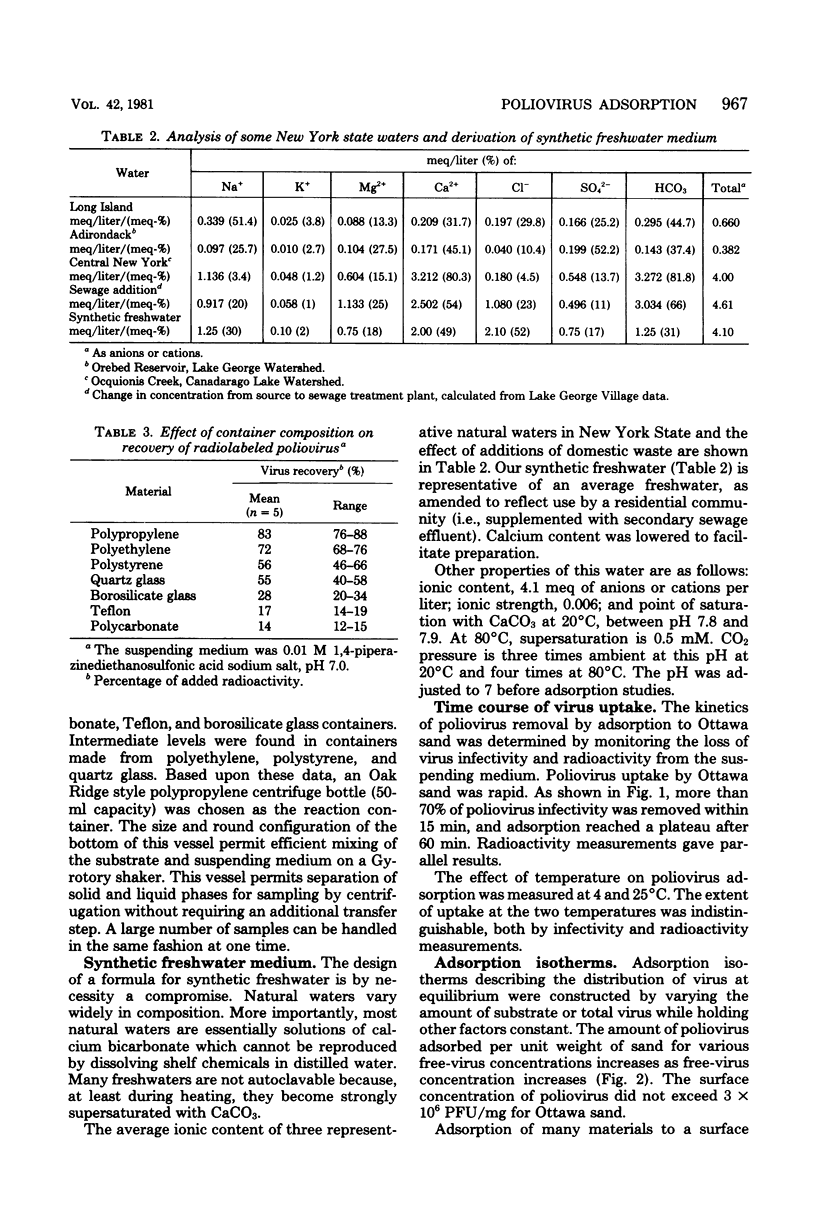
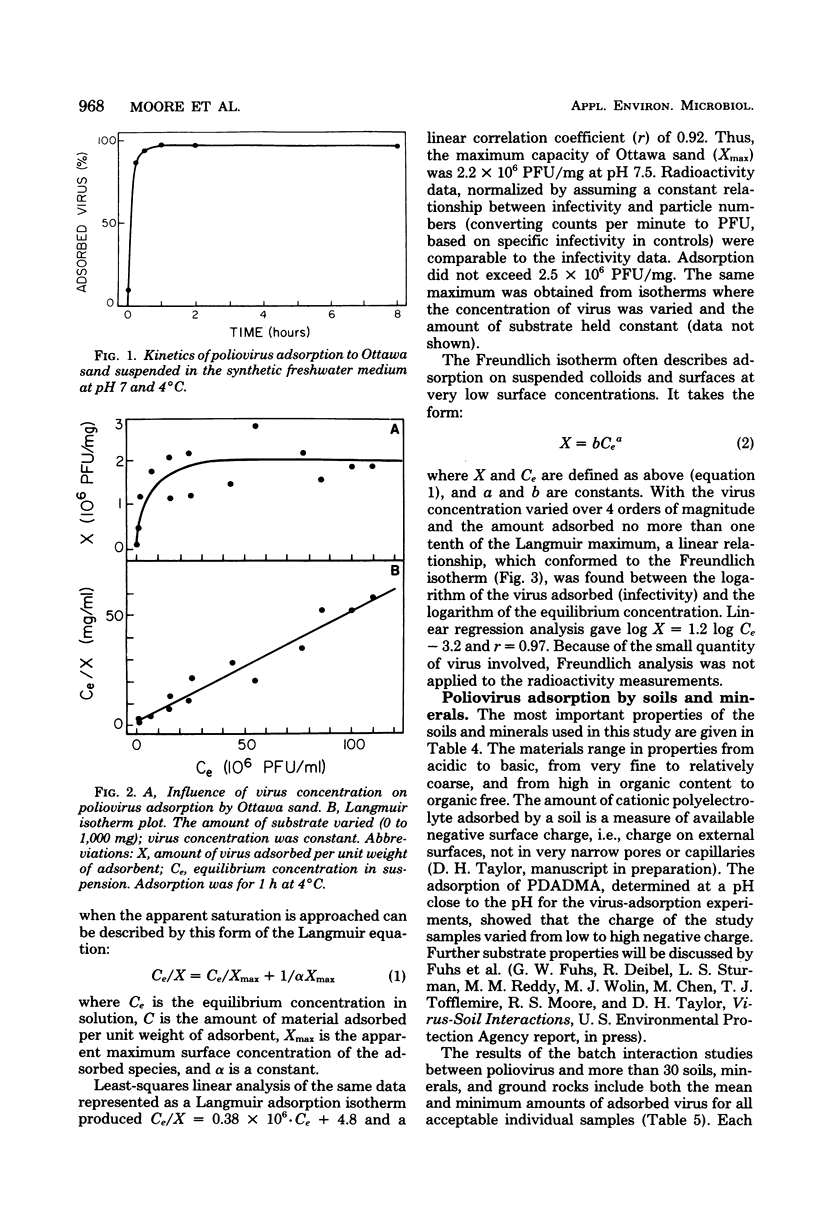
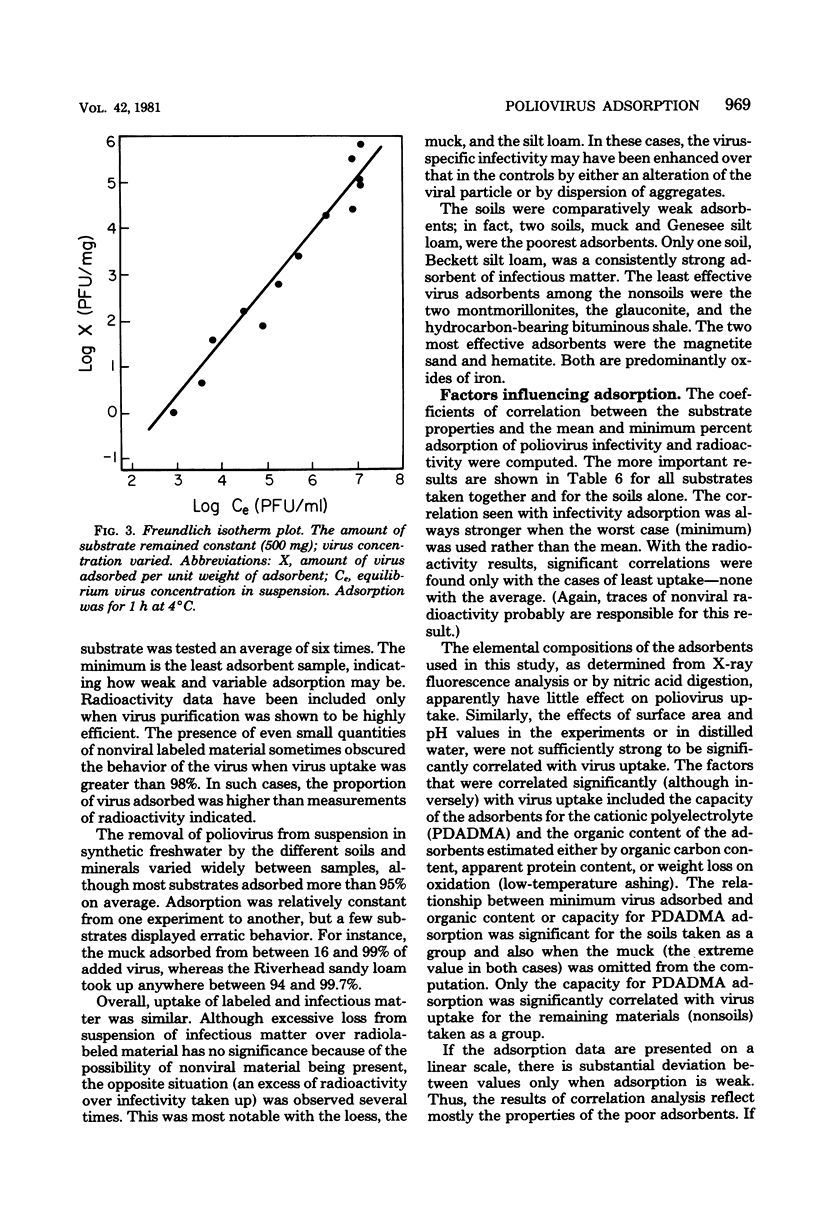
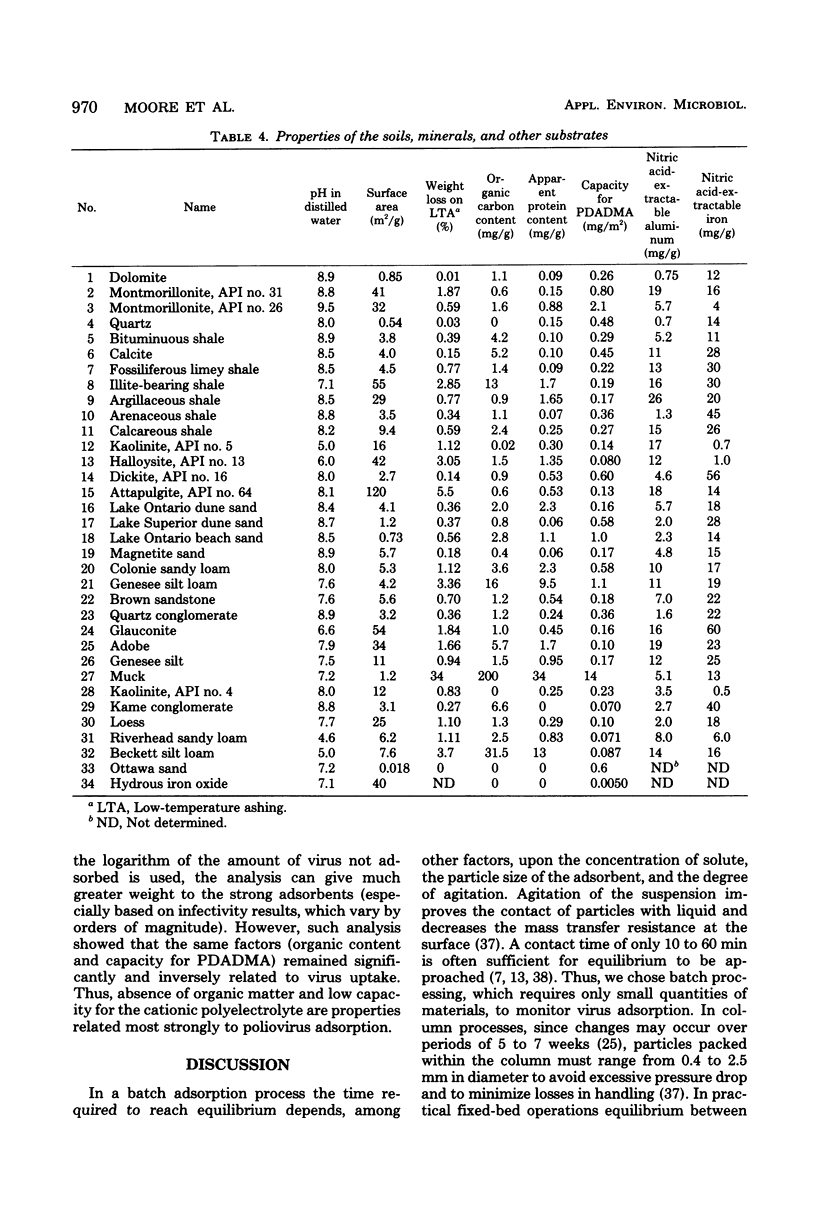
Abstract
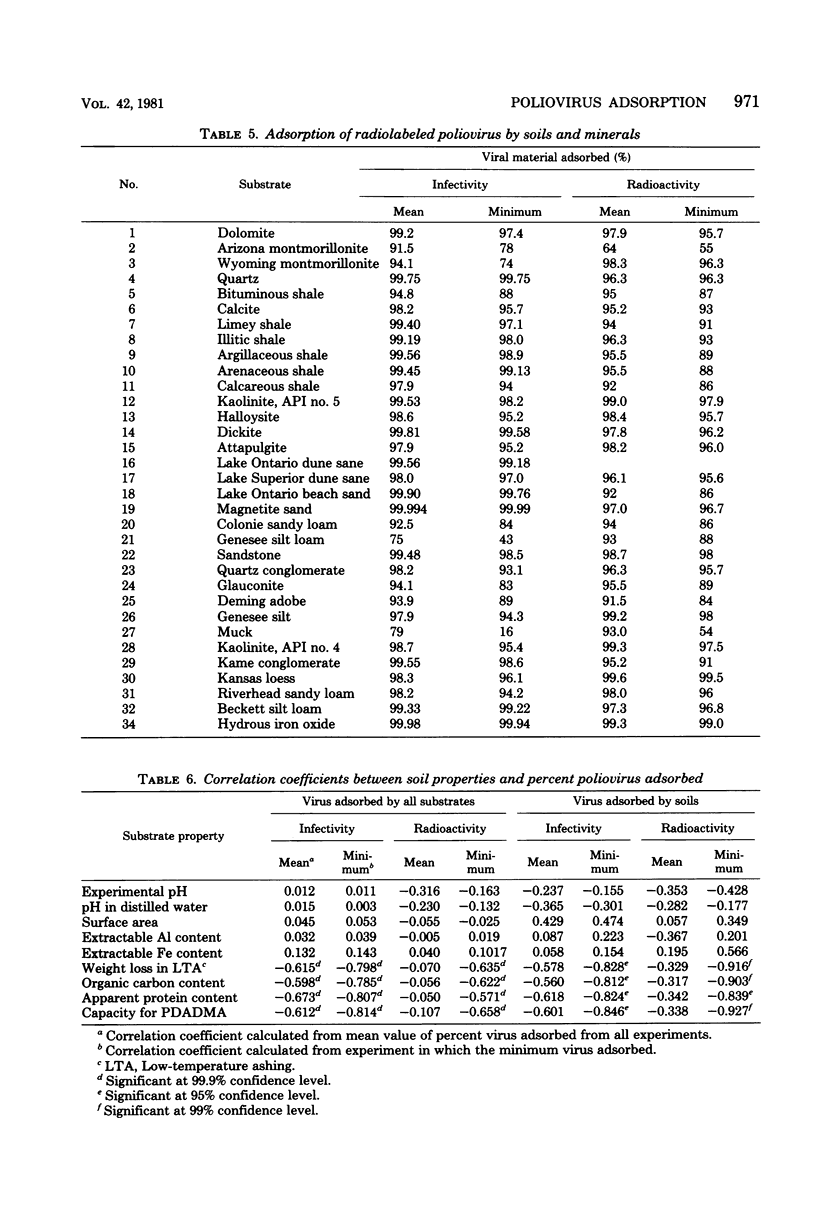
The adsorption of radiolabeled infectious poliovirus type 2 by 34 well-defined soils and mineral substrates was analyzed in a synthetic freshwater medium containing 1 mM CaCl2 and 1.25 mM NaHCO3 at pH 7. In a model system, adsorption of poliovirus by Ottawa sand was rapid and reached equilibrium within 1 h at 4°C. Near saturation, the adsorption could be described by the Langmuir equation; the apparent surface saturation was 2.5 × 106 plaque-forming units of poliovirus per mg of Ottawa sand. At low surface coverage, adsorption was described by the Freundlich equation. The soils and minerals used ranged from acidic to basic and from high