Abstract
Endothelial intracellular calcium ([Ca2+]i) plays an important role in the function of the juxtaglomerular vasculature. The present studies aimed to identify the existence and molecular elements of an endothelial calcium wave in cultured glomerular endothelial cells (GENC). GENCs on glass coverslips were loaded with Fluo-4/Fura red, and ratiometric [Ca2+]i imaging was performed using fluorescence confocal microscopy. Mechanical stimulation of a single GENC caused a nine-fold increase in [Ca2+]i, which propagated from cell to cell throughout the monolayer (7.9 ± 0.3 μm/s) in a regenerative manner (without decrement of amplitude, kinetics, and speed) over distances >400 μm. Inhibition of voltage-dependent calcium channels with nifedipine had no effect on the above parameters, but the removal of extracellular calcium reduced Δ[Ca2+]i by 50%. Importantly, the gap junction uncoupler α-glycyrrhetinic acid or knockdown of connexin 40 (Cx40) by transfecting GENCs with Cx40 short interfering RNA (siRNA) almost completely eliminated Δ [Ca2+]i and the calcium wave. Breakdown of extracellular ATP using a scavenger cocktail (apyrase and hexokinase) or nonselective inhibition of purinergic P2 receptors with suramin, had similar blocking effects. Scraping cells off along a line eliminated physical contact between cells but did not effect calcium wave propagation. Using an ATP biosensor technique, we detected a significant elevation in extracellular ATP (Δ = 76 ± 2 μM) during calcium wave propagation, which was abolished by Cx40 siRNA treatment (Δ = 6 ± 1 μM). These studies suggest that connexin 40 hemichannels and extracellular ATP are key molecular elements of the glomerular endothelial calcium wave, which may serve important juxtaglomerular functions.
Keywords: purinergic receptors, gap junction, connexin hemichannels, glomerular filtration, renin release
Autoregulatory mechanisms that maintain renal blood flow and glomerular filtration rate involve a propagating calcium wave in the juxtaglomerular apparatus (JGA) mediated by extracellular ATP, purinergic calcium signaling, and gap junctions (3, 21, 22). The calcium wave of tubuloglomerular feedback or direct vasoconstriction of the afferent arteriole (AA) propagates from contractile smooth muscle and glomerular mesangial cells to the underlying endothelium (22, 34), providing a vasodilatory feedback mechanism (34). Endothelial cells also receive stimuli from the vascular lumen that are mainly coupled to the intracellular calcium concentration, [Ca2+]i, a key determinant of vascular functions (12, 14, 38).
Calcium waves coordinate signals between functionally connected cells (syncytium) and have been described in many cell types and organs, including the lung, heart, brain, and kidney, where they play roles in modulating vascular responses, local blood flow, synaptic function, and secretion (6, 8, 9, 17, 22, 31, 32, 34, 35, 41). Cell-to-cell propagation generally involves gap junctional communication, as well as extracellular pathways (6, 9, 12, 31, 32, 41, 42). Like many vascular beds, cells of the juxtaglomerular vasculature are tightly coupled by gap junctions (33) and express a number of connexin (Cx) isoforms, including Cx37, Cx40, Cx43, and Cx45 (2, 5, 11–13, 15). Accordingly, an important feature of the afferent arteriole is the conduction of vascular responses to various stimuli (22, 27, 34), including propagation to endothelial cells (22, 34). However, calcium waves in the inaccessible and looping capillaries of the glomerular endothelium have not been studied.
One of the most prominent connexin isoforms in the kidney is Cx40, which is expressed in the preglomerular vascular endothelium, in renin-producing and mesangial cells of the JGA, and with particular abundance in the glomerular endothelium (2, 11, 12, 15). Cx40 was shown to contribute to the propagation of vasodilation (12, 26) and control of renin synthesis and release (19, 37), important functions of the JGA. Cx40 knockout mice are hypertensive (12, 26) and feature an elevated plasma renin concentration (37), due to an altered vasomotion of arterioles and the lack of the conventional inhibitory effects of ANG II and intarenal pressure on renin release (12, 26, 37).
Using the tsA58 mouse model, Akis and Madaio (1) recently developed a cell line of glomerular endothelial cells (GENC) that shows stable expression of the endothelial phenotype (1). Also, endothelial cells in the renin-producing, JG segment of the AA (in contrast to the proximal AA) appear to closely resemble GENCs. Common features include fenestrations, high permeability (24), and Cx40 expression (11, 12). The purpose of these studies was to identify the existence and molecular elements of the juxtaglomerular endothelial calcium wave using these cultured GENCs. In particular and in light of a recent study (37), we aimed to establish the roles of both extracellular ATP and the main JGA and glomerular endothelial connexin isoform, Cx40, in the regulation of JGA and glomerular functions. The present studies suggest that the juxtaglomerular endothelium, through Cx40 hemichannel-mediated ATP release and purinergic calcium signaling, may be an important component of the control of glomerular filtration and renin release.
MATERIALS AND METHODS
Cell cultures
GENCs (kind gift from N. Akis, Istanbul, Turkey) (1) and a mouse macula densa-derived cell line (MMDD1, kind gift from J. Schnermann, National Institutes of Health, Bethesda, MD) (40) were grown on circular glass coverslips (25 mm; VWR International Brisbane, CA) until reaching a confluent (90–100%) monolayer. Normal growth media for GENCs were prepared from DMEM with low glucose with the addition of 25 mM HEPES, 9 mM NaHCO3, 7.5% NCS (Gibco), and 1% penicillin- streptomycin (5% CO2 at 37°C). Normal growth media for MMDD1 cells were prepared from DMEM/F12 with the addition of 7.5% wt/vol NaHCO3, 10% FBS (Invitrogen), 1% penicillin-streptomycin, dexamethasone (50 μM). During experiments, a modified Krebs-Ringer HCO3 buffer was added to the top of the cells, containing (in mM) 115 NaCl, 5 KCl, 25 NaHCO3, 0.96 NaH2PO4, 0.24 Na2HPO4, 1.2 MgSO4, 2 CaCl2, 5.5 D-glucose, and 100 μM L-arginine. Calcium-free modified Krebs-Ringer-HCO3 buffer was made with the addition of 2 mM EGTA and the exclusion of the 2 mM CaCl2. All solutions were adjusted to pH = 7.4 using NaOH and HCl.
Confocal laser scanning fluorescence microscopy
Time-lapse ratiometric calcium imaging was performed with a Leica TCS SP2 AOBS MP confocal microscope system (Leica Microsystems, Heidelberg, Germany) using the dyes Fluo-4 (excitation at 488 nm, emission at 520 ± 20 nm) and Fura red (excitation at 488 nm, emission at >600 nm). A transmitted light detector and differential interference contrast imaging was used to control the position of the pipette during mechanical stimulation. All experiments were performed using the same instrument settings (laser power, offset, gain of both detector channels). Data acquisition and analysis were done using the Leica LCS imaging software LCS 2.61.1537. Cells were loaded with the ratiometric calcium dyes Fluo-4 AM and Fura red AM (10 μM each; Invitrogen) dissolved in DMSO, in modified Krebs-Ringer-HCO3 buffer at room temperature for ~20 min. After incubation, coverslips were transferred to the chamber of the Leica confocal microscope system for imaging with a 40× oil-immersion objective. Fluo-4/Fura red ratio values were converted to absolute [Ca2+]i using a calibration technique as described before (22). Calcium wave speed was calculated using the formula: speed = (distance/time) and the “stack profile” function of the Leica LCS analysis software package. Distance was defined as the space between the point of mechanical stimulation and the center of a cell demonstrating an increase in [Ca2+]i. In some experiments, the wave radius was measured as the farthest distance traveled by the front wave from the stimulated cell.
