Abstract
Human leucocyte antigen (HLA)-C is expressed at lower levels than other classical HLA-I molecules on somatic cells. Surface HLA-C proteins can occur as conventionally β2-microglobulin (β2m)-associated complexes or as open conformers dissociated from peptide and/or β2m. We investigated the conformation of HLA-C molecules on normal human trophoblast cells, which invade the maternal decidua during placentation. A panel of monoclonal antibodies to different conformations of HLA-I molecules was used in flow cytometry and surface immunoprecipitation experiments. On the surface of trophoblast cells only β2m-associated complexes of HLA-C molecules were detected. In contrast, both open conformers and β2m-associated HLA-C could be detected on other cells from the decidua, HLA-C-transfectants and cell lines. The levels of HLA-C expressed on primary trophoblast cells could be detected by antibodies specific to non-β2m-associated conformations because binding was seen after acid-induced denaturation of surface proteins. In contrast to HLA-G molecules on trophoblasts, we found no evidence for the presence of disulphide-linked multimers of HLA-C complexes. These results show that most HLA-C molecules present at the trophoblast cell surface are in the conventional β2m-associated conformation. These findings have implications regarding the stability of trophoblast HLA-C molecules and how they interact with receptors on decidual leucocytes during placentation.
Keywords: human leucocyte antigen-C, human trophoblast, killer immunoglobulin-like receptors, leucocyte immunoglobulin-like receptors
Introduction
During implantation, fetal trophoblast cells infiltrate the uterine mucosal lining, the decidua. Trophoblast cells express a distinctive combination of human leucocyte antigen (HLA) class I molecules (HLA-I), HLA-G, HLA-C and HLA-E.1–4 The receptors for these fetal ligands are found on maternal decidual natural killer (NK) and myelomonocytic cells and include killer immunoglobulin-like receptors (KIR), leucocyte immunoglobulin-like receptors (LILR) and CD94-associated receptors of the NKG2 complex.4–6
The only polymorphic trophoblast HLA-I molecule is HLA-C. The KIR, which are expressed by decidual NK cells and recognize HLA-C, are also highly polymorphic, so that unrelated individuals are unlikely to share a KIR genotype. The KIR are able to distinguish all HLA-C alleles as two groups, C1 and C2, based on a dimorphism at position 80 of the α1 domain.7 In an immunogenetic study we have shown that certain combinations of maternal KIR and fetal HLA-C genotypes are associated with pre-eclampsia,8 a disease where the trophoblast fails to invade correctly. Interestingly, the risk was conferred by the presence of an HLA-C2 group in the fetus independently of whether the mother also possessed a C2 group. In other words, trophoblast HLA-C2 was a risk factor whether or not it was a self, missing-self or non-self molecule in relation to maternal KIR recognition of self HLA-C molecules.8 This suggests that decidual NK cells might be able to distinguish HLA-C on trophoblast from that on maternal somatic cells in what could be a novel mechanism of immune recognition.
The defining trophoblast HLA-I molecule is HLA-G and this exists at the cell surface of normal trophoblast cells in various forms: the conventional heterotrimeric HLA-I complex, a disulphide-linked homodimer of this β2-microglobulin (β2m)-associated complex, and potentially as open conformers dissociated from peptide and/or β2m. The homodimeric HLA-G complexes of trophoblast preferentially bind the LILRB1 molecules expressed by decidual myelomonocytic cells.6,9,10 Unlike the β2m-associated forms, open conformers of HLA-G cannot bind LILRB1 and may interfere with the HLA-G–LILRB1 interaction.11
Open conformers of classical HLA-I molecules do occur and bind to immune receptors in trans.12 HLA-C has been shown to exist in these forms in vivo in various cell types.13,14 If the conformation of HLA-C on trophoblast cells is different from that on other cells, this may provide a mechanism for maternal NK-cell discrimination between HLA-C molecules of self and placental trophoblast cells. We have now further investigated the conformations of HLA-C molecules on normal human trophoblast.
Materials and methods
Cell lines and human tissue
We used the HLA-I null human B lymphoblastoid 721.221 line transfected with HLA-Cw*0401 (from Dr M. Lopez-Botet, Barcelona, Spain) and the choriocarcimoma cell line JEG-3 (American Type Culture Collection, Rockville, MD), which expresses the same HLA-Cw*0401 allele.15 Placental tissue was obtained from elective terminations of normal first-trimester pregnancies. Ethical approval for the use of these tissues was obtained from the Cambridge Local Research Ethics Committee and the cells were isolated as previously described.16 Briefly, trophoblast cells were released from chorionic villi by trypsin digestion, macrophages were depleted and the remaining trophoblast cells were cultured overnight on fibronectin. Maternal cells were also isolated from decidual curettings by collagenase digestion and were stained immediately.