in organic content to organic free. The available negative surface charge on each substrate was measured by the adsorption of a cationic polyelectrolyte, polydiallyldimethylammonium chloride. Most of the substrates adsorbed more than 95% of the virus. In general, soils, in comparison with minerals, were weak adsorbents. Among the soils, muck and Genesee silt loam were the poorest adsorbents; among the minerals, montmorillonite, glauconite, and bituminous shale were the least effective. The most effective adsorbents were magnetite sand and hematite, which are predominantly oxides of iron. Correlation coefficients for substrate properties and virus adsorption revealed that the elemental composition of the adsorbents had little effect on poliovirus uptake. Substrate surface area and pH, by themselves, were not significantly correlated with poliovirus uptake. A strong negative correlation was found between poliovirus adsorption and both the contents of organic matter and the available negative surface charge on the substrates as determined by their capacities for adsorbing the cationic polyelectrolyte, polydiallyldimethylammonium chloride.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- DRESCHER J. Comparison of the adsorption of influenza virus strain B/Berlin/2/55 on aluminum oxide and on aluminum hydroxide. Am J Hyg. 1961 Jul;74:104–118. doi: 10.1093/oxfordjournals.aje.a120197. [DOI] [PubMed] [Google Scholar]
- Farrah S. R., Goyal S. M., Gerba C. P., Conklin R. H., Smith E. M. Comparison between adsorption of poliovirus and rotavirus by aluminum hydroxide and activated sludge flocs. Appl Environ Microbiol. 1978 Feb;35(2):360–363. doi: 10.1128/aem.35.2.360-363.