Abstract
Toll-like receptors (TLRs) play an important role in innate immunity while, β2-adrenergic receptors (β2AR) provide the key linkages for the sympathetic nervous system to regulate the immune system. However, their role in macrophages remains uncertain. Here, we demonstrate the cross-talk between β2AR and TLR signalling pathways. Expression of β2AR was down-regulated by TLR4 ligand lipopolysaccharide (LPS) stimulation. To investigate the physiological consequence of this down-regulation RAW264 cells, a macrophage cell line, were transfected with a β2AR expression vector (RAWar). Both LPS-stimulated inducible nitric oxide synthase (NOS II) expression and NO production were markedly suppressed in the RAWar cells. The activation of nuclear factor-κB (NF-κB) and degradation of the inhibitor of NF-κB (IκBα) in response to LPS were markedly decreased in these cells. The level of β-arrestin 2, which regulates β2AR signalling, was also reduced in RAW264 cells after stimulation with LPS, but not in RAWar cells. Overexpression of β-arrestin 2 (RAWarr2) also inhibited NO production and NOS II expression. Furthermore, we demonstrated that β-arrestin 2 interacted with cytosolic IκBα and that the level of IκBα coimmunoprecipitated by anti-β-arrestin 2 antibodies was decreased in the RAW264 cells but not in RAWar or RAWarr2 cells. These findings suggest that LPS-stimulated signals suppress β2AR expression, leading to down-regulation of β-arrestin 2 expression, which stabilizes cytosolic IκBα and inhibits the NF-κB activation essential for NOS II expression, probably to ensure rapid and sufficient production of NO in response to microbial attack.
Keywords: β2-adrenergic receptor, monocytes/macrophages, nitric oxide, nuclear factor-κB, toll-like receptor
Introduction
The ability of the innate immune system to recognize and respond to microbial components has been chiefly attributed to a family of type I transmembrane receptors termed Toll-like receptors (TLRs) that are expressed abundantly on antigen-presenting cells such as macrophages and dendritic cells and can discriminate among the distinct molecular patterns associated with microbial components.1,2 The TLR-initiated activation of nuclear factor-κB (NF-κB) is essential for the regulation of inducible nitric oxide synthase (NOS II) and several proinflammatory cytokines, which are produced in response to invading pathogens. The NO produced by NOS II has a number of important biological functions, including roles in host defence against intracellular pathogens and tumour-cell killing. Although this basic definition is still accepted, over the past decade NO has been shown to play a much more diverse role not only in the immune system but also in other organ systems, including both beneficial and detrimental effects.3,4 For example, the systemic inflammatory response syndrome, which includes severe septic shock and multiple organ system failure, remains a leading cause of death in critically ill patients. Therefore, it is necessary to clarify the molecular mechanisms of TLR-initiated signalling that lead to NO production in response to microbial components.
Nuclear factor-κB is found predominantly in the cytoplasm complexed with members of the inhibitor of NF-κB (IκB) family. The release of NF-κB from IκB proteins is an essential step in the generation of transcriptionally competent NF-κB. The consensus is that IκB proteins mask the nuclear localization signals of NF-κB proteins, thereby regulating NF-κB activity, primarily by limiting their nuclear translocation. Recent studies, however, have indicated that IκBα is detected in both the nucleus and cytoplasm and that although the NF-κB complexes shuttle between the nucleus and cytoplasm under all conditions, they are unable to bind DNA because of their association with proteins of the IκB family.5–7 Nuclear IκBα is not sensitive to signal-induced degradation. Therefore, following stimulation, NF-κB activities are dependent on the level of cytoplasmic NF-κB/IκBα complexes.
Recently, we demonstrated that the level of β2-adrenergic receptor (β2AR) expression influences TLR4 signalling.8β2AR is a member of a family of G protein-coupled receptors (GPCRs) and is the key link involved in immune system regulation via the sympathetic nervous system.9,10 Primary and secondary lymphoid organs, such as the thymus, spleen and lymph nodes, receive extensive sympathetic/noradrenergic innervation, and lymphocytes, macrophages and many other immune cells bear functional β2AR. Therefore, β2AR stimulation regulates proinflammatory cytokine production, lymphocyte traffic and proliferation, and antibody secretion through cyclic adenosine monophosphate (cAMP) generation and protein kinase A (PKA) activation.10,11 However, the role of β2AR in the TLR signalling pathway in macrophages remains vague. On the other hand, arrestins are cytosolic proteins that play a critical role in the regulation of GPCR signalling.12,13 Recent studies have shown that they also interact with their partner molecules in a variety of signalling pathways, including NF-κB signalling.14–16 In the present study, we investigated the physiological consequence of the down-regulation of β2AR expression in macrophages and analysed the cross-talk between the signalling of β2AR and TLRs.