Flow cytometry
JEG-3 or primary trophoblast cells cultured overnight were removed from the plate with non-enzymic cell-dissociation solution (Sigma, Poole, UK). Fcγ receptors were blocked by an incubation in 200 μg/ml human immunoglobulin G (Sigma) and washed. Unlabelled monoclonal antibody (mAb) binding HLA-C or the BC-1 mAb (Table 1) was added and detected with phycoerythrin-conjugated secondary mAb (Sigma). Free secondary antibody-binding sites were blocked with mouse immunoglobulin G, before staining with directly conjugated mAb. The mAb G233 binds HLA-G molecules of trophoblast cells (Table 1). Fluorescein isothiocyanate (FITC) -conjugated CD3, CD56-Alexa 488 and a cocktail of FITC-conjugated lineage markers (CD3, CD14, CD16, CD19, CD20, CD56) were used to identify leucocyte populations (all Becton Dickinson, Oxford, UK). Data were acquired with a FACScan flow cytometer and analysed using CellQuest software (Becton Dickinson). For acid-induced denaturation, harvested cells were resuspended in a citric acid buffer (130 mm citric acid, 60 mm Na2HPO4 and 1% bovine serum albumin) for 60 seconds at 4°. Excess RPMI-1640 supplemented with 20% fetal calf serum was then added before blocking of the Fcγ receptors and staining as before. Flow cytometry was performed on primary cell preparations from individual donors. The experiments were performed on at least four independent donors and a representative result is shown.
Table 1.
Murine antibodies to HLA-I molecules; the conformations and alleles of HLA-I molecules recognized by each mAb are summarized
| mAb | Isotype | Antibody specificity | Original reference for mAb production |
|---|---|---|---|
| W6/32 | IgG2a | All alleles of β2m-associated HLA-I molecules are bound. The precise epitope is notknown, but is probably discontinuous and includes β2m, α2 and α3 domain residues.27 | 28 |
| B1.23.2 | IgG2a | β2m-associated conformers of HLA-B and HLA-C molecules.29 | 29 |
| Tü149 | IgG2a | β2m-associated HLA-I molecules are bound. Most HLA-B and HLA-C alleles arerecognized as well as some HLA-A alleles.30 | 31 |
| HC10 | IgG2a | Open conformers of HLA-I molecules are bound as this mAb binds an 57PxxWDR62epitope blocked by peptide binding. All HLA-B alleles, most HLA-C alleles and someHLA-A alleles but not HLA-E or G molecules are bound.32 | 33 |
| L31 | IgG1 | Open conformers of HLA-I molecules are bound13,34 as the L31 epitope includes residues66KYK68 in the peptide-binding groove of the α1 domain.35 This epitope is present inalmost all HLA-C alleles and some HLA-A and HLA-B allele sequences. | 36 |
| G233 | IgG2a | Specific to HLA-G molecules in a β2m-associated conformation.1 | 1 |
| BC-1 | IgG1 | Unidentified trophoblast antigen.1 | 19 |
β2m, β2-microglobulin; HLA, human leucocyte antigen; IgG, immunoglobulin G; mAb, monoclonal antibody.
Immunoprecipitation
For each immunoprecipitation 2 × 105 721.221 cells, 5 × 105 JEG-3 cells or 5 × 105 primary trophoblast cells pooled from several donors were washed with cold phosphate-buffered saline then biotinylated with 0·2 mg/ml EZ-Link Sulfo-NHS-LC-Biotin (Pierce, Tattenhall, UK) in pH 8·0 phosphate-buffered saline for 30 min, at 4° to prevent endocytic intracellular labelling. Unconjugated reagent was quenched by adding glycine to a concentration of 10 mm. Cells were then lysed in fresh ONYX [20 mm Tris–HCl (pH 7.4), 140 mm NaCl, 1 mm ethyleneglycoltetraacetic acid, 1% Triton X, 10% glycerol, 50 mm iodoacetamide and protease inhibitor cocktail (Roche Diagnostics, Lewes, UK)]. This concentration of iodoacetamide has been shown to prevent post-lysis HLA-I multimerization.17 Labelled lysates were stored at − 80°. On thawing, the lysates that were to be acidified were adjusted to pH 3·4 by the addition of HCl and were incubated on ice for 10 min before titration back to pH 7.4 with NaOH. Lysates were precleared by incubation for 90 min at 4° with protein G–Sepharose beads coated with isotype control mAb from Oxford Biotechnology (Oxford, UK). Precleared lysates were then immunoprecipitated for 90 min at 4° with protein G–Sepharose beads precoated with anti-HLA antibodies (Table 1). Immunoprecipitates were washed with ONYX containing 3 mm sodium dodecyl sulphate, bound proteins eluted with NuPAGE LDS Sample Buffer (Invitrogen, Paisley, UK) and denatured by treatment at 95° for 10 min. Denatured samples were stored at −20° overnight. Samples were resolved on non-reducing NuPAGE Bis–Tris 10% Gels (Invitrogen), electroblotted and visualized with a streptavidin–horseradish peroxidase conjugate followed by enhanced chemiluminescence (ECL) detection (Amersham Biosciences, Chalfont St Giles, UK).