Mechanical stimulation
A mechanical stimulus was applied by gently touching a single cell of the monolayer with a glass micropipette (Drummond Scientific), pulled to 2–3 μm in diameter. The micropipette was initially positioned just above the monolayer and was lowered to contact the target cell using a micromanipulator (ROE-200, Sutter Instruments).
Chemicals
The following pharmacological agents were used in the experiments: an ATP-scavenger cocktail consisting of hexokinase and apyrase (both 50 U/ml), the gap junction uncoupling agent 18α-glycyrrhetinic acid (α-GA; 25 μM), the voltage-dependent calcium channel blocker nifedipine (1 μM), and the nonselective purinergic receptor blocker suramin (50 μM). All treatments were applied to the chamber bathing solution and allowed to incubate for 10 min at room temperature. All reagents were purchased from Sigma, unless otherwise specified.
Immunocytochemistry
Cells on glass coverslips were fixed in 4% formaldehyde for 10 min and permeabilized with 0.1% Triton X-100 in PBS for 20 min at room temperature. Cells were blocked with a 1:20 dilution of goat serum for 20 min and incubated with a primary polyclonal antibody against Cx37, Cx40, or Cx43 (Zymed), or P2X1, P2X4, P2Y2, and P2Y4 (Alomone Laboratories) at 1:100 dilutions for 1 h at room temperature. This was followed by a 1-h incubation with a secondary anti-rabbit antibody conjugated with Alexa Fluor 488 (Invitrogen). Cells were then washed in PBS and mounted with a Vectashield medium containing 4,6-diamino-2-phenylindole for nuclear staining (Vector Laboratories). Confocal imaging was performed using the same Leica microscope system.
Cx40 short interfering RNA
GENCs were grown in 96-well plates at a concentration of 100,000 cells/ml in antibiotic-free complete DMEM medium for 24 h at 37°C in 5% CO2. During the next 48 h, GENCs were transfected using protocols and reagents included in the Silencer short interfering RNA (siRNA) transfection kit (Ambion). The next day, cells were transferred to larger plates and cultured in complete GENC medium for studies. RNA was extracted from cells and amplified to validate inhibition of Cx40. Functional confirmation of Cx40 knockdown was provided by fluorometric and immunocytochemistry studies. Cells retained adequate Cx40 silencing for at least three passages.
RT-PCR
Total RNA was purified from confluent tsA58 mouse-derived GENCs using a Total RNA Mini Kit (Bio-Rad). RNA was quantified using spectrophotometry and reverse-transcribed to single-stranded cDNA using avian reverse-transcritpase and random hexamers according to manufacturer’s instructions (Thermoscript RT-PCR system; Invitrogen). Two microliters of cDNA was amplified using a master mix containing Taq polymerase (Invitrogen) and the primers [summarized in Table 1 or as described before for β-actin (20)] each at a final concentration of 100 μM. The PCR reaction was carried out for 30 cycles of the following: 94°C for 30 s, 55.4°C for 30 s, and 72°C for 30 s. The PCR product was analyzed on a 2% agarose gel.
Table 1.
Connexin and purinergic receptor primers used to verify the expression of various connexins and P2 receptors in GENCs
| Primer Sequence | Expected Size, bp |
|---|---|
| Cx37 | |
| Fwd 5′-GGC TGG ACC ATG GAG CCG GT-3′ | 421 |
| Rev 5′-TTT CGG CCA CCC TGG GGA GC-3′ | |
| Cx40 | |
| Fwd 5′-CCA GCT TTT AAT GCC GAG AG-3′ | 311 |
| Rev 5′-ACC CTT CAC CCT CCT GAA CT-3′ | |
| Cx43 | |
| Fwd 5′-TAC CAC GCC ACC ACT GGC-3′ | 407 |
| Rev 5′-AAT CTC CAG GTC ATC AGG-3′ | |
| P2X1 | |
| Fwd 5′-TCA CCC AAT GAC GTA GAC CAG AAC-3′ | 316 |
| Rev 5′-AAC CCA GAT CC ACC AAC GAA C-3′ | |
| P2X4 | |
| Fwd 5′-ATC CTC CCC AAC ATC ACC ACG TCC TAC CTC AAA TCG TGC ATT TAC AAT-3′ | 555 |
| Rev 5′-TCA CTC GTT CAT CTC CCC CGA AAG ACC CTG CTC GTA ATC TTC CAC-3′ | |
| P2X7 | |
| Fwd 5′-GAA GTT AGG ACA CAG CAT C-3′ | 403 |
| Rev 5′-AGA GCC GTT CAT AGT TGG-3′ | |
| P2Y1 | |
| Fwd 5′-ACG TCA GAT GAG TAC CTG CG-3′ | 288 |
| Rev 5′-CCC TGT CGT TGA AAT CAC AC-3′ | |
| P2Y2 | |
| Fwd 5′-ATG GTG ATG TAG GAT GGA C-3′ | 232 |
| Rev 5′-GCA GGA GGC AGA GAT AAC-3′ | |
| P2Y4 | |
| Fwd 5′-TGT TCC ACC TGG CAT TGT CAG-3′ | 293 |
| Rev 5′-AAA GAT TGG GCA CGA GGC AG-3′ | |
ATP biosensor assay
The local concentration of ATP released extracellularly into the bathing solution was monitored at 37°C by a biosensor technique with PC12 cells, which express a variety of calcium-coupled purinergic receptors. Details of the technique were described earlier (3). Briefly, PC12 cells were loaded with Fluo-4 and Fura red as described above for GENCs and then injected on top of the GENC monolayer. Within a few seconds, PC12 cells freely settled on top of GENCs, with direct cell-to-cell contact. The release of ATP by mechanically stimulated GENCs causes activation of purinergic receptors on PC12 cells, which increases the PC12 calcium concentration. PC12 biosensor cell calcium responses were then converted to ATP levels using a dose-response calibration procedure as described before (3). ATP specificity of the PC12 biosensor cell calcium responses were confirmed using the purinergic receptor inhibitor, suramin (100 μM).
Data analysis
Data are expressed as means ± SE. Statistical significance was tested using ANOVA. Significance was accepted at P < 0.05.
