimilation by Pseudomonas oxalaticus (OX 1). 2. Formate and carbon dioxide utilization by cell-free extracts of the organism grown on formate. Biochem J. 1959 Aug;72:631–637. doi: 10.1042/bj0720631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahm H., Cox R. B., Quayle J. R. Metabolism of methanol by Rhodopseudomonas acidophila. J Gen Microbiol. 1976 Jun;94(2):313–322. doi: 10.1099/00221287-94-2-313. [DOI] [PubMed] [Google Scholar]
- Schroeder H. W., Cole R. J., Grigsby R. D., Hein H., Jr Inhibition of aflatoxin production and tentative identification of an aflatoxin intermediate "versiconal acetate" from treatment with dichlorvos. Appl Microbiol. 1974 Feb;27(2):394–399. doi: 10.1128/am.27.2.394-399.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh R., Hsieh D. P. Aflatoxin biosynthetic pathway: elucidation by using blocked mutants of Aspergillus parasiticus. Arch Biochem Biophys. 1977 Jan 15;178(1):285–292. doi: 10.1016/0003-9861(77)90193-x. [DOI] [PubMed] [Google Scholar]
- Singh R., Hsieh D. P. Enzymatic conversion of sterigmatocystin into aflatoxin B1 by cell-free extracts of Aspergillus parasiticus. Appl Environ Microbiol. 1976 May;31(5):743–745. doi: 10.1128/aem.31.5.743-745.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan N. C., Hsieh D. P. Enzymatic formation of the bisfuran structure in aflatoxin biosynthesis. Appl Environ Microbiol. 1980 Jan;39(1):109–112. doi: 10.1128/aem.39.1.109-112.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao R. C., Hsieh D. P. Step of dichlorvos inhibition in the pathway of aflatoxin biosynthesis. Appl Microbiol. 1974 Jul;28(1):52–57. doi: 10.1128/am.28.1.52-57.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamir L. O., Ginsburg R. Aflatoxin biosynthesis: detection of transient, acetate-dependent intermediates in Aspergillus by kinetic pulse-labeling. J Bacteriol. 1979 Jun;138(3):684–690. doi: 10.1128/jb.138.3.684-690.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]