Results and discussion
Isolated human trophoblast cells express HLA-C and HLA-G molecules at the cell surface
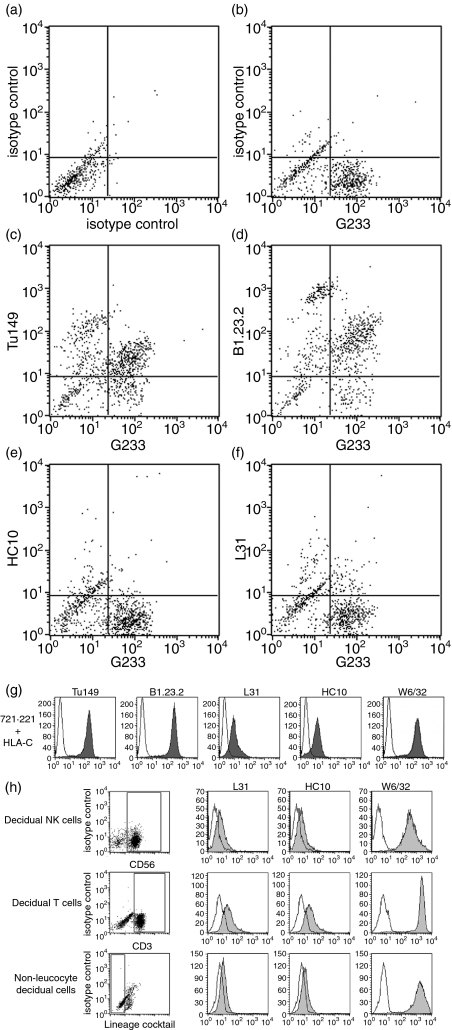
All mAb binding HLA-C also react with other HLA-I allotypes, especially certain HLA-B molecules. Unlike normal somatic cells, trophoblast cells never express HLA-B molecules and are therefore a unique cell type for the investigation of conformations of HLA-C in vivo.2,3 Primary trophoblast cells isolated from placental tissue of normal, first-trimester human pregnancies and cultured overnight were analysed by flow cytometry (Fig. 1). Extravillous trophoblast cells, which are the only normal cell type to express the non-classical HLA-G molecule,1 were identified by labelling with the HLA-G-specific mAb, G233 (Fig. 1b). Cells were then stained with two different mAb recognizing HLA-C molecules in a β2m-associated conformation, Tü149 and B1.23.2 (Fig. 1c and d; Table 1). The HLA-G+ extravillous trophoblast cells also bound both mAb-binding HLA-C, confirming that HLA-C was coexpressed at the surface of extravillous trophoblast cells.2,3 The primary trophoblast cultures also contained villous trophoblast (which express no surface HLA-I molecules3) and a population of HLA-C+ HLA-G− cells (which are leucocytes and stromal cells; data not shown).
Figure 1.
Surface human leucocyte antigen class I (HLA-I) molecules on primary cells from normal human pregnancies. Compared to isotype controls (a), extravillous trophoblast cells are identified by staining with the HLA-G specific monoclonal antibody (mAb) G233 (b). Double labelling with the mAb Tü149 (c) or B1.23.2 (d) confirms that these HLA-G+ cells also express HLA-C in the β2microglobulin-associated conformation. mAb HC10 (e) or L31 (f) that bind open conformers of HLA-C molecules do not bind trophoblast. The mAb recognizing all conformations of HLA-C molecules detect their antigens at the surface of 721.221 cells transfected with HLA-Cw4 (g). The mAb HC10 and L31 also detect their antigens on primary decidual cells identified by gates for CD56+, CD3+ or cells negative for leucocyte lineage markers (h). In histograms binding of the indicated mAb is shown in grey compared to staining of an isotype control in white.