RESULTS
Calcium wave in cultured GENCs
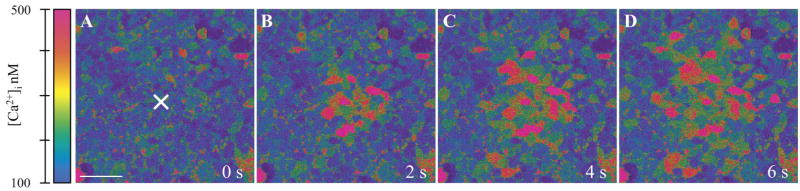
Resting [Ca2+]i in nearly confluent GENCs in culture was 67 ± 1 nM (n = 20). Mechanical stimulation of a single GENC with a glass micropipette caused a rapid and significant elevation of [Ca2+]i in the target cell, which was followed by the radial propagation of [Ca2+]i increases to adjacent cells in all directions (Fig. 1). The calcium wave traveled over distances at least 400 μm from the center with a constant front speed of about 8 μm/s. At this point, the wave moved out of the microscope field beyond further visualization. Interestingly, the peripheral cell [Ca2+]i responses did not show any signs of decrease in magnitude, speed, or kinetics with distance from the initial stimulus. Nearly identical responses in [Ca2+]i were observed adjacent to the central stimulated cell and 2–300 μm away in the periphery (Fig. 2). This phenomenon appeared to be specific for endothelial cells, since calcium waves under the same conditions in a MMDD1 epithelial cell line completely diminished over the same distances (Fig. 2).
Fig. 1.
Real-time fluorescence imaging of the mechanically induced calcium wave in cultured glomerular endothelial cells (GENCs). Calibrated, pseudocolor Fluo-4/Fura red ratio images are shown at the indicated time points after the stimulation of a single cell in the center of the field (labeled by ×). Scale bar = 20μm.
Fig. 2.
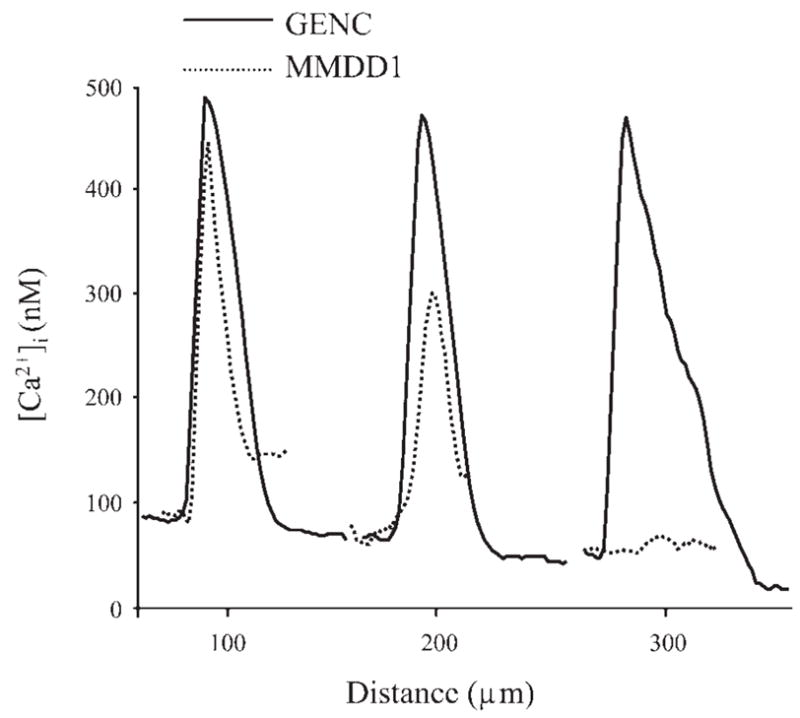
Representative recordings of the elevations in intracellular calcium concentrations, [Ca2+]i, in peripheral cells at various distances from the center during propagation of the calcium wave. [Ca2+]i responses in GENCs (solid line) and MMDD1 cells (dotted line) measured at the same distances are illustrated. The calcium wave showed different characteristics in the two cell types: there were no signs of attenuation in GENCs, but the wave rapidly waned over distance in MMDD1 cells.
Roles of connexins and extracellular ATP
Pharmacological experiments were performed to identify the key molecular elements of the endothelial calcium wave. Two parameters were analyzed: the speed of the front propagation (7.9 ± 0.3 μm/s in control) and the increase in [Ca2+]i in peripheral cells at various distances from the center (Δ [Ca2+]i = 599 ± 58 nM in control). To test the involvement of voltage-operated calcium channels in the propagation of the glomerular endothelial calcium wave, experiments were performed in the presence of 1 μM nifedipine. The addition of nifedipine failed to cause significant changes in the speed (7.4 ± 0.3 μm/s) or Δ [Ca2+]i (447 ± 74 nM, n = 6) (Fig. 3). Removal of extracellular calcium from the bathing solution had no effect on calcium wave propagation velocity (7.7 ± 0.3 μm/s), but Δ [Ca2+]i (321 ± 21 nM, n = 6) was significantly reduced (Fig. 3). Next, we used two approaches to study whether calcium waves propagated from cell to cell through gap junctions. First, a potent gap junction uncoupler, 25 μM α-GA, eliminated the calcium wave, and only a few cells adjacent to the stimulated cell responded with minor elevations in [Ca2+]i (Δ [Ca2+]i = 79 ± 13 nM) (Fig. 3). Also, siRNA knockdown of Cx40 in GENCs significantly reduced both the speed (2.0 ± 0.3 μm/s) and Δ [Ca2+]i (200 ± 12 nM) (Fig. 3). Treatment of GENCs with mismatch siRNA sequence and the void transfection vector served as controls, having no influence on calcium wave propagation (Fig. 3).
Fig. 3.

Summary of the effects of various pharmacological inhibitors on the calcium wave speed (open bars, left scale) and the increases in [Ca2+]i (Δ [Ca2+]i, solid bars, right scale) in peripheral cells at various distances from the center. The voltage-operated calcium channel inhibitor nifedipine, and a calcium-free bathing solution had no effect, while gap junction uncoupling with 18- α-glycyrrhetinic acid (18 α-GA), Cx40 silencing with Cx40 siRNA, scavenging extracellular ATP (apyrase, hexokinase cocktail), and the nonselective P2 receptor antagonist suramin caused significant decreases in the speed and Δ [Ca2+]i of the propagating calcium wave. The effects of ATP scavenging were reversible after enzyme washout (recovery) in paired experiments. Treatment of GENCs with mismatched siRNA sequence (MM) and the void transfection vector (-siRNA) had no influence on calcium wave propagation. n = 6 coverslips in each group, and at least 3 cells were analyzed per coverslip. *P < 0.05, compared with control.
To test the involvement of extracellular ATP, an ATP scavenger cocktail consisting of hexokinase and apyrase, 50 U/ml each, was used. The breakdown of ATP by these enzymes limited the effects of mechanical stimulation on propagation of the calcium wave (speed = 3.1 ± 0.5 μm/s, Δ [Ca2+]i = 70 ± 4 nM, n = 6) (Fig. 3). The same coverslips were used in paired experiments; subsequent washout of the ATP scavenging enzymes resulted in a recovery of both the calcium wave speed (9.6 ± 0.5 μm/s) and Δ [Ca2+]i (394 ± 39 nM, n = 6) nearly to control levels (Fig. 3). Nonselective inhibition of purinergic P2 receptors with 100 μM suramin almost completely abolished calcium responses (Δ [Ca2+]i = 100 ± 11 nM) and the propagation of the calcium wave (speed = 1.9 ± 0.1 μm/s) (Fig. 3). In these experiments, there was generally a strong correlation between calcium wave speed and Δ [Ca2+]i and the area of the monolayer to which the calcium wave propagated (not shown). In experiments with the inhibitors Δ-GA, Cx40 siRNA, ATP scavenger cocktail, and suramin, the calcium wave propagated to only a few cells adjacent to the stimulated cell.
