Abstract
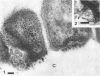
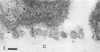
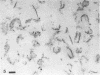
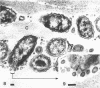
During growth of Bacteroides succinogenes in a liquid medium with cellulose as the source of carbohydrate, greater than 80% of the carboxymethylcellulase (endo-β-1,4-glucanase), xylanase, and aryl-β-xylosidase and 50% of the aryl-β-glucosidase released from cells into the culture fluid. Less than 25% of the cellobiase activity was detected in the culture fluid. Approximately 50% of each of the released enzymes measured was associated with sedimentable subcellular membrane vesicles. The vesicles appeared to be released from the outer membrane of intact cells by bleb formation, primarily in pockets between the cells and the cellulose, although a few unattached cells with blebs were seen. Many vesicles were seen adhering to cellulose, and they were also seen free in the culture fluid. These data suggest that B. succinogenes releases hydrolytic enzymes in nonsedimentable and particulate forms during growth by a mechanism which has until now received little attention. Cellulose incubated in a porous nylon bag in the rumen was colonized by bacteria resembling B. succinogenes, and subcellular vesicles were seen penetrating channels and fractures in the cellulose. On this basis, it is suggested that B. succinogenes cells in the rumen contribute to an extracellular population of subcellular vesicles that possess cellulolytic and hemicellulolytic activities which probably enhance polymer digestion and provide a source of sugars for microbes lacking polymer-degrading activity, thereby contributing to a stable heterogeneous microbial population.