Conformations of HLA-C molecules detected on the surface of trophoblast cells by flow cytometry
To investigate the conformation of HLA-C molecules on trophoblast cells, the binding of mAb recognizing HLA-C molecules in either β2m-associated or open conformations (Table 1) was first investigated by flow cytometry. Unlike the mAb Tü149 and B1.23.2 (Fig. 1c and d), the mAb HC10 and L31 did not bind to HLA-G+ extravillous trophoblast cells isolated from primary cell preparations (Fig. 1e and f). As a control, mAb to all conformations of HLA-C detected their antigen at the surface of 721.221 cells transfected with HLA-Cw4 (Fig. 1g). Interestingly, cells isolated from the maternal decidua did demonstrate HC10-reactive and L31-reactive antigens (Fig. 1h). From these flow cytometry experiments it appeared that most of the trophoblast HLA-C molecules were in the conventional β2m-associated conformation.
Conformations of HLA-C molecules detected at the surface of trophoblast cells by immunoprecipitation
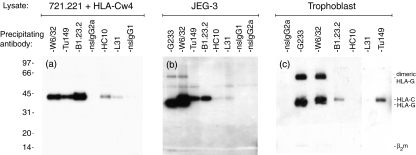
The conformation of HLA-C molecules was further investigated by biotin-labelling of surface proteins followed by immunoprecipitation and the development of Western blots with streptavidin–horseradish peroxidase (Fig. 2). From the HLA-Cw4 transfectants, the 45 000 Da molecular weight (MW) HLA-C heavy chain was brought down with mAb that bound the heterotrimeric β2m-associated molecules, W6/32, B1.23.2 and Tü149 (Fig. 2a). The 12 000 Da MW β2m band was only weakly visible, probably because of the low number of exposed lysine residues in native β2m available for biotin-label detection. HLA-C heavy chains with MW 45 000 Da were also immunoprecipitated with HC10 and L31, mAb which only bind peptide-free open conformers (Fig. 2a).
Figure 2.
Immunoprecipitation and Western blotting of surface-biotinylated human leucocyte antigen class I (HLA-I) molecules on trophoblast cells. 721.221 cells transfected with HLA-Cw4 (a), the JEG-3 choriocarcinoma line (b) and human trophoblast cells (c) were surface biotinylated, lysed and immunoprecipitated with the indicated monoclonal antibody (mAb). Precipitated complexes were resolved by non-reducing sodium dodecyl sulphate–polyacrylamide gel electrophoresis and surface complexes were detected by streptavidin-horseradish peroxidase. (a) From transfectants 45 000 Da molecular weight HLA-C molecules are detected by mAb to both β2microglobulin-associated (W6/32, B1.23.2, Tü149) as well as open conformations (L31, HC10) of the HLA-C molecule. (b,c) From both JEG-3 and primary trophoblast cells, W6/32, B1.23.2 and Tü149 mAb precipitate the 45 000 Da molecular weight HLA-C molecule. W6/32 and G233 also detect the 39 000 Da molecular weight HLA-G molecule expressed by trophoblast and JEG-3 cells. L31 and HC10 mAb do not detect their antigen on trophoblast cells and only very weakly on JEG-3 cells. Gels in this figure are each representative of three independent experiments, using in total trophoblast pooled from nine individual samples.
Similar precipitations were then performed with the choriocarcinoma cell line, JEG-3 (Fig. 2b), and primary trophoblast cells (Fig. 2c). All the mAb that recognized β2m-associated conformations of the HLA-C molecules clearly precipitated a 45 000 Da MW band from JEG-3 and primary trophoblast cells. In contrast, mAb L31 and HC10 did not significantly detect open conformers of HLA-C on trophoblast cells even after prolonged exposure of gels. There was only a very weak band with these mAb from JEG-3 cells. In addition to absolute detection, densitometry measurements indicated that the ratio of bands detected by HC10 and L31 relative to those reactive with Tü149, B1.23.2 and W6/32 was higher on transfectants compared to JEG-3 and trophoblast cells (data not shown). A doublet was seen with W6/32 because this mAb also binds to the 39 000 Da MW HLA-G molecule expressed by JEG-3 and trophoblast. Immunoprecipitation with the mAb G233, which is specific to HLA-G, confirms the identity of the 39 000 Da MW band in this doublet.