Connexin expression profile of GENCs
Because the above pharmacological experiments suggested the involvement of Cxs in the GENC calcium wave, the expression of the main vascular endothelial Cx isoforms Cx37, Cx40, and Cx43 in GENCs was examined using immunofluorescence and RT-PCR (Fig. 4). The protein (Fig. 4, A–C) and mRNA (Fig. 4E) of Cx40, but neither Cx37 nor Cx43, was detected in GENCs. Cell membranes robustly expressed Cx40 in a patchy pattern (Fig. 4B). After siRNA knockdown on Cx40 in GENCs, cells were devoid of Cx40 immunolabeling (Fig. 4D). Efficient silencing of Cx40 was also confirmed by RT-PCR (Fig. 4, E and F).
Fig. 4.

Localization of connexin isoforms and silencing of Cx40 in cultured GENCs. A–D: immunofluorescence of Cx37 (A), Cx40 (B), Cx43 (C) in wild-type GENCs and Cx40 immunofluorescence labeling in Cx40 siRNA-transfected GENCs (D). No Cx37 and Cx43 labeling was found. Mostly patchy Cx40 labeling (green) was found along the plasma membrane (B). There was no immunologically detectable Cx40 protein in Cx40 silenced cells (D). Cell nuclei are labeled with 4,6-diamino-2-phenylindole. Scale bar = 10 μm. E: RT-PCR detection of Cx37, 40, and 43 mRNA in GENCs. Only Cx40 mRNA was found. βactin served as a loading control (bottom). E–F: RT-PCR confirmation of effective Cx40 knockdown in GENCs transfected with Cx40 siRNA. Control (C), no treatment; -siRNA, transfection reagents, no siRNA; MM, mismatch sequence. β-actin served as a loading control.
Evidence for an extracellular, humoral mediator substance
Further experiments addressed the role and existence of an extracellular, humoral mediator vs. propagation through direct cell-to-cell contact via gap junctions. We used a glass micropipette and a micromanipulator to scrape off cells along a narrow, ~10- μm-wide line in the GENC monolayer (Fig. 5A), eliminating any physical contact between cells. Mechanical stimulation of a single cell on one side of the line triggered a calcium wave with a speed of 8.5 ± 0.2 μm/s, similar to that measured before (Fig. 3). Although the velocity was slower (4.5 ± 0.2 μm/s), the calcium wave did propagate to nonadjacent cells on the opposite side of the line (Fig. 5B). There was no significant difference in Δ [Ca2+]i between cells on the two sides (Fig. 5) (n = 15 from 5 different coverslips).
Fig. 5.
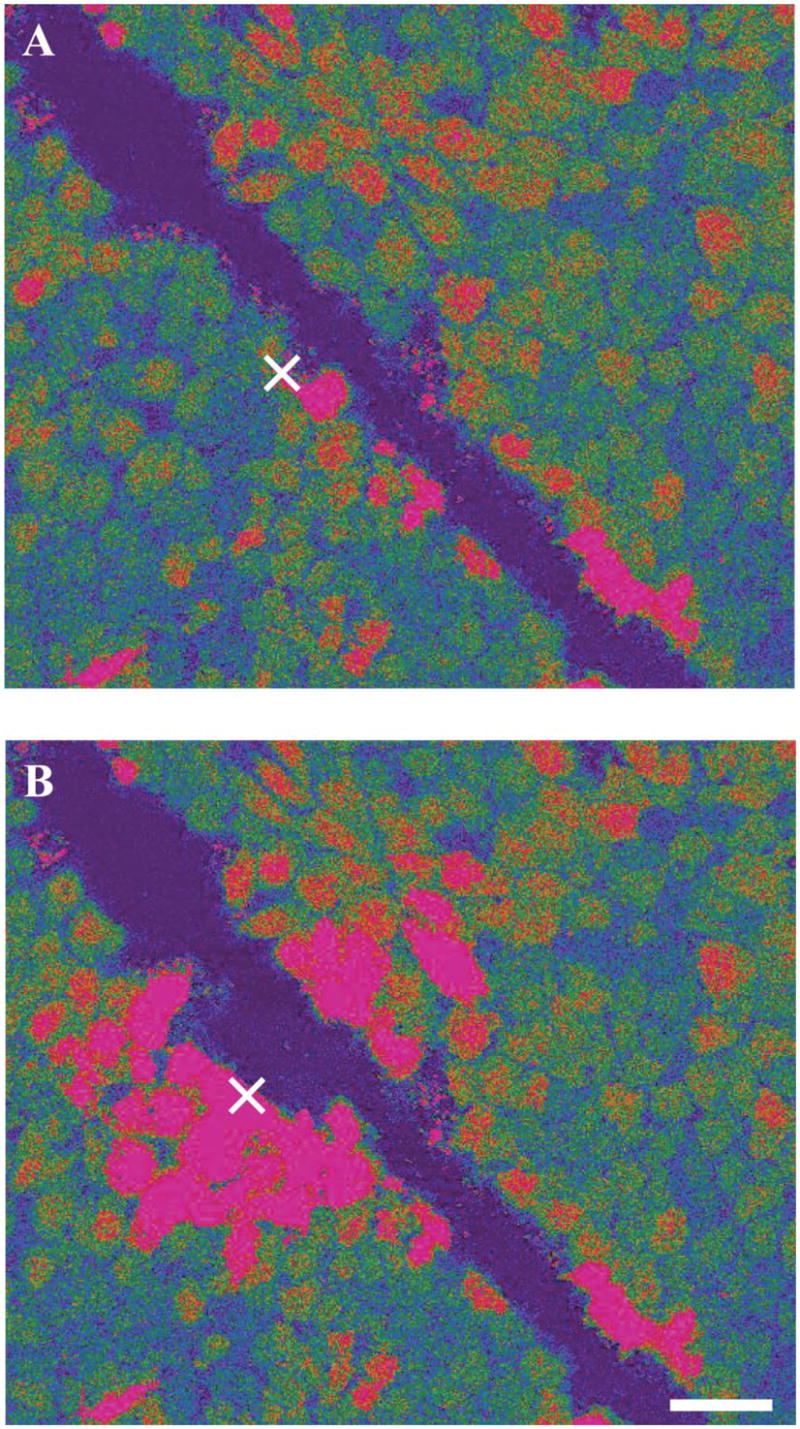
Evidence that an extracellular, humoral substance is mediating the calcium wave. Pseudocolor Fluo-4/Fura red ratio images before (A) and 5 s after (B) the stimulation of a single cell in the center of the field (labeled by ×). Some cells were scraped off along a narrow, about 10- μm-wide, line in the GENC monolayer (dark linear area), eliminating physical contact between cells. Mechanical stimulation of a single cell on one side of the line (×) triggered a calcium wave that propagated in all directions, including noncontiguous cells on the opposite side of the line. Scale bar = 20 μm.