Materials and methods
Cell culture
The murine macrophage cell line RAW264 (RCB0535) was purchased from RIKEN Cell Bank (Ibaraki, Japan) and cultured as described in our previous study.17 The cells were stimulated with 1 μg/ml lipopolysaccharide (LPS) from Escherichia coli 055 (Sigma-Aldrich, St Louis, MO). Cell viability was assessed using the trypan blue dye exclusion test and cell size was measured by flow cytometric analysis of forward light scatter characteristics using a FACSCalibur flow cytometer (Becton Dickinson, Mountain View, CA).
Electrophoretic mobility shift assay (EMSA)
Nuclear extracts were prepared as described elsewhere.18 The NF-κB oligonucleotide probe (5′-AGT TGA GGG GAC TTT CCC AGG-3′) was purchased from Promega (Madison, WI) and labelled with biotin at its 3′ end. The nuclear protein (2 μg) and excess amounts of labelled oligonucleotide probes were incubated in 20 μl EMSA buffer [20 mm HEPES, pH 7·6, 10 mm (NH4)2SO4, 1 mm dithiothreitol, 1 mm ethylenediaminetetraacetic acid (EDTA), 0·2% Tween, 30 mm KCl, 1 μg poly (dI-dC), 1 μg poly l-lysine] at room temperature for 15 min, electrophoresed in 7% polyacrylamide gels, transferred onto the Biodyne Plus Membane (Pall BioSupport Division, Port Washington, NY), and cross-linked in ultraviolet light. To detect signals, the blots were incubated with streptavidin–horseradish peroxidase conjugate in a blocking reagent for 15 min and with a chemiluminescent reagent for 5 min. The blots were then exposed to Kodak X Omat AR film (GE Healthcare Bio-Science, Piscataway, NJ).
Western blotting analysis
Cell membrane proteins were prepared using the Plasma Membrane Protein Extraction Kit (Bio Vision, Mountain View, CA). Cytoplasmic protein extracts were prepared as described previously (30). The protein concentration was determined using the Bradford reagent (BioRad, Hercules, CA), and equal amounts of membrane proteins or cytoplasmic proteins were loaded. The samples were separated by 10% sodium dodecyl sulphate–polyacrylamide gel electrophoresis (SDS–PAGE) and transferred on to polyvinylidene difluoride membranes (Applied Biosystems, Foster City, CA). The membranes were blocked with 10% non-fat dried milk in Tris-buffered saline and incubated with goat polyclonal antibodies against β2AR, goat polyclonal antibodies against β-arrestin 2, or rabbit polyclonal antibodies against IκBα and NOS II (Santa Cruz Biotechnology, Santa Cruz, CA); this was followed by incubation with appropriate secondary antibodies (horseradish peroxidase-conjugated rabbit anti-goat or goat anti-rabbit immunoglobulin G; Dako, Kyoto, Japan). To ensure equal protein loading, the membranes were incubated with rabbit anti-actin or anti-glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (Santa Cruz Biotechnology) for the detection of cytoplasmic or cell surface GAPDH19 after stripping. Immunoreactivity was visualized using an enhanced chemiluminescence reagent (ECL; GE Healthcare Bio-Science).
Immunoprecipitation
The cells were lysed with lysis buffer (20 mm Tris–HCl, pH 7·6, 150 mm NaCl, 2 mm EDTA, 0·5% Nonidet P-40 and protease inhibitors). The samples were clarified by centrifugation at 21 000 g at 4° for 30 min. The protein concentration was determined using the Bradford reagent (Bio-Rad). β-Arrestin 2 was immunoprecipitated with anti-β-arrestin 2 monoclonal antibodies (Santa Cruz Biotechnology) from equal samples, followed by treatment with 10 μl protein G–Sepharose beads (GE Healthcare Bio-Science). After extensive washing, the complexes were analysed by SDS–PAGE and Western blotting by using rabbit polyclonal antibodies against IκBα.
Determination of nitrite concentration
Nitrite in the cell culture supernatants was measured using the assay system of Ding et al.20 The nitrite concentration was calculated by comparison with sodium nitrite, which was used as a standard. In some experiments, 200 μm pyrrolidine dithiocarbamate (PDTC, Sigma) was added to the cultures.
Determination of intracellular cAMP concentration
Cells were cultured with or without LPS for 6 hr and were stimulated with Salbutamol (1 × 10−6 m) for the final 30 min. Cell supernatants were then removed and cells were lysed. Intracellular cAMP was determined with a commercially available enzyme immunoassay (GE Healthcare Bio-Science).
Real-time polymerase chain reaction (PCR)
Total cellular RNA was extracted from cells using the RNeasy Mini Kit (Qiagen, Hilden, Germany), and aliquots of 2 μg were reverse-transcribed with ReverScript I (Wako Pure Chemical Industries, Osaka, Japan) and an oligo-dT(15-mer) (Roche Diagnostics, Indianapolis, IN) at 42° for 50 min. The complementary DNAs (cDNAs) were amplified by PCR under the following conditions using the oligonucleotide primers and cycles listed in Table 1: 94° for 30 seconds, 55° for 30 seconds, and 72° for 30 seconds for NOS II and 18S ribosomal RNA (rRNA), and 94° for 30 seconds, 60° for 30 seconds, and 72° for 30 seconds for total and transfected β2AR and β-arrestin 2. The quantity of the cDNA template included in these reactions and the number of amplification cycles were optimized to ensure that the reactions were stopped during the linear phase of product amplification, thus permitting semiquantitative comparisons of messenger RNA (mRNA) abundance between different RNA preparations.