Full text
PDF


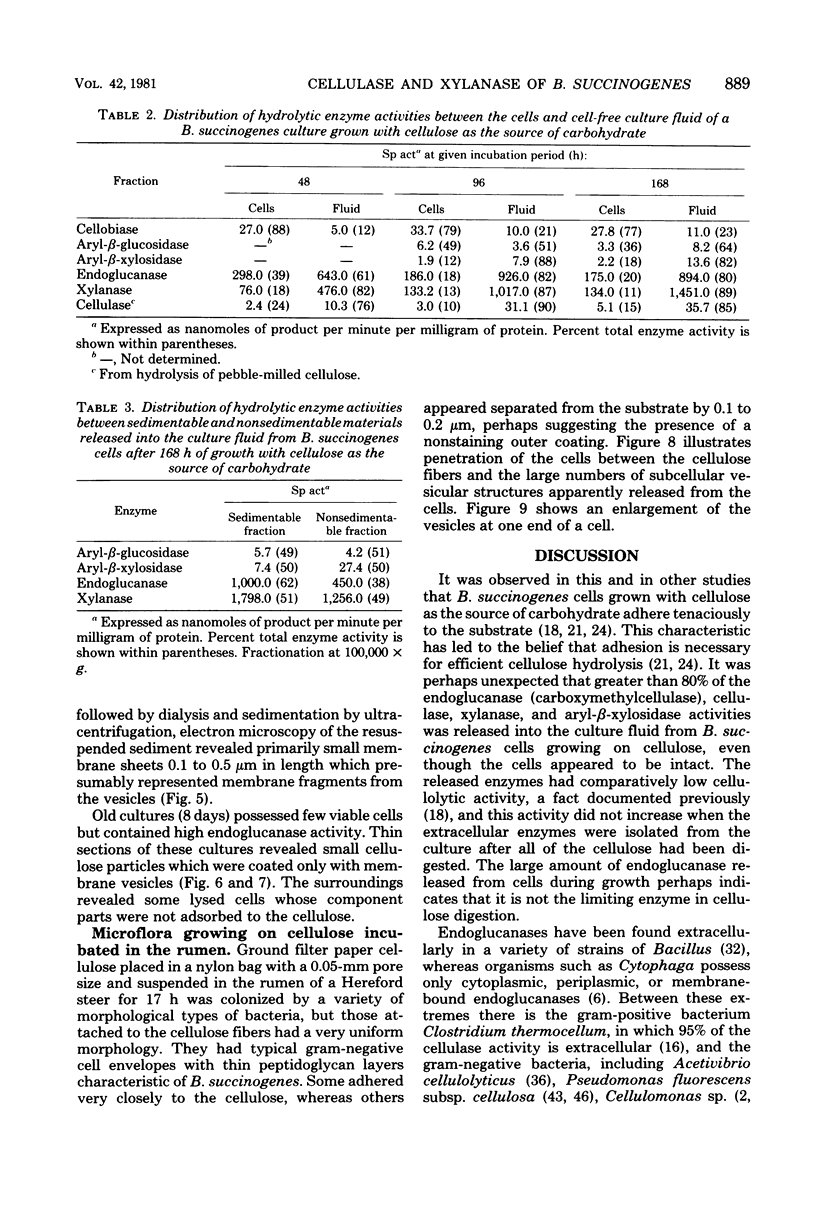
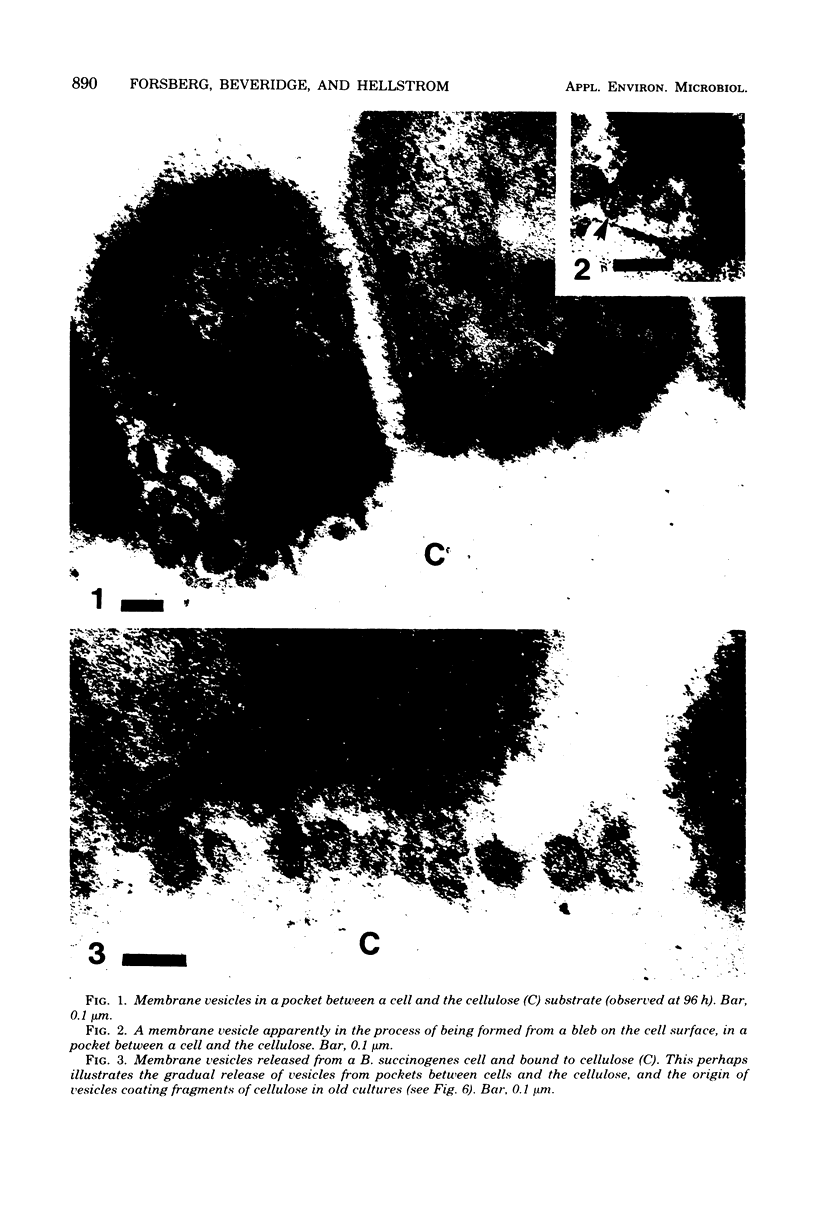
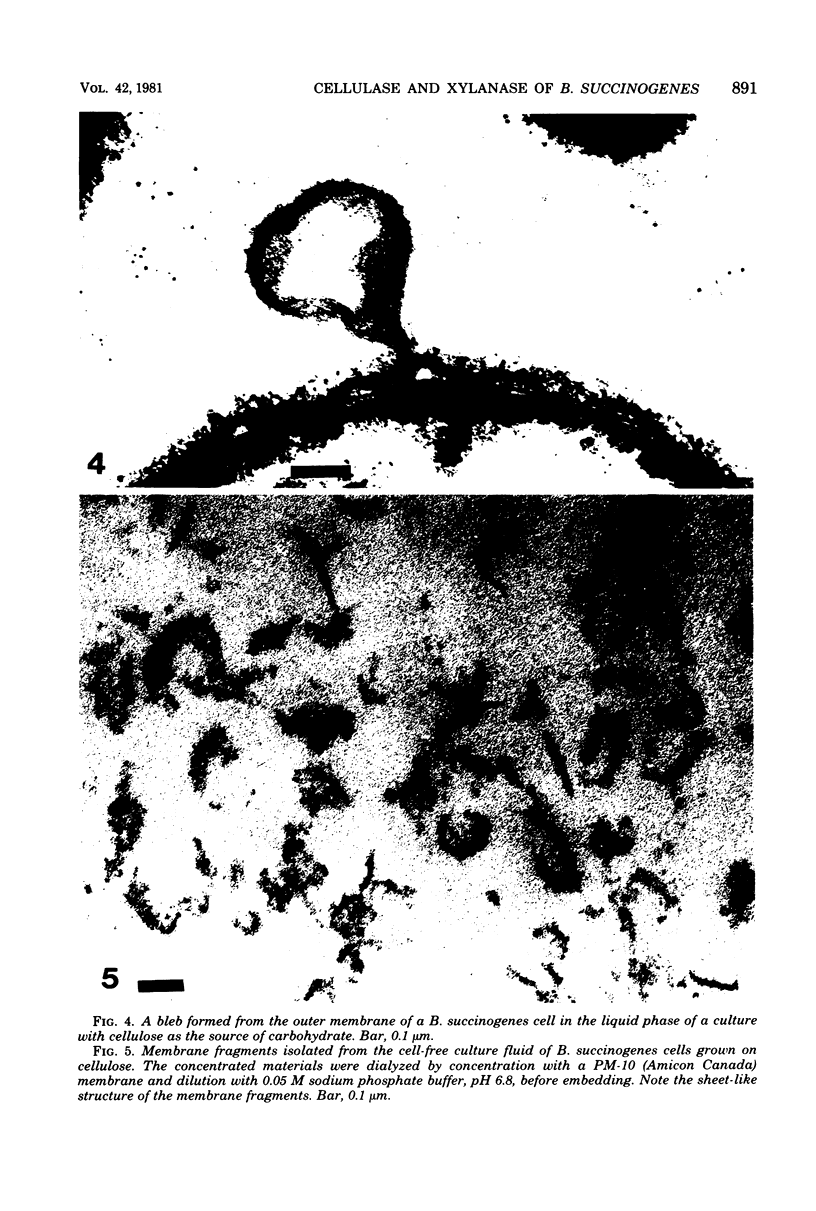

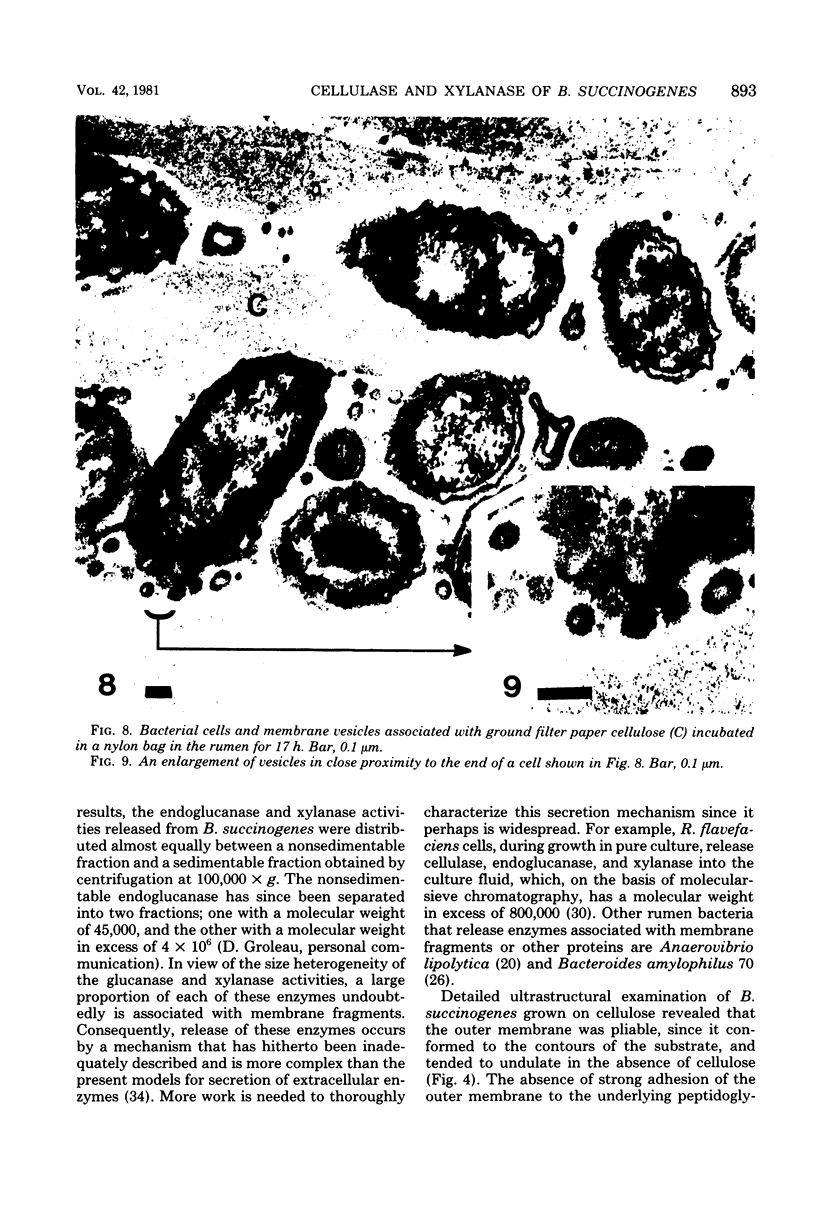



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berg B. Cellulase location in Cellvibrio fulvus. Can J Microbiol. 1975 Jan;21(1):51–57. doi: 10.1139/m75-007. [DOI] [PubMed] [Google Scholar]
- Beveridge T. J., Williams F. M., Koval J. J. The effect of chemical fixatives on cell walls of Bacillus subtilis. Can J Microbiol. 1978 Dec;24(12):1439–1451. doi: 10.1139/m78-233. [DOI] [PubMed] [Google Scholar]
- Bryant M. P. Nutritional requirements of the predominant rumen cellulolytic bacteria. Fed Proc. 1973 Jul;32(7):1809–1813. [PubMed] [Google Scholar]
- Béguin P., Eisen H. Purification and partial characterization of three extracellular cellulases from Cellulomonas sp. Eur J Biochem. 1978 Jul 3;87(3):525–531. doi: 10.1111/j.1432-1033.1978.tb12403.x. [DOI] [PubMed] [Google Scholar]
- Chang W. T., Thayer D. W. The cellulase system of a Cytophaga species. Can J Microbiol. 1977 Sep;23(9):1285–1292. doi: 10.1139/m77-192. [DOI] [PubMed] [Google Scholar]
- Cheng K. J., Costerton J. W. Localization of alkaline phosphatase in three gram-negative rumen bacteria. J Bacteriol. 1973 Oct;116(1):424–440. doi: 10.1128/jb.116.1.424-440.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng K. J., Hironaka R., Costerton J. W. Release of bacterial alkaline phosphatase in the rumen of cattle fed a feedlot bloat-provoking diet or a hay diet. Can J Microbiol. 1976 May;22(5):764–769. doi: 10.1139/m76-111. [DOI] [PubMed] [Google Scholar]