Purinergic receptor expression and the effects of exogenous ATP
To test whether silencing of Cx40 caused an altered sensitivity of endothelial cells to extracellular ATP, we first examined the profile of purinergic receptor expression in wild-type and Cx40 siRNA-treated GENCs. A variety of both P2X and P2Y receptors were detected using RT-PCR, including P2X1, P2X4, P2X7, P2Y1, P2Y2, and P2Y4 (Fig. 6A). Importantly, no change in purinergic receptor expression was observed in response to siRNA silencing of Cx40 (Fig. 6B). Similar results were obtained using immunohistochemistry for P2X1, P2X4, P2Y2, and P2Y4 (not shown). Next, we compared ATP-induced [Ca2+]i responses in wild-type and Cx40 siRNA-treated cells. The addition of exogenous 100 μM ATP to the bath solution caused a significant elevation of [Ca2+]i in wild-type GENCs. Nearly identical [Ca2+]i responses were observed in GENCs without Cx40 (Fig. 6C), further confirming intact purinergic signaling after siRNA treatment.
Fig. 6.
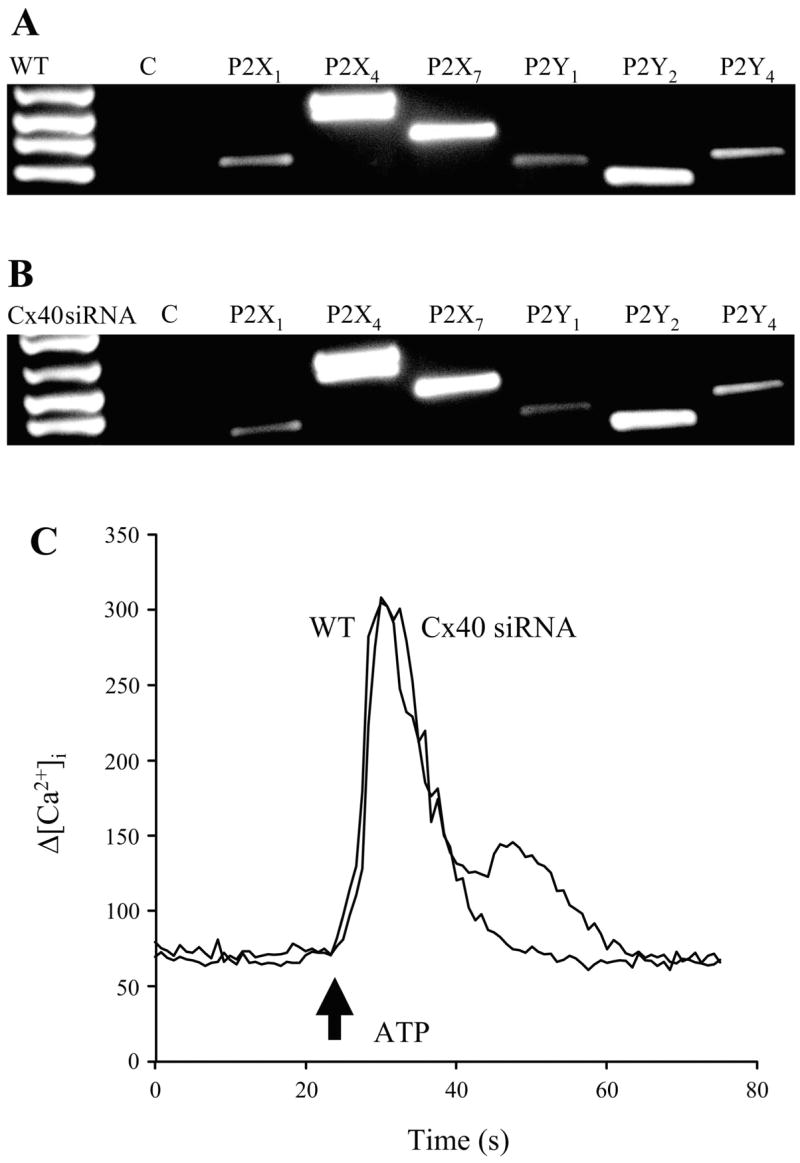
Purinergic receptor profile and signaling in cultured GENCs. A–B: RT-PCR detection of purinergic receptor mRNA in wild-type (A) and Cx40 siRNA-treated GENCs (B). No changes in receptor expression were found in response to siRNA knockdown of Cx40. C: representative recordings of 100 μM exogenous ATP-induced elevations in [Ca2+]i in wild-type (WT) and Cx40 siRNA-silenced GENCs (Cx40 siRNA). Arrow indicates the time ATP was added to the bathing solution. Responses were nearly identical.
Measurement of extracellular ATP
To confirm the involvement of extracellular ATP as a critical mediator of GENC calcium wave propagation, an ATP biosensor technique was used as described before (3) to measure local ATP levels in the bath solution during signal propagation. PC12 cells that express purinergic receptors were loaded with Fluo-4 and Fura red and positioned on top of GENCs in culture (Fig. 7A). Careful adjustment of focus allowed simultaneous fluorescence imaging of both PC12 and GENC calcium responses during the calcium wave. Mechanical stimulation induced a GENC calcium wave of similar characteristics as described above (Fig. 7B). PC12 cells detected significant elevations in extracellular ATP (Δ = 76 ± 2 μM) during calcium wave propagation (see supplemental video attachment online on the American Journal of Physiology—Regulatory, Integrative and Comparative Physiology Web site), which were abolished by Cx40 siRNA treatment (Δ = 6 ± 1 μM) (Fig. 7B). Even when the GENC calcium wave was intact, PC12 biosensor cell calcium responses were blocked by preincubation with the purinergic receptor inhibitor suramin (100 μM), confirming specificity of PC12 responses to extracellular ATP (Fig. 7B).
Fig. 7.

Extracellular ATP measurement in situ during the GENC calcium wave using a biosensor technique. A: representative image is shown of the fluorophore-loaded GENC monolayer (pseudocolor) and ATP-sensing PC12 cells atop also loaded with calcium dyes (red). A glass pipette was used for gentle mechanical stimulation of a single GENC. Differential interference contrast (DIC) overlay is shown for additional detail. Scale bar = 50 μm. A full video of the mechanically induced, propagating GENC calcium wave and ATP detection by PC12 cells can be found in a supplemental video file. B: summary of the speed of the GENC calcium wave (open bars, left scale) and simultaneous local, extracellular ATP measurements (solid bars, right scale) using the PC12 cell biosensor assay. siRNA silencing of Cx40 in GENCs significantly reduced the speed of the calcium wave and abolished elevations in bath ATP. Preincubation of PC12 cells with suramin (100 μM), a purinergic receptor inhibitor, blocked biosensor cell calcium responses, even when the GENC calcium wave was intact. n = 12 from four different coverslips in each group. *P < 0.05, compared with control.