Table 1.
Oligonucleotide sequences used for polymerase chain reaction
| Forward | Reverse | Cycle | |
|---|---|---|---|
| β2AR | GGAGCAGGATGGGCGGACGG | GCCTTCCATGCCTGGGGGAT | 34 |
| Transfected β2AR | GGAGCAGGATGGGCGGACGG | TGGTGATGGTGATGATGACC | 34 |
| β-arrestin 2 | GCAGCCAGGACCAGAGGACA | CCACGCTTCTCTCGGTTGTC | 35 |
| NOS II | CTTCCGAAGTTTCTGGCAGCAGCG | GAGCCTCGTGGCTTTGGGCTCCTC | 26 |
| 18S | GAGAAACGGCTACCACATCC | CCCAAGATCCAACTACGAGC | 26 |
β2AR, β2-adrenergic receptor; NOS II, nitric oxide synthase II.
β2AR and β-arrestin 2 plasmid constructs and stable transfection
Full-length murine β2AR (β2ar) and β-arrestin 2 (βarrestin2) cDNAs were obtained by PCR using the primers 5′-GCTGAATGAAGCTTCCAGGA-3′ (sense) and 5′-GCCTGTATTACAGTGGCGAG-3′ (antisense) for β2AR and 5′-GGCGGGCGGAGGGCGGCGAG-3′ (sense) and 5′-CGTCCTAGCAGAACTGGTCA-3′ (antisense) for β-arrestin 2. The amplified β2AR and β-arrestin 2 fragments were subcloned into the pGEM-T Easy vector (Promega) and then into NotI-digested pcDNA4 (Invitrogen, Carlsbad, CA). The amplified PCR products were sequenced using an automatic DNA sequencer (Applied Biosystems). The plasmid DNA used for transfection was prepared using the EndoFree Plasmid Kit (Qiagen). RAW264 cells were transfected with the pcDNA4 vector, pcDNA4-β2ar, or pcDNA4-βarrestin2 using LipofectAMINE Reagent (Invitrogen). Selection was initiated in a medium containing 500 μg/ml Zeocine (Invitrogen).
Luciferase assays
The full-length murine NOS II promoter fragment was cloned into the pGL3-enhancer luciferase reporter gene vector (Promega) (pGL3-NOS II) as described previously.21 RAW264 cells were transfected using the LipofectAMINE Reagent with constructs containing the luciferase reporter gene, and luciferase activity was determined using the Dual Luciferase Assay System Kit (Promega) as described elsewhere.21 Activity was normalized relative to an internal cotransfected constitutive control (Renilla luciferase expression vector, pRL-TK; Promega). In some experiments, RAW264 cells were transiently cotransfected with the NF-κB-responsive promoter reporter–luciferase construct pNF-κB-Luc (Clontech, Palo Alto, CA) or pGL3-NOS II and pcDNA4-β2ar or IκBα dominant-negative vector pCMV-IκBαM (Clontech).
Statistical analysis
Student's t-test for unpaired samples was used to compare two means. For more than two groups, statistical significance of the data was assessed by analysis of variance. Where significant differences were found, individual comparisons were made between groups using the t-statistic and adjusting the critical value according to the Bonferroni method. Differences were considered significant at P < 0·05. Data in the text and figures are expressed as means ± SEM.
Results
Preventing the down-regulation of β2AR inhibits LPS-stimulated NOS II expression
Levels of both β2AR protein and β2AR mRNA were markedly decreased in RAW264 cells following LPS stimulation (Fig. 1a). To investigate the role of β2AR down-regulation in response to LPS, a stable β2AR transfectant (RAWar) and a vector control (RAWvec) were established. Although the levels of both β2AR protein and mRNA expression were notably decreased in RAWvec cells following LPS stimulation, the down-regulation of β2AR expression was prevented in the RAWar cells (Fig. 1b). The transfected β2AR protein did not have a tag sequence capable of modifying β2AR function so the protein levels of only transfected β2AR could not be analysed. The mRNA levels of transfected β2AR were low in unstimulated RAWar cells but markedly increased in the cells following LPS stimulation (Fig. 1c). In our previous study, we showed that the levels of both protein and mRNA of transfected cDNA cloned into the pcDNA4 vector were low in unstimulated RAW264 cells but were markedly increased in the cells following LPS stimulation.17 Therefore, it appears that total β2AR expression in unstimulated RAWar cells was not much higher than in RAWvec cells and that the decrease in intrinsic β2AR expression in the LPS-stimulated RAWar cells was masked by the increased expression of transfected β2AR as the result of the LPS stimulation. Although, the intracellular cAMP concentration in RAWar cells stimulated with salbutamol was similar to that in RAWvec cells, LPS stimulation decreased the accumulation of intracellular cAMP in RAWvec cells but increased it in RAWar cells (Fig. 1d), suggesting that the transfected β2AR was functionally active. Similar histograms of the distribution of forward light scatter characteristics were observed in RAWvec and RAWar cells, suggesting that the β2AR transfection did not alter the cell size (Fig. 1e). In addition, cell viabilities were more than 98% in both cells.