- Costerton J. W., Ingram J. M., Cheng K. J. Structure and function of the cell envelope of gram-negative bacteria. Bacteriol Rev. 1974 Mar;38(1):87–110. doi: 10.1128/br.38.1.87-110.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dehority B. A. Hemicellulose degradation by rumen bacteria. Fed Proc. 1973 Jul;32(7):1819–1825. [PubMed] [Google Scholar]
- Dehority B. A. Mechanism of isolated hemicellulose and xylan degradation by cellulolytic rumen bacteria. Appl Microbiol. 1968 May;16(5):781–786. doi: 10.1128/am.16.5.781-786.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dekker R. F., Richards G. N. Hemicellulases: their occurrence, purification, properties, and mode of action. Adv Carbohydr Chem Biochem. 1976;32:277–352. doi: 10.1016/s0065-2318(08)60339-x. [DOI] [PubMed] [Google Scholar]
- Gawthorne J. M. Extracellular carbohydrase complex from rumen contents. Ann Rech Vet. 1979;10(2-3):249–250. [PubMed] [Google Scholar]
- Groleau D., Forsberg C. W. Cellulolytic activity of the rumen bacterium Bacteroides succinogenes. Can J Microbiol. 1981 May;27(5):517–530. doi: 10.1139/m81-077. [DOI] [PubMed] [Google Scholar]
- HALLIWELL G., BRYANT M. P. THE CELLULOLYTIC ACTIVITY OF PURE STRAINS OF BACTERIA FROM THE RUMEN OF CATTLE. J Gen Microbiol. 1963 Sep;32:441–448. doi: 10.1099/00221287-32-3-441. [DOI] [PubMed] [Google Scholar]
- Henderson C., Hodgkiss W. An electron microscopic study of Anaerovibrio lipolytica (strain 5S) and its lipolytic enzyme. J Gen Microbiol. 1973 Jun;76(2):389–393. doi: 10.1099/00221287-76-2-389. [DOI] [PubMed] [Google Scholar]