DISCUSSION
The present studies visualized and characterized the propagation of calcium waves in glomerular endothelial cells using a recently established cell line, since this experiment would not be feasible with the small and looping glomerular capillaries in situ. Mechanical stimulation, which is likely consistent with in vivo activation of endothelium by shear stress or contact with circulating blood cells, resulted in a regenerating calcium wave that propagated over distances >400 μm. Generation and propagation of the calcium wave were primarily due to an extracellular, humoral mediator, identified as ATP. For the first time, it was shown that Cx40 controls extracellular ATP-mediated calcium waves. Thus, Cx40 and extracellular ATP are key molecular elements of the juxtaglomerular vasculature and most likely play important roles in glomerular and JGA functions. Although the present studies do not provide direct evidence, the most likely explanation is that ATP was released from endothelial cells through Cx40 hemichannels.
Because they are difficult to unequivocally demonstrate, the existence and function of Cx hemichannels are still debated. Nevertheless, several studies suggest that functional Cx hemichannels exist and can mediate ATP release (6, 9, 32). The presence of Cx hemichannels in the kidney was recently proposed (20). Cx40 is a predominant endothelial Cx isoform in the kidney (11, 12, 15) and is essential for the pressure control of renin synthesis and release (37). It is well established that Cx40-deficient mice are hypertensive with high renin levels and renin-angiotensin system (RAS) activity, but it is not the only cause for hypertension (12, 18, 26, 37). Endothelial cells act as mechanosensors regulating the function of underlying vascular smooth muscle cells and vessel contractility (12, 38, 39). Importantly, it was recently speculated (37) that Cx40 is important for the induction and/or propagation of cytosolic calcium and calcium waves. The present studies fully complement this hypothesis and provide experimental evidence that Cx40 directly controls mechanically induced calcium waves in the juxtaglomerular endothelium. It is known that the JGA receives ATP-mediated purinergic calcium signals from the direction of the macula densa, which also propagates to renin-producing JG cells (22). Purinergic calcium signals spreading to JG cells from either the endothelium or the macula densa are consistent with the paradigm that increases in JG cell [Ca2+]i communicate various inhibitory mechanisms on renin synthesis and release, a phenomenon called the “calcium paradox” (10). The role of extracellular ATP-mediated calcium waves between renin-producing cells in controlling renin release has been demonstrated (42). ATP may effect JG cell [Ca2+]i and renin release directly, or through its degradation to adenosine (29, 30).
Similar to involvement in the control of renin release, the endothelial calcium wave may also be important in the regulation of JG vascular resistance and glomerular filtration. Cx40-mediated elevations in endothelial [Ca2+]i and the subsequent synthesis of vasodilator substances are important in vasomotor function (12, 26). On the basis of the present data, the lack of Cx40 causes defective ATP-mediated purinergic calcium signaling, and impaired vasodilatation is a possible mechanism for the increased vascular tone and hypertension in Cx40 knockout animals, in addition to RAS activation (12, 13, 18, 26, 37). Glomerular endothelium-derived vasodilators may act on the glomerular mesangium locally, or may diffuse back to preglomerular and JGA environments with the help of significant interstitial fluid flow in this area (25). Glomerular endothelial Cx40 expression is increased in certain pathophysiological conditions (11, 43), which through ATP purinergic calcium signaling and vasodilators, could contribute to glomerular hyperfiltration (43). Impaired flow-dependent control of vascular tone in mice lacking the ATP receptor P2X4 (38), the major contributor to ATP and flow-induced calcium influx in endothelial cells (4, 39), provides further strong support for the critical role of Cx40-mediated endothelial ATP release.
Propagation characteristics of the endothelial calcium wave observed in cultured GENCs in the present experiments (including speed, ATP, and connexin dependence) are similar to data obtained with the in vitro microperfused glomerulus preparation (22), indicating relevance of present cell culture data to conditions in situ. One discrepancy is the regenerative pattern, which was not observed in a recent microperfusion study (34) and could be reconciled by differences in cell culture conditions (8). Nevertheless, regenerating calcium waves have been described in cultured vascular endothelial cells (8). In GENCs, this could play a significant role in the potent conduction of vasodilator signals over long distances in the glomerular capillary network. Regenerating calcium waves may be specific for the endothelium, as responses in an epithelial cell line were much different (Fig. 2). The use of calcium-free medium reduced Δ [Ca2+]i about 50% (Fig. 3), which is consistent with the involvement of both calcium entry and release from intracellular stores in calcium waves (8, 17, 31, 32). We cannot exclude the possibility, however, that the calcium chelator EGTA used in these experiments caused a depletion of intracellular calcium stores directly and reduced the responsiveness of GENCs. Nevertheless, no reductions in baseline GENC [Ca2+]i were detected in response to calcium-free medium (data not shown), a fact that argues against this possibility. Nifedipine had no effect on the calcium wave, in accord with the minor functional importance of voltage-dependent calcium channels in endothelial cells (14, 34). Effective silencing of Cx40 was achieved in GENCs using the siRNA technique (Figs. 3 and 4) and provided evidence for the important role of Cx40 in ATP-mediated purinergic calcium signaling. This most likely involved ATP release through Cx40 hemichannels, as well as propagation of the calcium wave through Cx40 gap junctions. Supporting this is the finding that calcium wave speed was nearly double in physically connected GENCs compared with conditions when only extracellular humoral mediation was present (Fig. 5). Also, the measurement of local, bath ATP levels during calcium wave propagation using the PC12 biosensor technique (Fig. 7) provided evidence for Cx40-dependent extracellular ATP release during calcium wave propagation. Our data are also consistent with reports that gap junction uncouplers like 18 α-GA can inhibit gap junctional communication, as well as Cx hemichannels (9, 22, 41). In terms of the mechanical stimulus used in these experiments, a single GENC was gently touched by a glass micropipette as shown in Fig. 7A and in the supplementary video. The cells remained intact and responsive after mechanical stimulation.
Because Cx deficiency can alter the expression of various genes (16), including purinergic P2 receptors (28), the profile of P2 receptor expression and the effects of exogenous ATP were tested on wild-type and Cx40-silenced GENCs (Fig. 6). The unaltered P2 receptor profile (Fig. 6, A and B) and the essentially identical responses in [Ca2+]i to exogenous ATP (Fig. 6C) indicate that defective ATP release rather than reduced ATP sensitivity of GENCs was responsible for the diminished Δ [Ca2+]i and calcium wave after Cx40 siRNA treatment (Fig. 3). Experimental maneuvers that supported the critical role of extracellular ATP, including ATP scavenging enzymes and purinergic receptor inhibition have been successfully used before (22, 31).
Localization of Cx40 in GENCs is consistent with earlier Cx40 immunohistochemical localization in various species (11, 12, 15, 43). The punctate pattern of Cx40 labeling observed in the present study is also consistent with other studies (11, 12, 15, 43). Further support of the key role of Cx40 in the GENC calcium wave is that other major vascular Cx isoforms, specifically Cx37 and Cx43, were not expressed in detectable amounts in these cells (Fig. 4).
Perspectives and Significance
The present study suggests that interendothelial calcium signaling in the juxtaglomerular vasculature is mediated by extracellular ATP and purinergic P2 receptors. Moreover, extracellular ATP and purinergic calcium signaling are controlled by Cx40, which may involve ATP release through Cx40 hemichannels. Additional work is needed to confirm the role of endothelium-derived ATP and subsequent purinergic calcium signaling in the control of glomerular filtration and renin release by physiological stimuli, including blood pressure.