Figure 1.
Lipopolysaccharide (LPS) stimulation down-regulates β2-adrenergic receptor (β2AR) expression. (a) RAW264 cells were stimulated with LPS. The protein levels of β2AR and GAPDH (loading control) in the plasma membrane were analysed by Western blotting (left panel). The β2AR messenger RNA (mRNA) and 18S ribosomal RNA (rRNA; loading control) were analysed by reverse transcription–polymerase chain reaction (RT-PCR; right upper panel). Bar graphs show the relative intensity of the PCR bands from three separate experiments (mean ± SEM) (right lower panel). *P < 0·01 versus 0 hr. (b) RAW264 cells were transfected with the β2ar construct or vector alone. The protein levels of β2AR and GAPDH (left panel) and mRNA expressions of β2AR and 18S rRNA (right upper panel) were analysed as in (a). Bar graphs show the relative intensities of the PCR bands from three separate experiments (mean ± SEM) (right lower panel). *P < 0·01 versus 0 hr. (c) mRNA expressions of β2AR and 18S rRNA (upper panel) were analysed as in (a). Bar graphs show the relative intensities of the PCR bands from three separate experiments (mean ± SEM) (lower panel). *P < 0·01 versus 0 hr. (d) Cells were cultured with or without LPS for 6 hr and were stimulated with salbutamol (1 × 10−6 m) for the final 30 min. Then, intracellular cyclic AMP concentrations were analysed. *P < 0·05 versus without LPS. (e) Cell size was measured by flow cytometric analysis of forward light scatter characteristics (FSC).
The effects of forced β2AR expression on NO production were examined. The nitrite concentration in the culture supernatants of the LPS-stimulated RAWar cells was considerably lower than in the culture supernatants of the RAWvec cells (Fig. 2a). After stimulation with LPS for 6 hr, a distinct 130 000 molecular weight NOS II protein band was observed in the RAWvec cells but not in the RAWar cells (Fig. 2b). Although a protein band corresponding to NOS II was observed in the RAWar cells after stimulation with LPS for 24 hr, the expression level was apparently lower than in the RAWvec cells. Similar results were obtained on reverse transcription PCR analysis of NOS II mRNA expression (Fig. 2b).
Figure 2.
Forced β2-adenergic receptor (β2AR) expression suppresses nitric oxide (NO) production and nitric oxide synthase II (NOS II) expression. (a) Cells were stimulated with lipopolysaccharide (LPS) for 24 hr, and nitrite accumulation in the supernatants was measured using the Griess reagent. The results are expressed as means ± SEM from three-well cultures. *P < 0·001 versus LPS-stimulated RAW264 or RAWvec cells. (b) The protein levels of NOS II and GAPDH (left panel) and messenger RNA expressions of NOS II and 18S ribosomal RNA were analysed as in A (right upper panel). Bar graphs show the relative intensity of the polymerase chain reaction bands from four separate experiments (mean ± SEM) (right lower panel). *P < 0·01 versus corresponding RAWvec cells. Data shown are representative of three or four separate experiments.
Preventing the down-regulation of β2AR inhibits LPS-stimulated NF-κB activation
Next, the effects of forced β2AR expression on NF-κB activation in response to LPS were analysed. As illustrated in Fig. 3(a), marked NF-κB activation was observed in the RAWvec cells stimulated with LPS for 3 and 6 hr but not in the RAWar cells. The level of cytoplasmic IκBα was decreased in the RAWvec cells after LPS stimulation for 6 hr but this level was not decreased in the RAWar cells (Fig. 3b). To further confirm the role of β2AR in LPS-stimulated NF-κB activation, the effects of forced β2AR expression on NF-κB-dependent gene transcription were analysed. NF-κB-mediated-luciferase reporter activity (Fig. 3c) and NOS II promoter activity (Fig. 3d) after stimulation with LPS were inhibited in cells that were cotransfected with the pcDNA4-β2ar construct (AR) as well as in cells cotransfected with pCMV-IκBαM (DN-κB). These findings suggested that β2AR functions as a negative regulator of NF-κB activation by inhibiting IκBα degradation in LPS-stimulated macrophages. Previously, it has been shown that PDTC blocks NF-κB activation by inhibiting IκBα degradation and subsequently the translocation of NF-κB subunits to the nucleus.22 To elucidate the effects of NF-κB activation on the expression of the responsive gene, Nos2, PDTC was added to the RAW264 cell cultures at several time-points after the addition of LPS, and accumulation of NO in the supernatants was analysed after LPS stimulation for 24 hr. As illustrated in Fig. 3(e), when PDTC was added to cultures at 0–9 hr after the addition of LPS, the NO concentrations in these cultures were markedly lower than those in cultures stimulated with LPS for 24 hr without PDTC (right column), indicating that continuous NF-κB activation is essential for adequate NOS II induction.
