Abstract
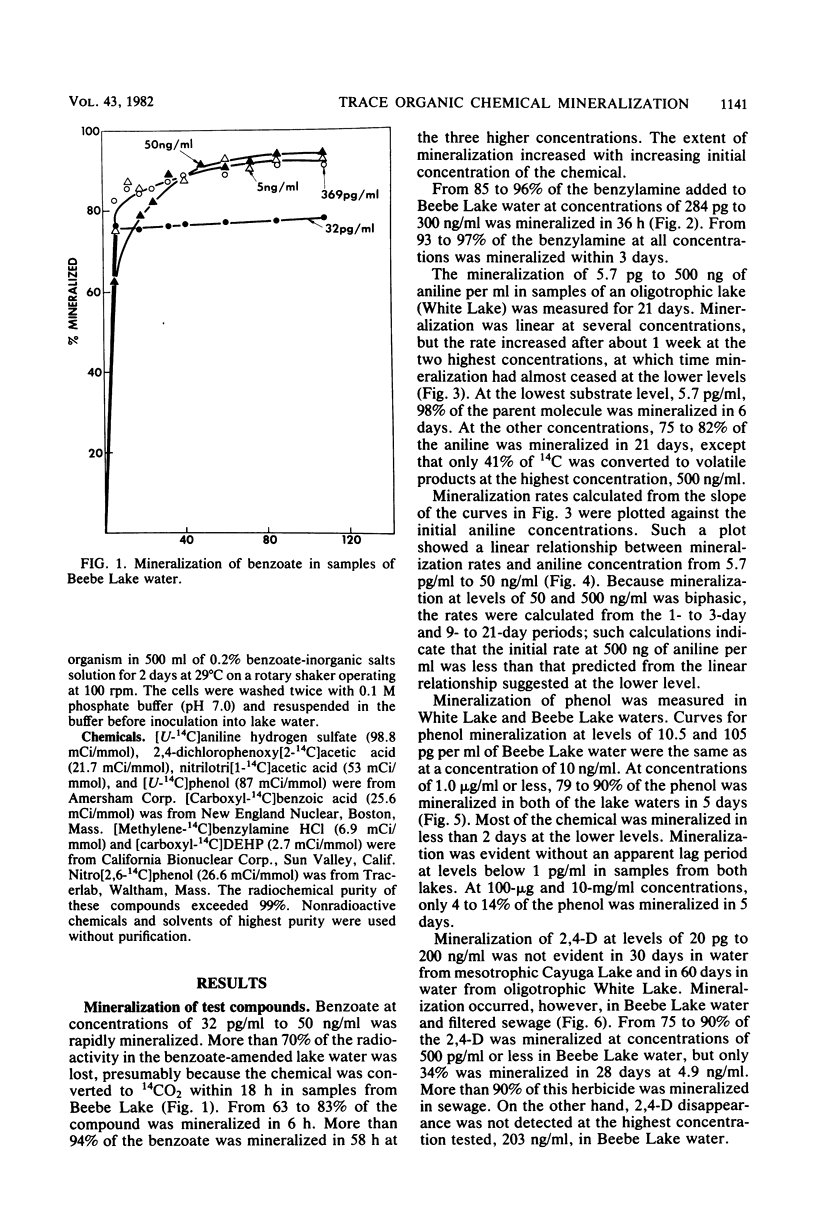
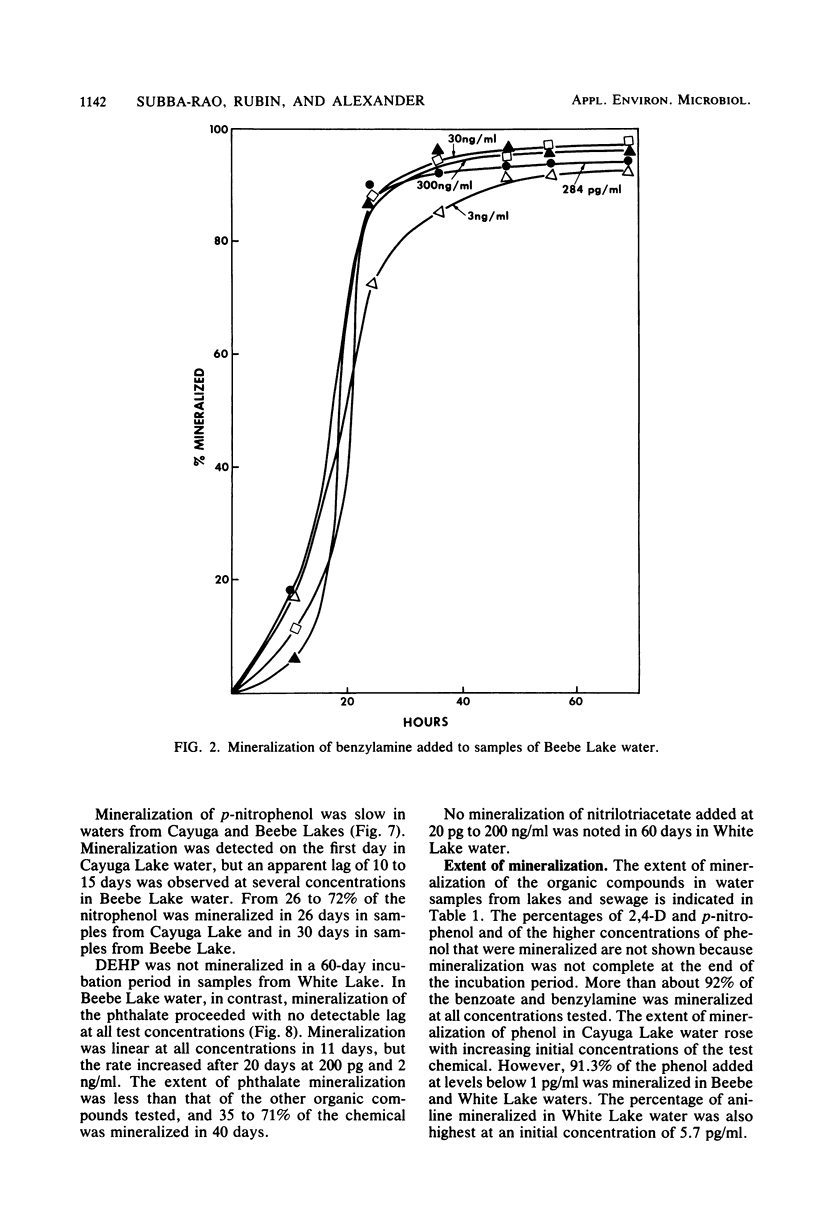
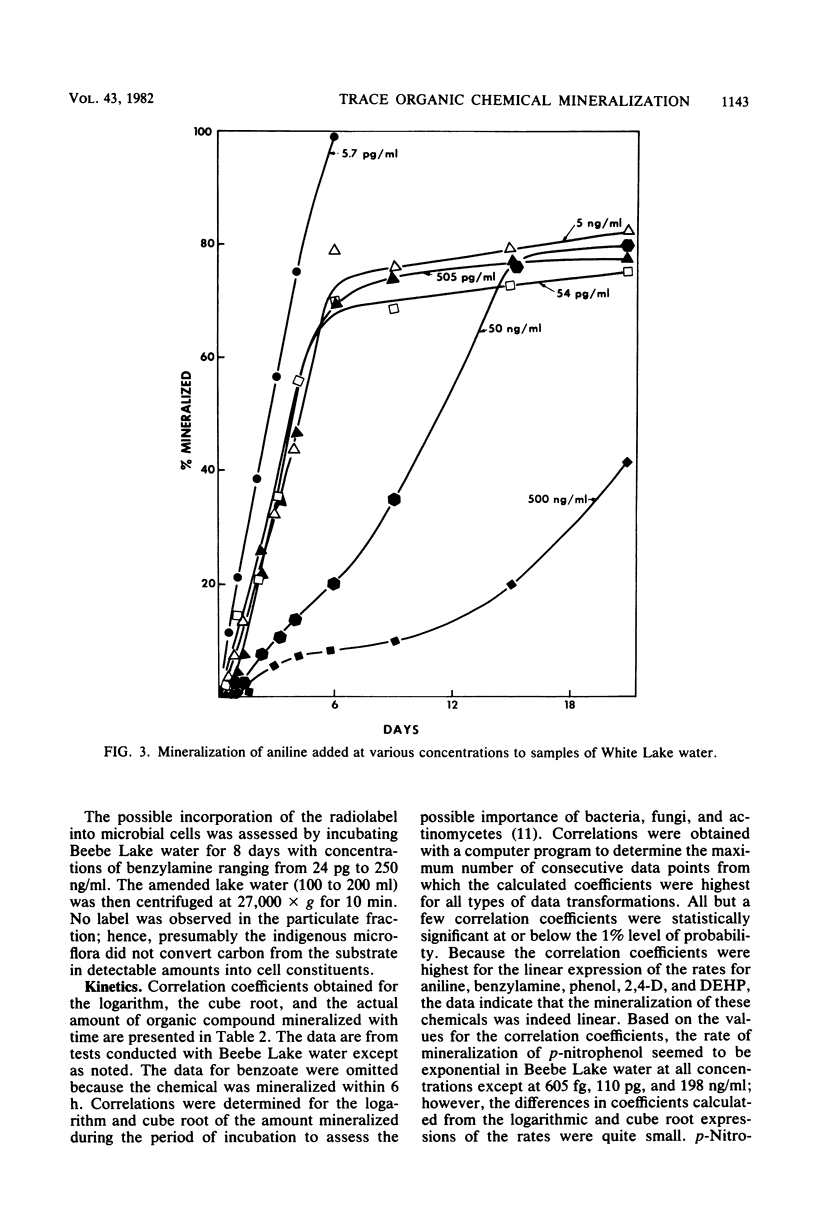
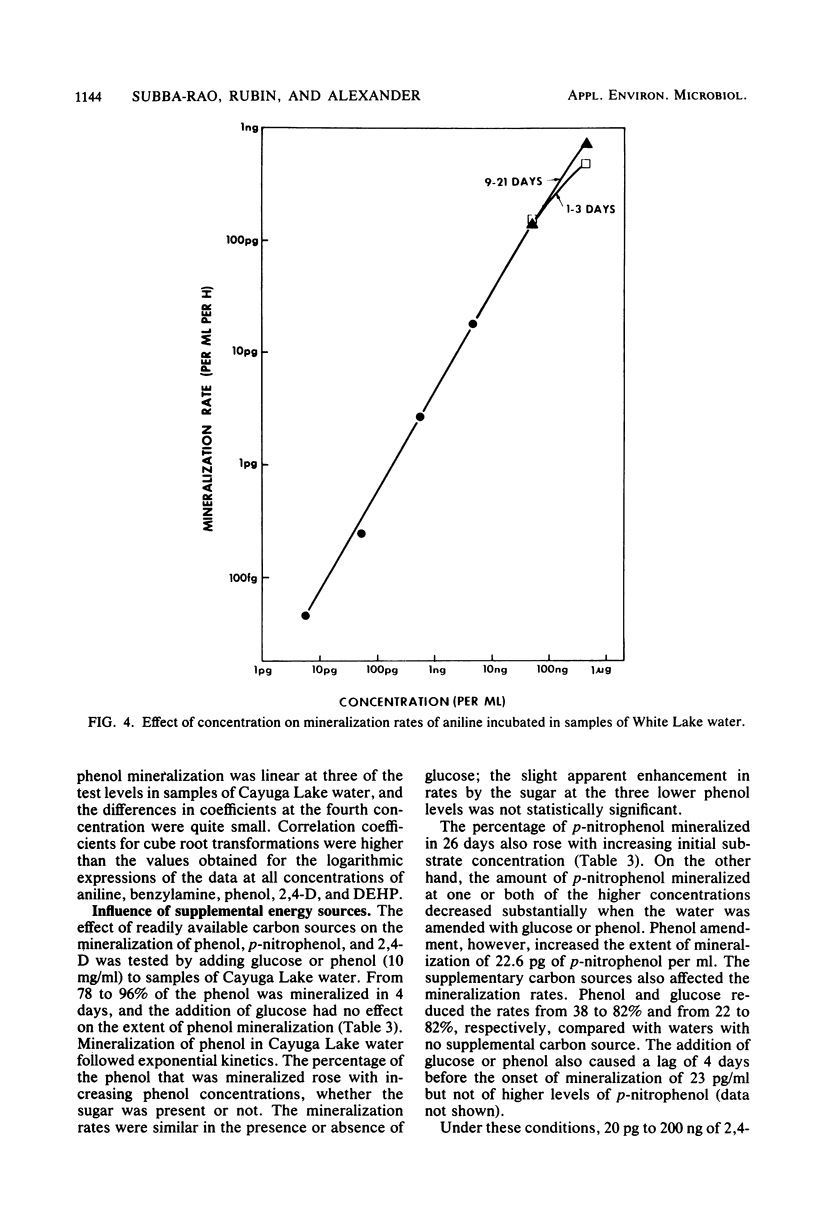
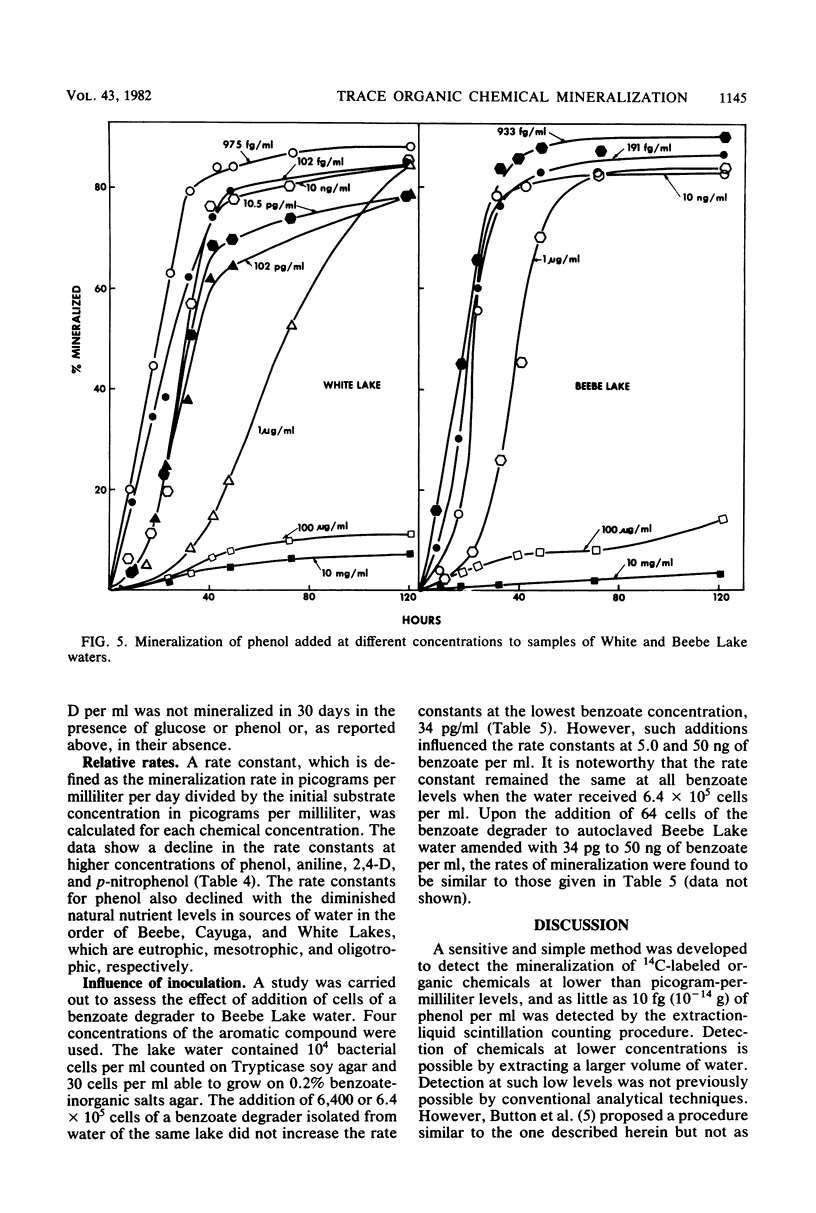
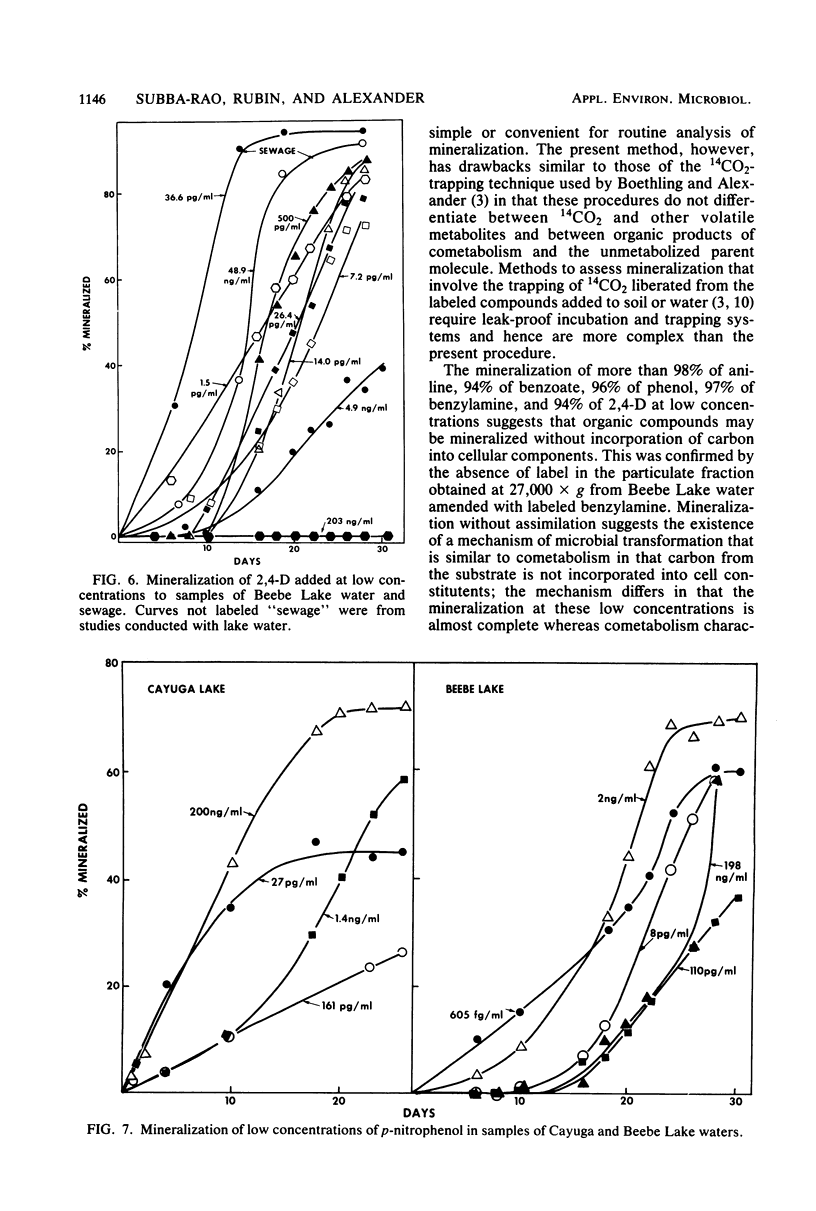
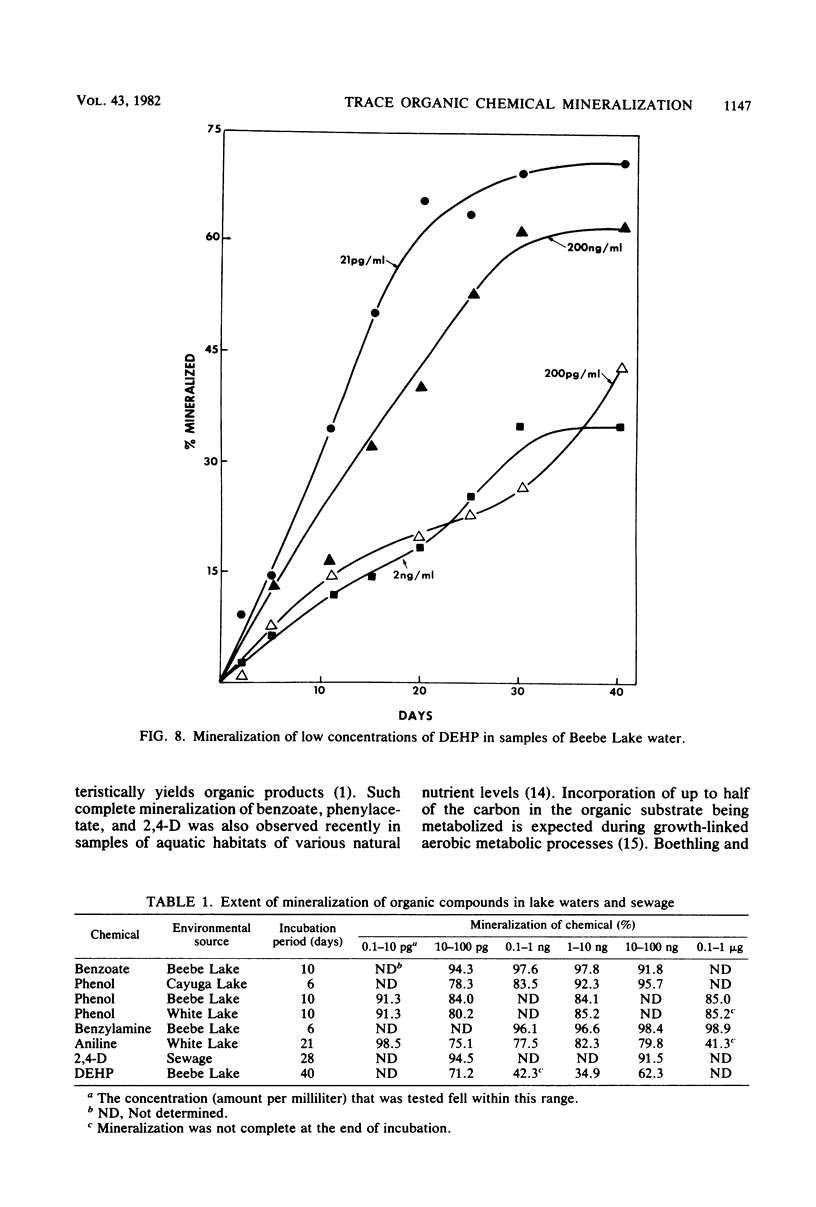
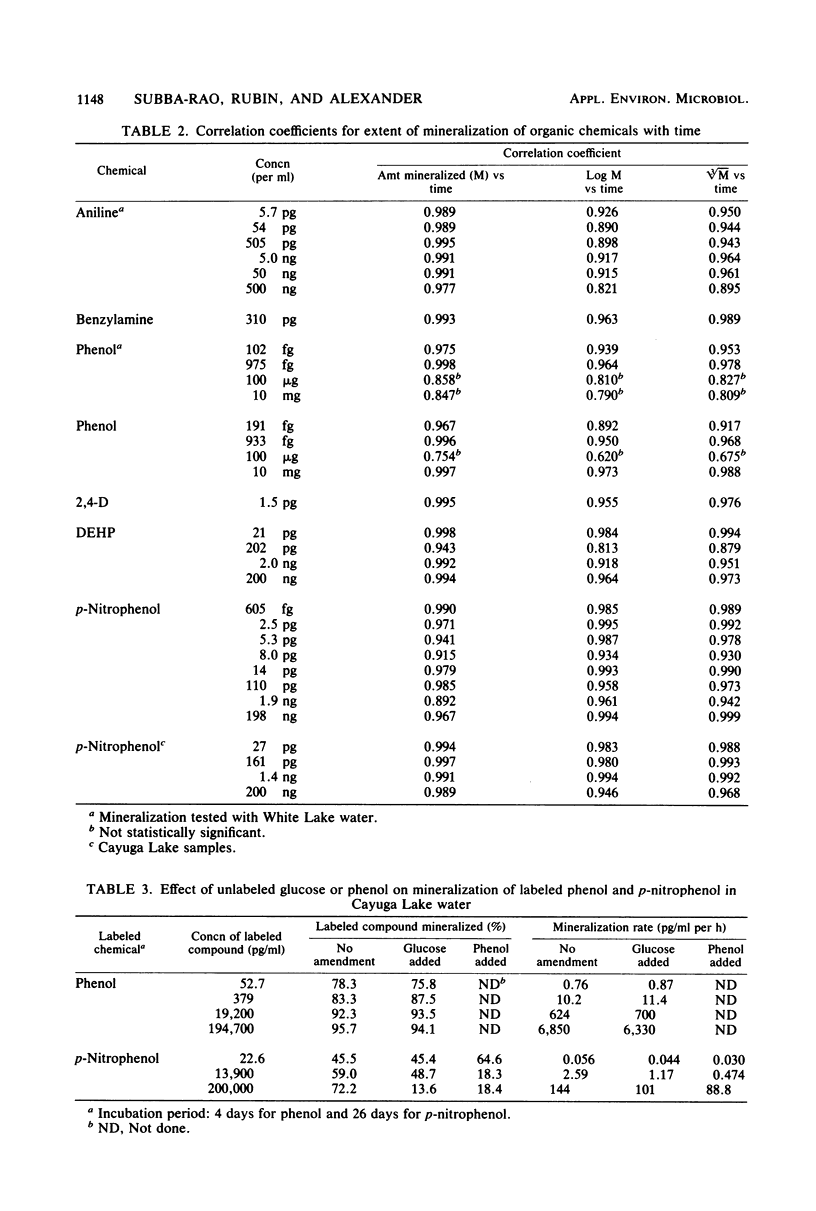
A sensitive and rapid method was developed to measure the mineralization of 14C-labeled organic compounds at picogram-per-milliliter or lower levels in samples of natural waters and sewage. Mineralization was considered to be equivalent to the loss of radioactivity from solutions. From 