The immunoprecipitated molecules were resolved in non-reducing electrophoresis conditions to determine whether any multimeric complexes of HLA-C were present. A proportion of HLA-G molecules exist in an 80 000 Da MW complex, which is linked by disulphide bonding.6,9,10 No evidence for disulphide-linked multimers of HLA-C molecules at the surface of transfectants, JEG-3 or trophoblast cells was found, using mAb capable of detecting β2m-associated or open conformers (Fig. 2a–c). In summary, immunoprecipitation of HLA-I molecules on HLA-C transfectants, JEG-3 cells and normal trophoblast supports the flow cytometry findings that HLA-C molecules at the surface of trophoblast cells are associated with β2m.
Detection of HLA-C after acid-induced denaturation
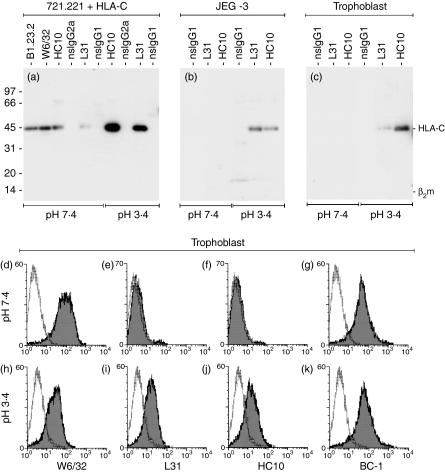
To demonstrate that mAb L31 and HC10 can detect the levels of HLA-C molecules present on trophoblast cells in both immunoprecipitation and flow cytometry experiments, the same assays were repeated after a brief incubation of the isolated primary cells at an acidic pH to induce denaturation, which results in dissociation of β2m from the HLA-I heavy chain.18 After this treatment, mAb L31 and HC10 clearly immunoprecipitated HLA-C from both JEG-3 and trophoblast cell lysates as well as from transfected cells (Fig. 3a–c). In flow cytometry the same results were seen when HLA-G+ extravillous trophoblast cells were specifically analysed by gating these cells from the primary cell preparation. There was significant binding of mAb L31 and HC10 to trophoblast cells after the acid exposure had induced surface protein denaturation (Fig. 3e, f, i and j). Staining for unrelated trophoblast surface antigens was not changed, as shown by the mAb BC-119 (Fig. 3g and k). These results conclusively proved that the β2m-associated molecules detected were indeed HLA-C and not HLA-E or HLA-G. Furthermore, they showed that the flow cytometry and immunoprecipitation methods used would be adequate to detect open conformers if they were present on trophoblast.
Figure 3.
Monoclonal antibody (mAb) to open conformers of human leucocyte antigen (HLA)-C bind their antigen on trophoblast cells after acid-induced denaturation of the HLA-I complexes. The mAb binding after a brief acid incubation was investigated by immunoprecipitation (a–c) and flow cytometry (d–k). (a) For HLA-C transfected cells, mAb recognizing both β2microgobulin-associated (W6/32, B1.23.2) and open conformations (L31, HC10) of HLA-C immunoprecipitate their 45 000 Da molecular weight antigen from untreated lysates. The mAb to open conformers clearly detect more antigen after acid treatment. (b,c) L31 and HC10 only immunoprecipitate their 45 000 Da molecular weight antigen from acidified lysates of JEG-3 (b) and primary trophoblast cells (c), but not from untreated lysates. Flow cytometry staining of HLA-G+ cells gated from the primary trophoblast preparations is shown, for cells kept at pH 7·4 (d–g) or briefly incubated in acidic conditions (h–k). (d,h) W6/32 mAb staining decreases after acid exposure, consistent with the partial denaturation of some HLA-I complexes. (e, f, i, j) HC10 and L31 mAb clearly bind trophoblast only after acid treatment. (g, k) Detection of the non-HLA-I antigen BC-1 is not influenced by acid treatment.
Final comments
Our experiments show that the majority of HLA-C molecules at the trophoblast cell surface are present in a conventional β2m-associated conformation. We found no evidence that disulphide-linked multimeric complexes or open conformers were present. In comparison with HLA-A and HLA-B, HLA-C molecules have reduced surface expression on somatic cells.20,21 The reasons for this are not entirely clear22 but some data imply that restricted peptide binding limits egress from the endoplasmic reticulum and affects the stability of the heterotrimeric complexes at the cell surface.23,24 Our studies of trophoblast cells suggest a stable HLA-C complex with little degradation to free heavy chains at the cell surface. This may result from the different peptides available in trophoblast compared to 721.221 cells, as has been shown for HLA-G molecules.25 The stable β2m-associated conformation of HLA-C molecules at the trophoblast cell surface appears to be different from that in normal somatic cells, where open conformers are present as shown by reactivity with mAb HC10 and L31. It will be difficult to definitively confirm these results because somatic cells express both HLA-A and HLA-B molecules and there are no HLA-C-specific mAb. Trophoblast cells are unique in not only lacking HLA-A or HLA-B expression but also expressing HLA-G and HLA-E.