Figure 3.
Forced β2-adrenergic receptor (β2AR) expression suppresses nuclear factor-κB (NF-κB) activation. (a) The vector control cells and β2AR transfectant were stimulated with lipopolysaccharide (LPS), and NF-κB activation was analysed by electrophoretic mobility shift assay. (b) The vector control cells and β2AR transfectant were stimulated with LPS, and cytoplasmic inhibitor of NF-κB (IκBα) and GAPDH (loading control) were analysed by Western blotting. (c, d) RAW264 cells were cotransfected with the pNF-κB-Luc vector (c) or the NOS II promoter–luciferase construct (d) and vector (Vec), pcDNA4-β2AR (AR) or pCMV-IκBαM (DN-IκB). The cells were cultured with LPS for 24 hr, and luciferase activities were determined. The results are expressed as means ± SEM from six-well cultures. *P < 0·001 versus cells cotransfected with Vec. (e) Pyrrolidine dithiocarbamate (PDTC) was added to the cultures at the indicated time-points after addition of LPS. Nitrite accumulation in the supernatants at 24 hr of culture was measured using the Griess reagent. The results are expressed as means ± SEM from three-well cultures. The error bars are too small to be distinguishable in the figure (numeric data from the left bar: 3·75 ± 0·18, 5·07 ± 0·22, 4·22 ± 0·07, 5·69 ± 0·12, 10·38 ± 0·06, 15·00 ± 0·05, and 25·20 ±0·28). *P < 0·001 versus LPS-stimulated cells without PDTC. Data shown are representative of two or three separate experiments.
β2AR regulates NF-κB activation through β-arrestins
As β-arrestin 2 has been reported to interact with IκBα,15,16 we examined whether β-arrestin 2 participates in the β2AR-mediated regulation of IκBα degradation and NF-κB activation in response to LPS. The expression of β-arrestin 2 was also down-regulated in the LPS-stimulated RAW264 cells (Fig. 4, left panels). Forced β2AR expression abolished the down-regulation of β-arrestin 2 expression (middle panels), suggesting that β-arrestin 2 expression was regulated by β2AR. Deletion of β2AR by small interfering RNA (siRNA) decreased β-arrestin 2 expression (data not shown), supporting the theory that β-arrestin 2 expression is regulated by β2AR. To investigate the role of β-arrestin 2 down-regulation in response to LPS, a stable β-arrestin 2 transfectant (RAWarr2) was established (Fig. 4, right panels). Since transfection with the vector did not influence NO production (Fig. 1c), cells transfected with β-arrestin 2 were compared with RAW264 cells. As shown in the RAWar cells (Fig. 2), NO production (Fig. 5a) and NOS II protein and mRNA expressions (Fig. 5b) were definitely decreased in the RAWarr2 cells.
Figure 4.
Lipopolysaccharide (LPS) stimulation down-regulates β-arrestin 2 expression. RAW264, RAWar, and RAWarr2 cells were stimulated with LPS, and the protein levels of β-arrestin 2 and GAPDH (upper panel) and messenger RNA expressions of β-arrestin 2 and 18S ribosomal RNA (middle panel) were analysed as in Fig. 1(a). Bar graphs show the relative intensity of the band from three separate experiments (mean ± SEM) (lower panel). *P < 0·01 versus 0 hr.
Figure 5.
Forced β-arrestin 2 expression suppresses nitric oxide (NO) production and nitric oxide synthase II (NOS II) expression. (a) Cells were stimulated with lipopolysaccharide (LPS) for 24 hr, and nitrite accumulation in the supernatants was measured using the Griess reagent. The results are expressed as means ± SEM from three-well cultures. *P < 0·001 versus LPS-stimulated RAW264 cells. (b) The protein levels of NOS II and GAPDH (left panel) and messenger RNA expressions of NOS II and 18S ribosomal RNA (light upper panel) were analysed as in Fig. 1(a). Bar graphs show the relative intensity of the polymerase chain reaction bands from three separate experiments (mean ± SEM) (right lower panel). *P < 0·01 versus corresponding RAW264 cells. Data shown are representative of three to four separate experiments.
Anti-β-arrestin 2 antibodies coimmunoprecipitated IκBα in RAW264 cells before, but not after, LPS stimulation for 6 hr (Fig. 6). On the other hand, the amount of IκBα coprecipitated by anti-β-arrestin 2 antibodies was not reduced but rather was increased in the RAWar and RAWarr2 cells after LPS stimulation, indicating that the LPS-stimulated down-regulation of β2AR and β-arrestin 2 is essential for IκBα degradation.
Figure 6.