93 to 98% of benzoate, benzylamine, aniline, phenol, and 2,4-dichlorophenoxyacetate at one or more concentrations below 300 ng/ml was mineralized in samples of lake waters and sewage, indicating little or no incorporation of carbon into microbial cells. Assimilation of 14C by the cells mineralizing benzylamine in lake water was not detected. Mineralization in lake waters was linear with time for aniline at 5.7 pg to 500 ng/ml, benzylamine at 310 ng/ml, phenol at 102 fg to 10 mg/ml, 2,4-dichlorophenoxyacetate at 1.5 pg/ml, and di-(2-ethylhexyl) phthalate at 21 pg to 200 ng/ml, but it was exponential at several p-nitrophenol concentrations. The rate of mineralization of 50 and 500 ng of aniline per ml and 200 pg and 2.0 ng of the phthalate per ml increased with time in lake waters. The phthalate and 2,4-dichlorophenoxyacetate were mineralized in samples from a eutrophic but not an oligotrophic lake. Addition to eutrophic lake water of a benzoate-utilizing bacterium did not increase the rate of benzoate mineralization at 34 and 350 pg/ml but did so at 5 and 50 ng/ml. Glucose and phenol reduced the percentage of p-nitrophenol mineralized at p-nitrophenol concentrations of 200 ng/ml but not at 22.6 pg/ml and