Abstract
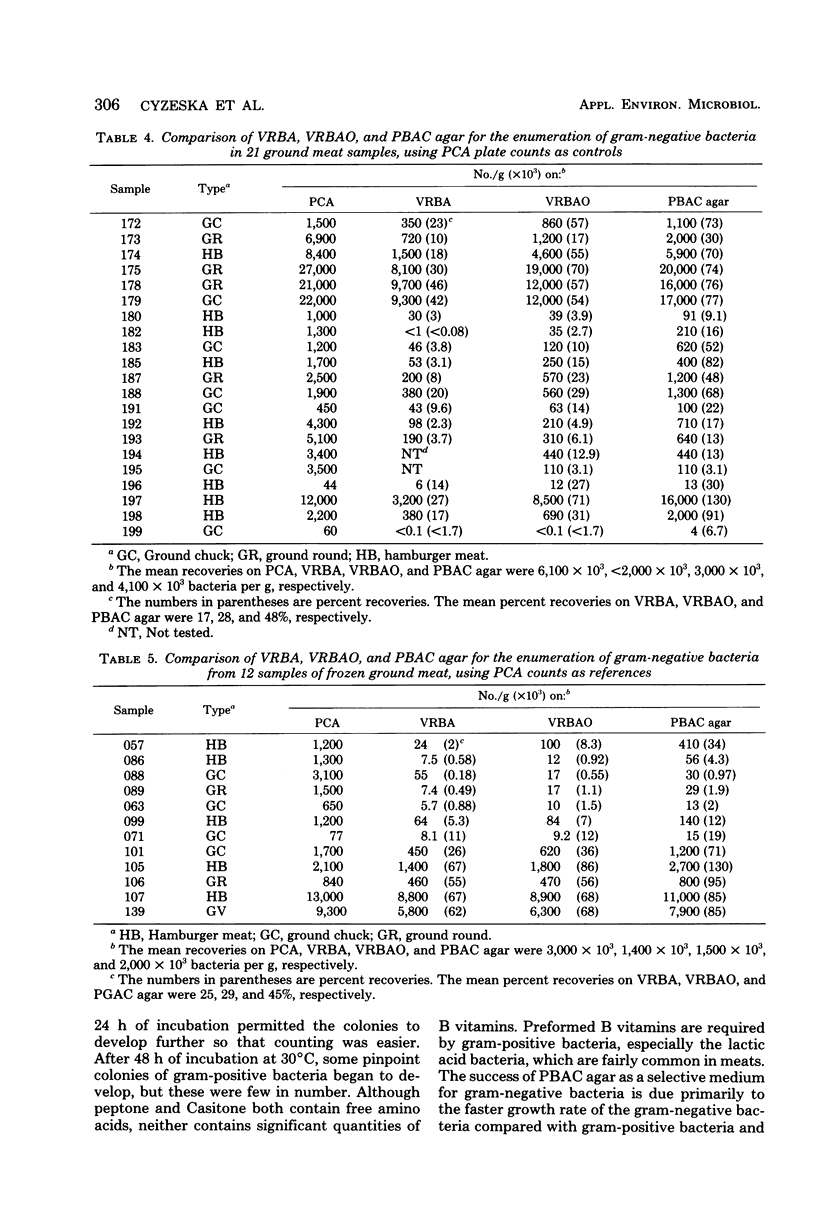
We developed a new medium, designated peptone bile amphotericin cycloheximide (PBAC) agar, which contains (per liter) 10 g of peptone, 300 mg of bile salts, 1 mg of amphotericin B, 1 g of cycloheximide, and 15 g of agar. When 21 samples of fresh ground beef were studied and plate count agar counts were used as references, we obtained a mean recovery of 28% of total counts with violet red bile agar overlay, whereas we obtained 48% recovery with PBAC agar. With 12 samples of frozen ground beef, recovery on violet red bile agar overlay was 29% of the recovery on plate count agar, whereas the corresponding value on PBAC agar was 45%. PBAC agar allowed the enumeration of 1.4 times as many gram-negative bacteria as violet red bile agar overlay. None of eight strains of gram-positive bacteria and none of eight strains of yeasts grew on PBAC agar. Of 158 colonies randomly selected from pour plates of eight fresh ground meat samples, 95% stained gram negative. In comparison, only 70% of 151 colonies selected from corresponding plate count agar plates were gram negative. The lack of background color, turbidity, and ease of use make PBAC agar easier to handle than other media used for gram-negative bacteria, such as violet red bile agar, violet red bile agar overlay, and crystal violet tetrazolium agar. In the preparation PBAC agar, all ingredients are autoclaved together except amphotericin B, which is filter sterilized and added before the plates are poured.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Hartman P. A., Hartman P. S., Lanz W. W. Violet red bile 2 agar for stressed coliforms. Appl Microbiol. 1975 Apr;29(4):537–539. doi: 10.1128/am.29.4.537-539.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jay J. M., Margitic S. Comparison of homogenizing, shaking, and blending on the recovery of microorganisms and endotoxins from fresh and frozen ground beef as assessed by plate counts and the Limulus amoebocyte lysate test. Appl Environ Microbiol. 1979 Nov;38(5):879–884. doi: 10.1128/aem.38.5.879-884.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puchelle E., Aug F., Pham Q. T., Bertrand A. Comparison of three methods for measuring nasal mucociliary clearance in man. Acta Otolaryngol. 1981 Mar-Apr;91(3-4):297–303. doi: 10.3109/00016488109138511. [DOI] [PubMed] [Google Scholar]
- Ray B., Speck M. L. Plating procedure for the enumeration of coliforms from dairy products. Appl Environ Microbiol. 1978 Apr;35(4):820–822. doi: 10.1128/aem.35.4.820-822.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speck M. L., Ray B., Read R. B., Jr Repair and enumeration of injured coliforms by a plating procedure. Appl Microbiol. 1975 Apr;29(4):549–550. doi: 10.1128/am.29.4.549-550.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]