β-arrestin 2 interacts with cytosolic inhibitor of NF-κB (IκBα). Before and after stimulation with lipopolysaccharide (LPS) for 6 hr, cells were lysed and immunoprecipitated with anti-β-arrestin 2 antibodies. Western blotting analysis was performed using anti-IκBα antibodies (upper panel). The protein levels of GAPDH in equal amounts of lysates were used for control (lower panel).
Discussion
In this study, we investigated the role played by β2AR in the antimicrobial responses of macrophages. First, we demonstrated that β2AR expression is decreased by LPS stimulation. To investigate the role of β2AR down-regulation in response to LPS directly, we established a macrophage cell line, RAWar. Prevention of the down-regulation of β2AR expression in RAWar cells resulted in reduced NO production, suggesting that the LPS-associated down-regulation of β2AR expression plays an important role in NO production in macrophages.
Decreases in NOS II mRNA expression were observed in the RAWar cells, indicating that NOS II expression was transcriptionally down-regulated by forced β2AR expression. Prevention of the down-regulation of β2AR expression in the RAWar cells resulted in a marked decrease in NF-κB activation and inhibited cytosolic IκBα degradation, indicating that the forced β2AR expression inhibited LPS-induced NF-κB activation by IκBα stabilization.
On the other hand, β-arrestins, which are universally expressed members of the arrestin family, are the major regulators of GPCR signalling and bind to activated GPCRs, causing receptor desensitization and internalization.14 Recently, β-arrestins have been shown to play functional roles in the regulation of a variety of signalling pathways and in the mediation of cross-talk between signalling pathways. Moreover, there is accumulating evidence that β-arrestin 2, which is expressed abundantly in the spleen, is functionally involved in some important immune responses.23–26 We have demonstrated that β-arrestin 2 is down-regulated in LPS-stimulated RAW264 cells. Down-regulation of β-arrestin 2 was abolished in RAWar cells, suggesting that β-arrestin 2 expression is regulated by β2AR. These findings suggest that β2AR participates in signal transduction pathways from TLR4 by regulating the level of β-arrestin 2 expression. Meanwhile, the amount of IκBα coimmunoprecipitated by anti-β-arrestin 2 antibodies was decreased in the RAW264 cells after their stimulation with LPS but not in the RAWar or RAWarr2 cells, suggesting that β2AR inhibited LPS-induced NF-κB activation by stabilizing IκBα through β-arrestin 2. The release of NF-κB following the degradation of IκBα proteins is an essential step in the generation of transcriptionally competent NF-κB. In addition, NF-κB activity following stimulation is dependent on the level of cytoplasmic NF-κB/IκBα complexes free from stabilizing factors. Therefore, the following appear likely: (1) LPS-stimulated signals suppress β2AR expression, (2) the reduction of β2AR results in the down-regulation of β-arrestin 2 expression, (3) β-arrestin 2 stabilizes cytoplasmic IκBα and inhibits NF-κB activation (so reduction in the level of β-arrestin 2 accelerates IκBα degradation and NF-κB activation in LPS-stimulated cells) and (4) nuclear translocation of NF-κB enhances NOS II expression.
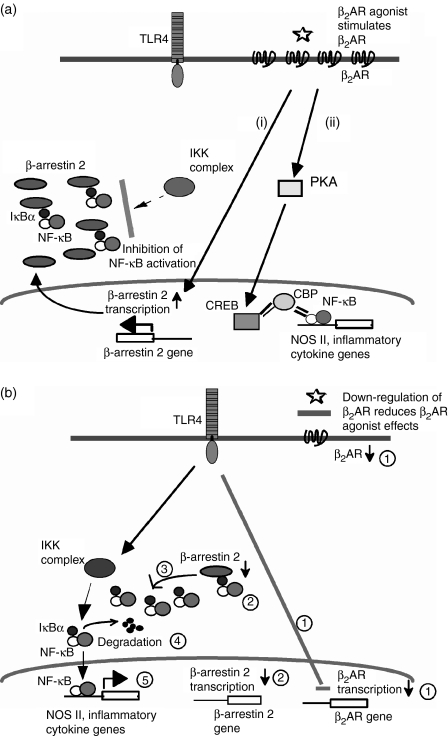
The cross-talk between β2AR and the TLR signalling pathways is schematically summarized in Fig. 7.
Figure 7.
Cross-talk between β2-adrenergic receptor (β2AR) and Toll-like receptor (TLR) signalling pathways. (a) β2AR agonists suppress nuclear factor-κB (NF-κB) activation by increasing cytoplasmic β-arrestin 2, which stabilizes the NF-κB/inhibitor of NF-κB (IκBα) complexes in cytoplasm (i) or by activating cAMP response element binding protein (CREB), which then produces competition between CREB-binding protein (CBP) and NF-κB in the nucleus (ii). (b) TLR4-dependent signals lead to the following steps both in the presence or absence of β2AR agonists:  TLR4-dependent down-regulation of β2AR expression,
TLR4-dependent down-regulation of β2AR expression,  down-regulation of β-arrestin 2,
down-regulation of β-arrestin 2,  release of NF-κB/IκBα complexes in the cytoplasm,
release of NF-κB/IκBα complexes in the cytoplasm,  degradation of IκBα, and
degradation of IκBα, and  translocation of NF-κB to the nucleus and transcription of its target genes.
translocation of NF-κB to the nucleus and transcription of its target genes.