inhibited the rates of mineralization at both concentrations. These results show that the kinetics of mineralization, the capacity of the aquatic community to assimilate carbon from the substrate or the extent of assimilation, and the sensitivity of the mineralizing populations to organic compounds are different at trace levels than at higher concentrations of organic compounds.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alexander M. Biodegradation of chemicals of environmental concern. Science. 1981 Jan 9;211(4478):132–138. doi: 10.1126/science.7444456. [DOI] [PubMed] [Google Scholar]
- BAUCHOP T., ELSDEN S. R. The growth of micro-organisms in relation to their energy supply. J Gen Microbiol. 1960 Dec;23:457–469. doi: 10.1099/00221287-23-3-457. [DOI] [PubMed] [Google Scholar]
- Boethling R. S., Alexander M. Effect of concentration of organic chemicals on their biodegradation by natural microbial communities. Appl Environ Microbiol. 1979 Jun;37(6):1211–1216. doi: 10.1128/aem.37.6.1211-1216.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Button D. K., Schell D. M., Robertson B. R. Sensitive and accurate methodology for measuring the kinetics of concentration-dependent hydrocarbon metabolism rates in seawater by microbial communities. Appl Environ Microbiol. 1981 Apr;41(4):936–941. doi: 10.1128/aem.41.4.936-941.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juengst F. W., Jr, Alexander M. Conversion of 1,1,1-trichloro-2,2-bis(p-chlorophenyl)ethane (DDT) to water-soluble products by microorganisms. J Agric Food Chem. 1976 Jan-Feb;24(1):111–115. doi: 10.1021/jf60203a009. [DOI] [PubMed] [Google Scholar]
- Law A. T., Button D. K. Multiple-carbon-source-limited growth kinetics of a marine coryneform bacterium. J Bacteriol. 1977 Jan;129(1):115–123. doi: 10.1128/jb.129.1.115-123.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARSHALL K. C., ALEXANDER M. Growth characteristics of fungi and actinomycetes. J Bacteriol. 1960 Sep;80:412–416. doi: 10.1128/jb.80.3.412-416.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marinucci A. C., Bartha R. Biodegradation of 1,2,3- and 1,2,4-trichlorobenzene in soil and in liquid enrichment culture. Appl Environ Microbiol. 1979 Nov;38(5):811–817. doi: 10.1128/aem.38.5.811-817.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paris D. F., Steen W. C., Baughman G. L., Barnett J. T. Second-order model to predict microbial degradation of organic compounds in natural waters. Appl Environ Microbiol. 1981 Mar;41(3):603–609. doi: 10.1128/aem.41.3.603-609.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfaender F. K., Alexander M. Effect of nutrient additions on the apparent cometabolism of DDT. J Agric Food Chem. 1973 May-Jun;21(3):397–399. doi: 10.1021/jf60187a046. [DOI] [PubMed] [Google Scholar]
- Rubin H. E., Subba-Rao R. V., Alexander M. Rates of mineralization of trace concentrations of aromatic compounds in lake water and sewage samples. Appl Environ Microbiol. 1982 May;43(5):1133–1138. doi: 10.1128/aem.43.5.1133-1138.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]