These findings have obvious implications for the recognition of invading trophoblast by decidual leucocytes. Stability of the β2m-associated conformation of HLA-C may be the result of trophoblast-derived peptides and it is also known that the peptides bound to HLA-C molecules influence the affinity of KIR binding.26 The KIR specific for HLA-C are up-regulated on decidual NK cells compared to peripheral blood.5 These variations might partially explain why, in our immunogenetic study, the risk of pre-eclampsia was associated with HLA-C2 in the fetus independently of the maternal HLA-C group.8 Other innate receptors in the leucocyte receptor complex are LILR, which can also discriminate between different conformations of HLA-I molecules and bind with high affinity to HLA-G dimers, a situation unique to the decidua.6 As about 70% of decidual leucocytes are CD56+ it is also clear that in the placental bed NK cells expressing high levels of KIR will bind to stable HLA-C heterotrimers in an immune synapse that lacks HLA-A or HLA-B molecules. This situation is unique to the maternal–fetal interaction during placentation.
Acknowledgments
This study was funded by the Wellcome Trust and Wellbeing of Women. We would also like to thank Dr B. Uchanska-Ziegler, Dr P. Le Bouteiller, Dr L. Boyle and Dr P. Giacomini for providing the Tü149, B1.23.2, HC10 and L31 mAb, respectively, as well as Jacqui Northfield for technical assistance.
Glossary
Abbreviations:
- HLA
human leucocyte antigen
- HLA-I
HLA class-I
- KIR
killer immunoglobulin-like receptor
- LILR
leucocyte immunoglobulin-like receptor
- LRC
leucocyte receptor complex
- β2m
β2-microglobulin
- mAb
monoclonal antibody
References
- 1.Loke YW, King A, Burrows T, et al. Evaluation of trophoblast HLA-G antigen with a specific monoclonal antibody. Tissue Antigens. 1997;50:135–46. doi: 10.1111/j.1399-0039.1997.tb02852.x. [DOI] [PubMed] [Google Scholar]
- 2.King A, Boocock C, Sharkey AM, Gardner L, Beretta A, Siccardi AG, Loke YW. Evidence for the expression of HLA-C Class I mRNA and protein by human first trimester trophoblast. J Immunol. 1996;156:2068–76. [PubMed] [Google Scholar]
- 3.King A, Burrows TD, Hiby SE, et al. Surface expression of HLA-C antigen by human extravillous trophoblast. Placenta. 2000;21:376–87. doi: 10.1053/plac.1999.0496. [DOI] [PubMed] [Google Scholar]
- 4.King A, Allan DS, Bowen M, et al. HLA-E is expressed on trophoblast and interacts with CD94/NKG2 receptors on decidual NK cells. Eur J Immunol. 2000;30:1623–31. doi: 10.1002/1521-4141(200006)30:6<1623::AID-IMMU1623>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 5.Verma S, King A, Loke YW. Expression of killer cell inhibitory receptors on human uterine natural killer cells. Eur J Immunol. 1997;27:979–83. doi: 10.1002/eji.1830270426. [DOI] [PubMed] [Google Scholar]
- 6.Apps R, Gardner L, Sharkey AM, Holmes N, Moffett A. A homodimeric complex of HLA-G on normal trophoblast modulates antigen-presenting cells via LILRB1. Eur J Immunol. 2007;37:1924–37. doi: 10.1002/eji.200737089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Colonna M, Borsellino G, Falco M, Ferrara GB, Strominger JL. HLA-C is the inhibitory ligand that determines dominant resistance to lysis by NK1- and NK2-specific natural killer cells. Proc Natl Acad Sci U S A. 1993;90:12000–4. doi: 10.1073/pnas.90.24.12000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hiby SE, Walker JJ, O'Shaughnessy KM, Redman CW, Carrington M, Trowsdale J, Moffett A. Combinations of maternal KIR and fetal HLA-C genes influence the risk of preeclampsia and reproductive success. J Exp Med. 2004;200:957–65. doi: 10.1084/jem.20041214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gonen-Gross T, Achdout H, Gazit R, et al. Complexes of HLA-G protein on the cell surface are important for leukocyte Ig-like receptor-1 function. J Immunol. 2003;171:1343–51. doi: 10.4049/jimmunol.171.3.1343. [DOI] [PubMed] [Google Scholar]