Catecholamines increase cAMP via β2AR activation, and PKA activation inhibits NF-κB-induced transcription by phosphorylating cAMP responsive element binding protein (CREB), which competes with p65 for the limited amounts of CREB-binding protein (CBP) (Fig. 7a(ii)).27 However, β2AR agonists did not suppress NO production (unpublished observation). In the present study, we demonstrated that LPS stimulation suppressed the cAMP accumulation in RAWvec cells stimulated with a β2AR agonist. In addition, we showed that prevention of the down-regulation of β2AR inhibits the degradation of IκBα through β-arrestin 2, which stabilizes IκBα in the steady state (Fig. 7a(ii)). Therefore, the down-regulation of expression of both β2AR and β-arrestin 2 by the TLR4-dependent pathway might provide a mechanism for ‘escaping’ anti-proinflammatory signals, such as the β2AR–cAMP–PKA pathway27 or the β2AR–β-arrestin 2–IκBα pathway. As the levels of β2AR ligands vary under different conditions, understanding the cross-talk between TLRs and β2AR pathways may have both physiological and pathophysiological importance. Taken together, the observations of the present study regarding the regulation of TLR4 signalling through β2AR appear to provide another therapeutic target for the regulation of inflammatory disease conditions.
Acknowledgments
We thank Dr T. Seya (Hokkaido University, Sapporo, Japan) for providing helpful comments. This study was supported in part by Grants-in-Aid for Scientific Research (including the Academic Frontier Project) from the Japanese Ministry of Education, Culture, Sports, Science and Technology and by a Grant-in-Aid for Promotion and Mutual Aid Corporation for Private Schools of Japan (to T.K. and H.O.).
References
- 1.Takeda K, Kaisho T, Akira S. Toll-like receptors. Annu Rev Immunol. 2003;21:335–76. doi: 10.1146/annurev.immunol.21.120601.141126. [DOI] [PubMed] [Google Scholar]
- 2.Janeway CA, Jr, Medzhitov R. Innate immune recognition. Annu Rev Immunol. 2002;20:197–216. doi: 10.1146/annurev.immunol.20.083001.084359. [DOI] [PubMed] [Google Scholar]
- 3.Hierholzer C, Harbrecht B, Menezes JM, et al. Essential role of induced nitric oxide in the initiation of the inflammatory response after hemorrhagic shock. J Exp Med. 1998;187:917–28. doi: 10.1084/jem.187.6.917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bogdan C. Nitric oxide and the immune response. Nat Immunol. 2001;2:907–16. doi: 10.1038/ni1001-907. [DOI] [PubMed] [Google Scholar]
- 5.Lain de Lera T, Folgueira L, Martin AG, et al. Expression of IkappaBalpha in the nucleus of human peripheral blood T lymphocytes. Oncogene. 1999;18:1581–8. doi: 10.1038/sj.onc.1202455. [DOI] [PubMed] [Google Scholar]
- 6.Tergaonkar V, Correa RG, Ikawa M, Verma IM. Distinct roles of IkappaB proteins in regulating constitutive NF-kappaB activity. Nat Cell Biol. 2005;7:921–3. doi: 10.1038/ncb1296. [DOI] [PubMed] [Google Scholar]
- 7.Rodriguez MS, Thompson J, Hay RT, Dargemont C. Nuclear retention of IkappaBalpha protects it from signal-induced degradation and inhibits nuclear factor kappaB transcriptional activation. J Biol Chem. 1999;274:9108–15. doi: 10.1074/jbc.274.13.9108. [DOI] [PubMed] [Google Scholar]
- 8.Itoh CE, Kizaki T, Hitomi Y, et al. Down-regulation of beta2-adrenergic receptor expression by exercise training increases IL-12 production by macrophages following LPS stimulation. Biochem Biophys Res Commun. 2004;322:979–84. doi: 10.1016/j.bbrc.2004.08.050. [DOI] [PubMed] [Google Scholar]
- 9.Downing JE, Miyan JA. Neural immunoregulation: emerging roles for nerves in immune homeostasis and disease. Immunol Today. 2000;21:281–9. doi: 10.1016/s0167-5699(00)01635-2. [DOI] [PubMed] [Google Scholar]
- 10.Elenkov IJ, Wilder RL, Chrousos GP, Vizi ES. The sympathetic nerve – an integrative interface between two supersystems: the brain and the immune system. Pharmacol Rev. 2000;52:595–638. [PubMed] [Google Scholar]
- 11.Kohm AP, Sanders VM. Norepinephrine and beta 2-adrenergic receptor stimulation regulate CD4+ T and B lymphocyte function in vitro and in vivo. Pharmacol Rev. 2001;53:487–525. [PubMed] [Google Scholar]