Supplementary Material
Acknowledgments
GRANTS
These studies were supported by Grants DK-64324 and DK-74754 from National Institutes of Health, and by an Established Investigator Award 0640056N from the American Heart Association to J. Peti-Peterdi. I. Toma was a National Kidney Foundation Postdoctoral Research Fellow during these studies.
References
- 1.Akis N, Madaio MP. Isolation, culture, and characterization of endothelial cells from mouse glomeruli. Kidney Int. 2004;65:2223–2227. doi: 10.1111/j.1523-1755.2004.00634.x. [DOI] [PubMed] [Google Scholar]
- 2.Arensback B, Mikkelsen HB, Gustafsson F, Christensen T, Holstein-Rathlou NH. Expression of connexin 37, 40, and 43 mRNA and protein in renal preglomerular arterioles. Histochem Cell Biol. 2001;115:479–487. doi: 10.1007/s004180100275. [DOI] [PubMed] [Google Scholar]
- 3.Bell PD, Lapointe JY, Sabirov R, Hayashi S, Peti-Peterdi J, Manabe K, Kovacs G, Okada Y. Macula densa cell signaling involves ATP release through a maxi anion channel. Proc Natl Acad Sci USA. 2003;100:4322–4327. doi: 10.1073/pnas.0736323100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Burnstock G. Vessel tone and remodeling. Nat Med. 2006;12:16–17. doi: 10.1038/nm0106-16. [DOI] [PubMed] [Google Scholar]
- 5.Butterweck A, Gerg U, Elfgang C, Willecke K, Traub O. Immunochemical characterization of the gap junction protein connexin45 in mouse kidney and transfected human HeLa cells. J Membrane Biol. 1994;141:247–256. doi: 10.1007/BF00235134. [DOI] [PubMed] [Google Scholar]
- 6.Cotrina ML, Lin JH, Alves-Rodrigues A, Liu S, Li J, Azmi-Ghadimi H, Kang J, Naus CC, Nedergaard M. Connexins regulate calcium signaling by controlling ATP release. Proc Natl Acad Sci USA. 1998;95:15735–15740. doi: 10.1073/pnas.95.26.15735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Coutinho-Saliva R, Ojcius DM, Górecki DC, Persechini PM, Bisaggio RC, Mendes AN, Marks J, Burnstock G, Dunn PM. Multiple P2X and P2Y receptor subtypes in mouse J774, spleen and peritoneal macrophages. Biochem Pharmacol. 2005;69:641–655. doi: 10.1016/j.bcp.2004.11.012. [DOI] [PubMed] [Google Scholar]
- 8.Domenighetti AA, Beny JL, Chabaud F, Frieden M. An intercellular regenerative calcium wave in porcine coronary artery endothelial cells in primary culture. J Physiol. 1998;513:103–116. doi: 10.1111/j.1469-7793.1998.103by.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ebihara L. New roles for connexons. News Physiol Sci. 2003;18:100–103. doi: 10.1152/nips.01431.2002. [DOI] [PubMed] [Google Scholar]
- 10.Grunberger C, Obermayer B, Klar J, Kurtz A, Schweda F. The calcium paradoxon of renin release: calcium suppresses renin exocytosis by inhibition of calcium-dependent adenylate cyclases AC5 and AC6. Circ Res. 2006;99:1197–1206. doi: 10.1161/01.RES.0000251057.35537.d3. [DOI] [PubMed] [Google Scholar]
- 11.Haefliger JA, Demotz S, Braissant O, Suter E, Waeber B, Nicod P, Meda P. Connexins 40 and 43 are differentially regulated within the kidneys of rats with renovascular hypertension. Kidney Int. 2001;60:190–201. doi: 10.1046/j.1523-1755.2001.00786.x. [DOI] [PubMed] [Google Scholar]
- 12.Haefliger JA, Nicod P, Meda P. Contribution of connexins to the function of the vascular wall. Cardiovasc Res. 2004;62:345–356. doi: 10.1016/j.cardiores.2003.11.015. [DOI] [PubMed] [Google Scholar]
- 13.Haefliger JA, Krattinger N, Martin D, Pedrazzini T, Capponi A, Döring B, Plum A, Charollais A, Willecke K, Meda P. Connexin 43-dependent mechanism modulates renin secretion and hypertension. J Clin Invest. 2006;116:405–413. doi: 10.1172/JCI23327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Himmel HM, Whorton AR, Strauss HC. Intracellular calcium, currents, and stimulus-response coupling in endothelial cells. Hypertension. 1993;21:112–127. doi: 10.1161/01.hyp.21.1.112. [DOI] [PubMed] [Google Scholar]
- 15.Hwan Seul K, Beyer EC. Heterogeneous localization of connexin40 in the renal vasculature. Microvasc Res. 2000;59:140–148. doi: 10.1006/mvre.1999.2216. [DOI] [PubMed] [Google Scholar]
- 16.Iacobas DA, Urban-Maldonado M, Iacobas S, Scemes E, Spray DC. Array analysis of gene expression in connexin-43 null astrocytes. Physiol Genomics. 2003;15:177–190. doi: 10.1152/physiolgenomics.00062.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ichimura H, Parthasarathi K, Lindert J, Bhattacharya J. Lung surfactant secretion by interalveolar Ca2+ signaling. Am J Physiol Lung Cell Mol Physiol. 2006;291:L596–L601. doi: 10.1152/ajplung.00036.2006. [DOI] [PubMed] [Google Scholar]
- 18.Krattinger N, Capponi A, Mazzolai L, Aubert JF, Caille D, Nicod P, Waeber G, Meda P, Haefliger JA. Connexin 40 regulates renin production and blood pressure. Kidney Int. 2007;72:814–822. doi: 10.1038/sj.ki.5002423. [DOI] [PubMed] [Google Scholar]
- 19.Kurtz L, Schweda F, de Wit C, Kriz W, Witzgall R, Warth R, Sauter A, Kurtz A, Wagner C. Lack of connexin 40 causes displacement of renin-producing cells from afferent arterioles to the extraglomerular mesangium. J Am Soc Nephrol. 2007;18:1103–1111. doi: 10.1681/ASN.2006090953. [DOI] [PubMed] [Google Scholar]
- 20.McCulloch F, Chambrey R, Eladari D, Peti-Peterdi J. Localization of connexin 30 in the luminal membrane of cells in the distal nephron. Am J Physiol Renal Physiol. 2005;289:F1304–F1312. doi: 10.1152/ajprenal.00203.2005. [DOI] [PubMed] [Google Scholar]
- 21.Nishiyama A, Rahman M, Inscho EW. Role of interstitial ATP and adenosine in the regulation of renal hemodynamics and microvascular function. Hypertens Res. 2004;27:791–804. doi: 10.1291/hypres.27.791. [DOI] [PubMed] [Google Scholar]
- 22.Peti-Peterdi J. Calcium wave of tubuloglomerular feedback. Am J Physiol Renal Physiol. 2006;291:F473–F480. doi: 10.1152/ajprenal.00425.2005. [DOI] [PubMed] [Google Scholar]