- 10.Shiroishi M, Kuroki K, Ose T, et al. Efficient leukocyte Ig-like receptor signaling and crystal structure of disulfide-linked HLA-G dimer. J Biol Chem. 2006;281:10439–47. doi: 10.1074/jbc.M512305200. [DOI] [PubMed] [Google Scholar]
- 11.Gonen-Gross T, Achdout H, Arnon TI, et al. The CD85J/leukocyte inhibitory receptor-1 distinguishes between conformed and beta 2-microglobulin-free HLA-G molecules. J Immunol. 2005;175:4866–74. doi: 10.4049/jimmunol.175.8.4866. [DOI] [PubMed] [Google Scholar]
- 12.Arosa FA, Santos SG, Powis SJ. Open conformers: the hidden face of MHC-I molecules. Trends Immunol. 2007;28:115–23. doi: 10.1016/j.it.2007.01.002. [DOI] [PubMed] [Google Scholar]
- 13.Giacomini P, Beretta A, Nicotra MR, et al. HLA-C heavy chains free of beta2-microglobulin: distribution in normal tissues and neoplastic lesions of non-lymphoid origin and interferon-gamma responsiveness. Tissue Antigens. 1997;50:555–66. doi: 10.1111/j.1399-0039.1997.tb02913.x. [DOI] [PubMed] [Google Scholar]
- 14.Raine T, Brown D, Bowness P, Hill Gaston JS, Moffett A, Trowsdale J, Allen RL. Consistent patterns of expression of HLA class I free heavy chains in healthy individuals and raised expression in spondyloarthropathy patients point to physiological and pathological roles. Rheumatology (Oxford) 2006;45:1338–44. doi: 10.1093/rheumatology/kel305. [DOI] [PubMed] [Google Scholar]
- 15.Sernee MF, Ploegh HL, Schust DJ. Why certain antibodies cross-react with HLA-A and HLA-G: epitope mapping of two common MHC class I reagents. Mol Immunol. 1998;35:177–88. doi: 10.1016/s0161-5890(98)00026-1. [DOI] [PubMed] [Google Scholar]
- 16.Trundley A, Gardner L, Northfield J, Chang C, Moffett A. Methods for isolation of cells from the human fetal–maternal interface. Methods Mol Med. 2006;122:109–22. doi: 10.1385/1-59259-989-3:109. [DOI] [PubMed] [Google Scholar]
- 17.Bird LA, Peh CA, Kollnberger S, Elliott T, McMichael AJ, Bowness P. Lymphoblastoid cells express HLA-B27 homodimers both intracellularly and at the cell surface following endosomal recycling. Eur J Immunol. 2003;33:748–59. doi: 10.1002/eji.200323678. [DOI] [PubMed] [Google Scholar]
- 18.Polakova K, Karpatova M, Russ G. Dissociation of beta 2-microglobulin is responsible for selective reduction of HLA class I antigenicity following acid treatment of cells. Mol Immunol. 1993;30:1223–30. doi: 10.1016/0161-5890(93)90037-c. [DOI] [PubMed] [Google Scholar]
- 19.Loke YW, His BL, Bulmer JN, Grivaux C, Hawley S, Gardner L, King A, Carter NP. Evaluation of a monoclonal antibody BC-1, which identifies an antigen expressed on the surface membrane of human extravillous trophoblast. Am J Reprod Immunol. 1992;27:77–81. doi: 10.1111/j.1600-0897.1992.tb00727.x. [DOI] [PubMed] [Google Scholar]
- 20.Snary D, Barnstable CJ, Bodmer WF, Crumpton MJ. Molecular structure of human histocompatibility antigens: the HLA-C series. Eur J Immunol. 1977;7:580–5. doi: 10.1002/eji.1830070816. [DOI] [PubMed] [Google Scholar]
- 21.Zemmour J, Parham P. Distinctive polymorphism at the HLA-C locus: implications for the expression of HLA-C. J Exp Med. 1992;176:937–50. doi: 10.1084/jem.176.4.937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McCutcheon JA, Gumperz J, Smith KD, Lutz CT, Parham P. Low HLA-C expression at cell surfaces correlates with increased turnover of heavy chain mRNA. J Exp Med. 1995;181:2085–95. doi: 10.1084/jem.181.6.2085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Neisig A, Melief CJ, Neefjes J. Reduced cell surface expression of HLA-C molecules correlates with restricted peptide binding and stable TAP interaction. J Immunol. 1998;160:171–9. [PubMed] [Google Scholar]