- Hungate R. E. Studies on Cellulose Fermentation: III. The Culture and Isolation for Cellulose-decomposing Bacteria from the Rumen of Cattle. J Bacteriol. 1947 May;53(5):631–645. doi: 10.1128/jb.53.5.631-645.1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Latham M. J., Brooker B. E., Pettipher G. L., Harris P. J. Adhesion of Bacteroides succinogenes in pure culture and in the presence of Ruminococcus flavefaciens to cell walls in leaves of perennial ryegrass (Lolium perenne). Appl Environ Microbiol. 1978 Jun;35(6):1166–1173. doi: 10.1128/aem.35.6.1166-1173.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McWethy S. J., Hartman P. A. Purification and some properties of an extracellular alpha-amylase from Bacteroides amylophilus. J Bacteriol. 1977 Mar;129(3):1537–1544. doi: 10.1128/jb.129.3.1537-1544.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberkotter L. V., Rosenberg F. A. Extracellular endo-beta-1,4-glucanase in Cellvibrio vulgaris. Appl Environ Microbiol. 1978 Jul;36(1):205–209. doi: 10.1128/aem.36.1.205-209.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Priest F. G. Extracellular enzyme synthesis in the genus Bacillus. Bacteriol Rev. 1977 Sep;41(3):711–753. doi: 10.1128/br.41.3.711-753.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REYNOLDS E. S. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol. 1963 Apr;17:208–212. doi: 10.1083/jcb.17.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramaley R. F. Molecular biology of extracellular enzymes. Adv Appl Microbiol. 1979;25:37–55. doi: 10.1016/s0065-2164(08)70145-x. [DOI] [PubMed] [Google Scholar]