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floyd R., Sharp D. G. Aggregation of poliovirus and reovirus by dilution in water. Appl Environ Microbiol. 1977 Jan;33(1):159–167. doi: 10.1128/aem.33.1.159-167.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goyal S. M., Gerba C. P. Comparative adsorption of human enteroviruses, simian rotavirus, and selected bacteriophages to soils. Appl Environ Microbiol. 1979 Aug;38(2):241–247. doi: 10.1128/aem.38.2.241-247.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- LaBelle R. L., Gerba C. P. Influence of pH, salinity, and organic matter on the adsorption of enteric viruses to estuarine sediment. Appl Environ Microbiol. 1979 Jul;38(1):93–101. doi: 10.1128/aem.38.1.93-101.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray J. P., Laband S. J. Degradation of poliovirus by adsorption on inorganic surfaces. Appl Environ Microbiol. 1979 Mar;37(3):480–486. doi: 10.1128/aem.37.3.480-486.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson R. K. Automated Pregl-Dumas technique for determining total carbon, hydrogen, and nitrogen in atmospheric aerosols. Anal Chem. 1973 Mar;45(3):605–609. doi: 10.1021/ac60325a050. [DOI] [PubMed] [Google Scholar]
- Sobsey M. D., Dean C. H., Knuckles M. E., Wagner R. A. Interactions and survival of enteric viruses in soil materials. Appl Environ Microbiol. 1980 Jul;40(1):92–101. doi: 10.1128/aem.40.1.92-101.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor D. H., Moore R. S., Sturman L. S. Influence of pH and electrolyte composition on adsorption of poliovirus by soils and minerals. Appl Environ Microbiol. 1981 Dec;42(6):976–984. doi: 10.1128/aem.42.6.976-984.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]