- 24.Sibilio L, Martayan A, Setini A, Lo Monaco E, Tremante E, Butler RH, Giacomini P. A single bottleneck in HLA-C assembly. J Biol Chem. 2007 doi: 10.1074/jbc.M708068200. DOI: 10.1074/jbc.M708068200 in press. [DOI] [PubMed] [Google Scholar]
- 25.Ishitani A, Sageshima N, Lee N, Dorofeeva N, Hatake K, Marquardt H, Geraghty DE. Protein expression and peptide binding suggest unique and interacting functional roles for HLA-E, -F, and -G in maternal–placental immune recognition. J Immunol. 2003;171:1376–84. doi: 10.4049/jimmunol.171.3.1376. [DOI] [PubMed] [Google Scholar]
- 26.Hansasuta P, Dong T, Thananchai H, Weekes M, Willberg C, Aldemir H, Rowland-Jones S, Braud V. Recognition of HLA-A3 and HLA-A11 by KIR3DL2 is peptide-specific. Eur J Immunol. 2004;34:1673–9. doi: 10.1002/eji.200425089. [DOI] [PubMed] [Google Scholar]
- 27.Tran TM, Ivanyi P, Hilgert I, et al. The epitope recognized by pan-HLA class I-reactive monoclonal antibody W6/32 and its relationship to unusual stability of the HLA-B27/beta2-microglobulin complex. Immunogenetics. 2001;53:440–6. doi: 10.1007/s002510100353. [DOI] [PubMed] [Google Scholar]
- 28.Barnstable CJ, Bodmer WF, Brown G, Galfre G, Milstein C, Williams AF, Ziegler A. Production of monoclonal antibodies to group A erythrocytes, HLA and other human cell surface antigens – new tools for genetic analysis. Cell. 1978;14:9–20. doi: 10.1016/0092-8674(78)90296-9. [DOI] [PubMed] [Google Scholar]
- 29.Rebai N, Malissen B. Structural and genetic analyses of HLA class I molecules using monoclonal xenoantibodies. Tissue Antigens. 1983;22:107–17. doi: 10.1111/j.1399-0039.1983.tb01176.x. [DOI] [PubMed] [Google Scholar]
- 30.Hutter H, Hammer A, Blaschitz A, Hartmann M, Ebbesen P, Dohr G, Ziegler A, Uchanska-Ziegler B. Expression of HLA class I molecules in human first trimester and term placenta trophoblast. Cell Tissue Res. 1996;286:439–47. doi: 10.1007/s004410050713. [DOI] [PubMed] [Google Scholar]
- 31.Uchanska-Ziegler B, Nossner E, Schenk A, Ziegler A, Schendel DJ. Soluble T cell receptor-like properties of an HLA-B35-specific monoclonal antibody (TU165) Eur J Immunol. 1993;23:734–8. doi: 10.1002/eji.1830230325. [DOI] [PubMed] [Google Scholar]
- 32.Perosa F, Luccarelli G, Prete M, Favoino E, Ferrone S, Dammacco F. Beta 2-microglobulin-free HLA class I heavy chain epitope mimicry by monoclonal antibody HC-10-specific peptide. J Immunol. 2003;171:1918–26. doi: 10.4049/jimmunol.171.4.1918. [DOI] [PubMed] [Google Scholar]
- 33.Stam NJ, Spits H, Ploegh HL. Monoclonal antibodies raised against denatured HLA-B locus heavy chains permit biochemical characterization of certain HLA-C locus products. J Immunol. 1986;137:2299–306. [PubMed] [Google Scholar]
- 34.Martayan A, Fiscella M, Setini A, et al. Conformation and surface expression of free HLA-CW1 heavy chains in the absence of beta 2-microglobulin. Hum Immunol. 1997;53:23–33. doi: 10.1016/S0198-8859(96)00256-X. [DOI] [PubMed] [Google Scholar]
- 35.Marozzi A, Meneveri R, De Santis C, Robbioni P, Molteni E, Beretta A, Siccardi AG, Ginelli E. Expression of distinct conformations of free HLA-Cw4 heavy chains in transfected neuroblastoma cells. Immunogenetics. 1996;43:289–95. doi: 10.1007/BF02440996. [DOI] [PubMed] [Google Scholar]
- 36.Beretta A, Grassi F, Pelagi M, et al. HIV env glycoprotein shares a cross-reacting epitope with a surface protein present on activated human monocytes and involved in antigen presentation. Eur J Immunol. 1987;17:1793–8. doi: 10.1002/eji.1830171218. [DOI] [PubMed] [Google Scholar]