- SCOTT H. W., DEHORITY B. A. VITAMIN REQUIREMENTS OF SEVERAL CELLULOLYTIC RUMEN BACTERIA. J Bacteriol. 1965 May;89:1169–1175. doi: 10.1128/jb.89.5.1169-1175.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheifinger C. C., Wolin M. J. Propionate formation from cellulose and soluble sugars by combined cultures of Bacteroides succinogenes and Selenomonas ruminantium. Appl Microbiol. 1973 Nov;26(5):789–795. doi: 10.1128/am.26.5.789-795.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanton T. B., Canale-Parola E. Treponema bryantii sp. nov., a rumen spirochete that interacts with cellulolytic bacteria. Arch Microbiol. 1980 Sep;127(2):145–156. doi: 10.1007/BF00428018. [DOI] [PubMed] [Google Scholar]
- Stewart C. S., Paniagua C., Dinsdale D., Cheng K. J., Garrow S. H. Selective isolation and characteristics of Bacteriodes succinogenes from the rumen of a cow. Appl Environ Microbiol. 1981 Feb;41(2):504–510. doi: 10.1128/aem.41.2.504-510.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker D. J. Some properties of xylanase and xylobiase from mixed rumen organisms. Aust J Biol Sci. 1967 Aug;20(4):799–808. doi: 10.1071/bi9670799. [DOI] [PubMed] [Google Scholar]
- Yoshikawa T., Suzuki H., Nisizawa K. Biogenesis of multiple cellulase components of Pseudomonas fluorescens var. cellulosa. I. Effects of culture conditions on the multiplicity of cellulase. J Biochem. 1974 Mar;75(3):531–540. doi: 10.1093/oxfordjournals.jbchem.a130421. [DOI] [PubMed] [Google Scholar]