- 23.Rivera I, Zhang S, Fuller BS, Edwards B, Seki T, Wang MH, Marrero MB, Inscho EW. P2 receptor regulation [Ca2+]i in cultured mouse mesangial cells. Am J Physiol Renal Physiol. 2007;292:F1380–F1389. doi: 10.1152/ajprenal.00349.2006. [DOI] [PubMed] [Google Scholar]
- 24.Rosivall L, Peti-Peterdi J. Heterogeneity of the afferent arteriole—correlations between morphology and function. Nephrol Dial Transplant. 2006;21:2703–2707. doi: 10.1093/ndt/gfl308. [DOI] [PubMed] [Google Scholar]
- 25.Rosivall L, Mirzahosseini S, Toma I, Sipos A, Peti-Peterdi J. Fluid flow in the juxtaglomerular interstitium visualized in vivo. Am J Physiol Renal Physiol. 2006;291:F1241–F1247. doi: 10.1152/ajprenal.00203.2006. [DOI] [PubMed] [Google Scholar]
- 26.Rummery NM, Hill CE. Vascular gap junctions and implications for hypertension. Clin Exp Pharmacol Physiol. 2004;31:659–667. doi: 10.1111/j.1440-1681.2004.04071.x. [DOI] [PubMed] [Google Scholar]
- 27.Salomonsson M, Gustafsson F, Andreasen D, Jensen BL, Holstein-Rathlou NH. Local electric stimulation causes conducted calcium response in rat interlobular arteries. Am J Physiol Renal Physiol. 2002;283:F473–F480. doi: 10.1152/ajprenal.00247.2001. [DOI] [PubMed] [Google Scholar]
- 28.Scemes E, Suadicani SO, Spray DC. Intercellular communication in spinal cord astrocytes: fine tuning between gap junctions and P2 nucleotide receptors in calcium wave propagation. J Neurosci. 2000;20:1435–1445. doi: 10.1523/JNEUROSCI.20-04-01435.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schweda F, Segerer F, Castrop H, Schnermann J, Kurtz A. Blood pressure-dependent inhibition of renin secretion requires A1 adenosine receptors. Hypertension. 2005;46:780–786. doi: 10.1161/01.HYP.0000183963.07801.65. [DOI] [PubMed] [Google Scholar]
- 30.Schweda F, Wagner C, Kramer BK, Schnermann J, Kurtz A. Preserved macula densa-dependent renin secretion in A1 adenosine receptor knockout mice. Am J Physiol Renal Physiol. 2003;284:F770–F777. doi: 10.1152/ajprenal.00280.2002. [DOI] [PubMed] [Google Scholar]
- 31.Schwiebert EM. Extracellular ATP-mediated propagation of Ca2+ waves. Focus on “mechanical strain-induced Ca2+ waves are propagated via ATP release and purinergic receptor activation. Am J Physiol Cell Physiol. 2000;279:C281–C283. doi: 10.1152/ajpcell.2000.279.2.C281. [DOI] [PubMed] [Google Scholar]
- 32.Stout CE, Costantin JL, Naus CC, Charles AC. Intercellular calcium signaling in astrocytes via ATP release through connexin hemichannels. J Biol Chem. 2002;277:10482–10488. doi: 10.1074/jbc.M109902200. [DOI] [PubMed] [Google Scholar]
- 33.Taugner R, Kirchheim H, Forssmann WG. Myoendothelial contacts in glomerular arterioles and in renal interlobular arteries of rat, mouse and Tupaia belangeri. Cell Tissue Res. 1984;235:319–325. doi: 10.1007/BF00217856. [DOI] [PubMed] [Google Scholar]
- 34.Uhrenholt TR, Schjerning J, Vanhoutte PM, Jensen BL, Skott O. Intercellular calcium signaling and nitric oxide feedback during constriction of rabbit renal afferent arterioles. Am J Physiol Renal Physiol. 2007;292:F1124–F1131. doi: 10.1152/ajprenal.00420.2006. [DOI] [PubMed] [Google Scholar]
- 35.Uhrenholt TR, Domeier TL, Segal SS. Propagation of calcium waves along endothelium of hamster feed arteries. Am J Physiol Heart Circ Physiol. 2007;292:H1634–H1640. doi: 10.1152/ajpheart.00605.2006. [DOI] [PubMed] [Google Scholar]
- 36.Urban M, Rozental R, Spray DC. A simple RT-PCR-based strategy for screening connexin identity. Braz J Med Biol Res. 1999;32:1029–1037. doi: 10.1590/s0100-879x1999000800014. [DOI] [PubMed] [Google Scholar]
- 37.Wagner C, de Wit C, Kurtz L, Grunberger C, Kurtz A, Schweda F. Connexin 40 is essential for the pressure control of renin synthesis and secretion. Circ Res. 2007;100:556–563. doi: 10.1161/01.RES.0000258856.19922.45. [DOI] [PubMed] [Google Scholar]
- 38.Yamamoto K, Sokabe T, Matsumoto T, Yoshimura K, Shibata M, Ohura N, Fukuda T, Sato T, Sekine K, Kato S, Isshiki M, Fujita T, Kobayashi M, Kawamura K, Masuda H, Kamiya A, Ando J. Impaired flow-dependent control of vascular tone and remodeling in P2X4-deficient mice. Nat Med. 2006;12:133–137. doi: 10.1038/nm1338. [DOI] [PubMed] [Google Scholar]
- 39.Yamamoto K, Sokabe T, Ohura N, Nakatsuka H, Kamiya A, Ando J. Endogenously released ATP mediates shear stress-induced Ca2+ influx into pulmonary artery endothelial cells. Am J Physiol Heart Circ Physiol. 2003;285:H793–H803. doi: 10.1152/ajpheart.01155.2002. [DOI] [PubMed] [Google Scholar]
- 40.Yang T, Park JM, Arend L, Huang Y, Topaloglu R, Pasumarthy A, Praetorius H, Spring K, Briggs JP, Schnermann J. Low chloride stimulation of prostaglandin E2 release and cyclooxygenase-2 expression in a mouse macula densa cell line. J Biol Chem. 2000;275:37922–37929. doi: 10.1074/jbc.M006218200. [DOI] [PubMed] [Google Scholar]
- 41.Yao J, Morioka T, Li B, Oite T. Coordination of mesangial cell contraction by gap junction-mediated intercellular Ca2+ wave. J Am Soc Nephrol. 2002;13:2018–2026. doi: 10.1097/01.asn.0000023436.71816.56. [DOI] [PubMed] [Google Scholar]
- 42.Yao J, Suwa M, Li B, Kawamura K, Morioka T, Oite T. ATP-dependent mechanism for coordination of intercellular Ca2+ signaling and renin secretion in rat juxtaglomerular cells. Circ Res. 2003;93:338–345. doi: 10.1161/01.RES.0000086802.21850.5D. [DOI] [PubMed] [Google Scholar]
- 43.Zhang J, Hill CE. Differential connexin expression in preglomerular and postglomerular vasculature: accentuation during diabetes. Kidney Int. 2005;68:1171–1185. doi: 10.1111/j.1523-1755.2005.00509.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.