- 12.Ferguson SS, Downey WE, 3rd, Colapietro AM, Barak LS, Menard L, Caron MG. Role of beta-arrestin in mediating agonist-promoted G protein-coupled receptor internalization. Science. 1996;271:363–6. doi: 10.1126/science.271.5247.363. [DOI] [PubMed] [Google Scholar]
- 13.Ogasawara J, Sanpei M, Rahman N, Sakurai T, Kizaki T, Hitomi Y, Ohno H, Izawa T. β-adrenergic receptor trafficking by exercise in rat adipocytes: roles of G-protein-coupled receptor kinase-2, β-arrestin-2, and the ubiquitin–proteasome pathway. FASEB J. 2006;20:350–2. doi: 10.1096/fj.05-4688fje. [DOI] [PubMed] [Google Scholar]
- 14.Luttrell LM, Lefkowitz RJ. The role of β-arrestins in the termination and transduction of G-protein-coupled receptor signals. J Cell Sci. 2002;115:455–65. doi: 10.1242/jcs.115.3.455. [DOI] [PubMed] [Google Scholar]
- 15.Witherow DS, Garrison TR, Miller WE, Lefkowitz RJ. β-arrestin inhibits NF-κB activity by means of its interaction with the NF-κB inhibitor IκBα. Proc Natl Acad Sci U S A. 2004;101:8603–7. doi: 10.1073/pnas.0402851101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gao H, Sun Y, Wu Y, Luan B, Wang Y, Qu B, Pei G. Identification of β-arrestin2 as a G protein-coupled receptor-stimulated regulator of NF-κB pathways. Mol Cell. 2004;14:303–17. doi: 10.1016/s1097-2765(04)00216-3. [DOI] [PubMed] [Google Scholar]
- 17.Kizaki T, Suzuki K, Hitomi Y, et al. Uncoupling protein 2 plays an important role in nitric oxide production of lipopolysaccharide-stimulated macrophages. Proc Natl Acad Sci U S A. 2002;99:9392–7. doi: 10.1073/pnas.142206299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kizaki T, Ookawara T, Iwabuchi K, et al. Age-associated increase of basal corticosterone levels decreases ED2high, NF-κBhigh activated macrophages. J Leukoc Biol. 2000;68:21–30. [PubMed] [Google Scholar]
- 19.Raje CI, Kumar S, Harle A, Nanda JS, Raje M. The macrophage cell surface glyceraldehyde-3-phosphate dehydrogenase is a novel transferrin receptor. J Biol Chem. 2007;282:3252–61. doi: 10.1074/jbc.M608328200. [DOI] [PubMed] [Google Scholar]
- 20.Ding AH, Nathan CF, Stuehr DJ. Release of reactive nitrogen intermediates and reactive oxygen intermediates from mouse peritoneal macrophages. comparison of activating cytokines and evidence for independent production. J Immunol. 1988;141:2407–12. [PubMed] [Google Scholar]
- 21.Kizaki T, Suzuki K, Hitomi Y, et al. Negative regulation of LPS-stimulated expression of inducible nitric oxide synthase by AP-1 in macrophage cell line J774A.1. Biochem Biophys Res Commun. 2001;289:1031–8. doi: 10.1006/bbrc.2001.6123. [DOI] [PubMed] [Google Scholar]
- 22.Staal FJ, Roederer M, Herzenberg LA, Herzenberg LA. Intracellular thiols regulate activation of nuclear factor κB and transcription of human immunodeficiency virus. Proc Natl Acad Sci U S A. 1990;87:9943–7. doi: 10.1073/pnas.87.24.9943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sun Y, Cheng Z, Ma L, Pei G. β-arrestin2 is critically involved in CXCR4-mediated chemotaxis, and this is mediated by its enhancement of p38 MAPK activation. J Biol Chem. 2002;277:49212–9. doi: 10.1074/jbc.M207294200. [DOI] [PubMed] [Google Scholar]
- 24.Barlic J, Andrews JD, Kelvin AA, et al. Regulation of tyrosine kinase activation and granule release through β-arrestin by CXCRI. Nat Immunol. 2000;1:227–33. doi: 10.1038/79767. [DOI] [PubMed] [Google Scholar]
- 25.Fong AM, Premont RT, Richardson RM, Yu YR, Lefkowitz RJ, Patel DD. Defective lymphocyte chemotaxis in β-arrestin2- and GRK6-deficient mice. Proc Natl Acad Sci U S A. 2002;99:7478–83. doi: 10.1073/pnas.112198299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Walker JK, Fong AM, Lawson BL, Savov JD, Patel DD, Schwartz DA, Lefkowitz RJ. β-arrestin-2 regulates the development of allergic asthma. J Clin Invest. 2003;112:566–74. doi: 10.1172/JCI17265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Parry GC, Mackman N. Role of cyclic AMP response element-binding protein in cyclic AMP inhibition of NF-κB-mediated transcription. J Immunol. 1997;159:5450–6. [PubMed] [Google Scholar]