Abstract
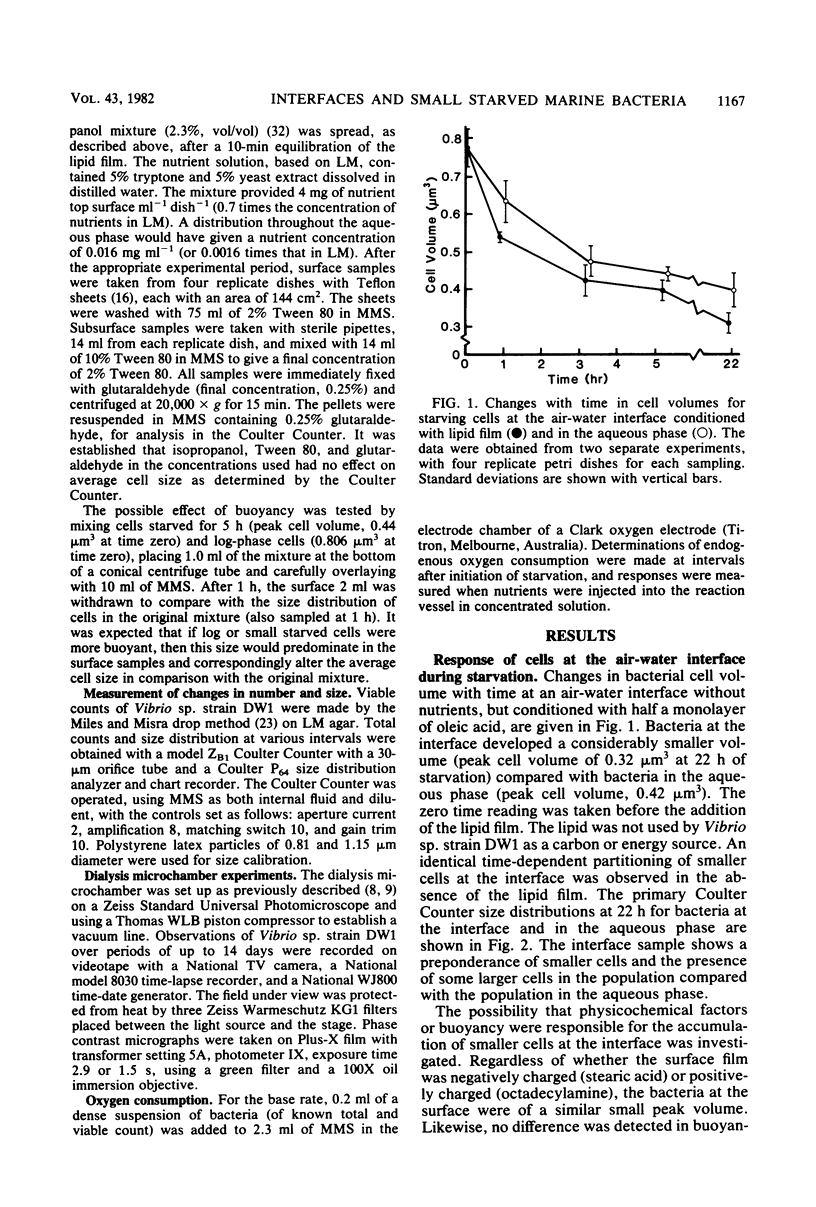
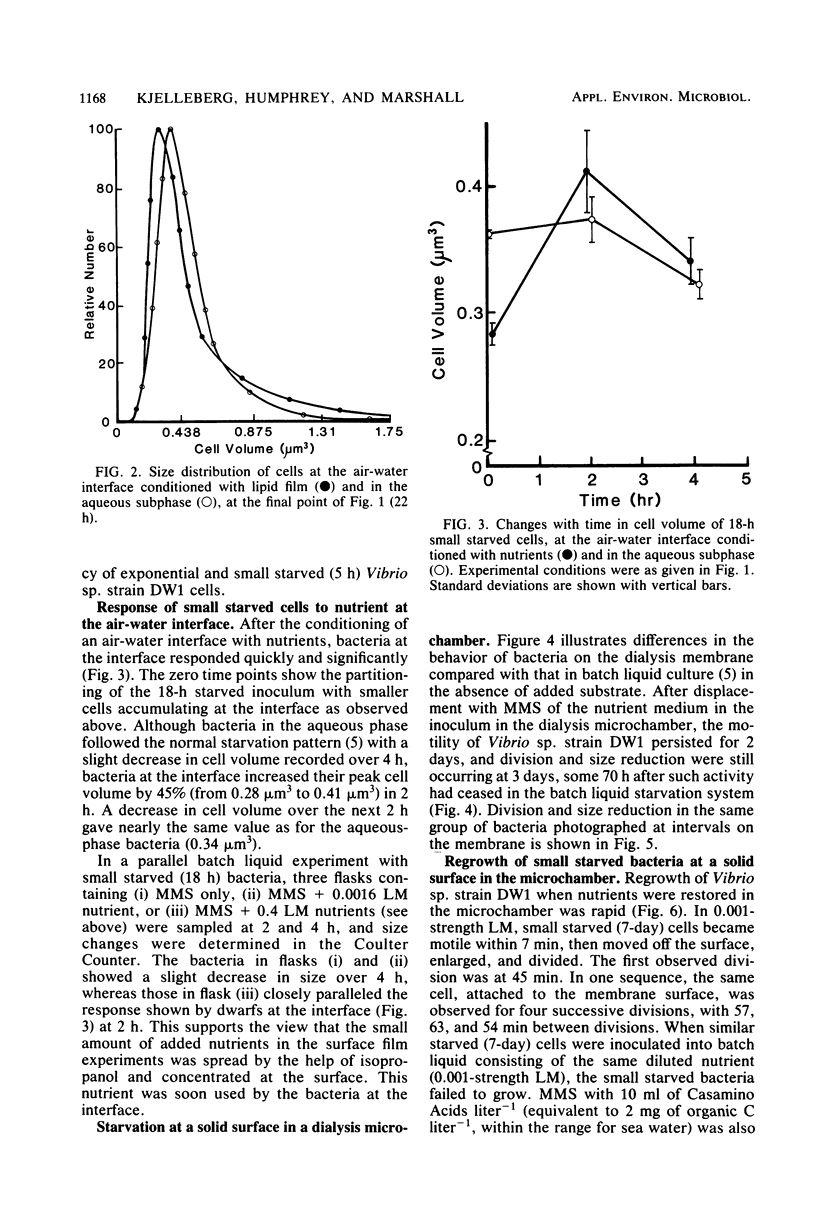
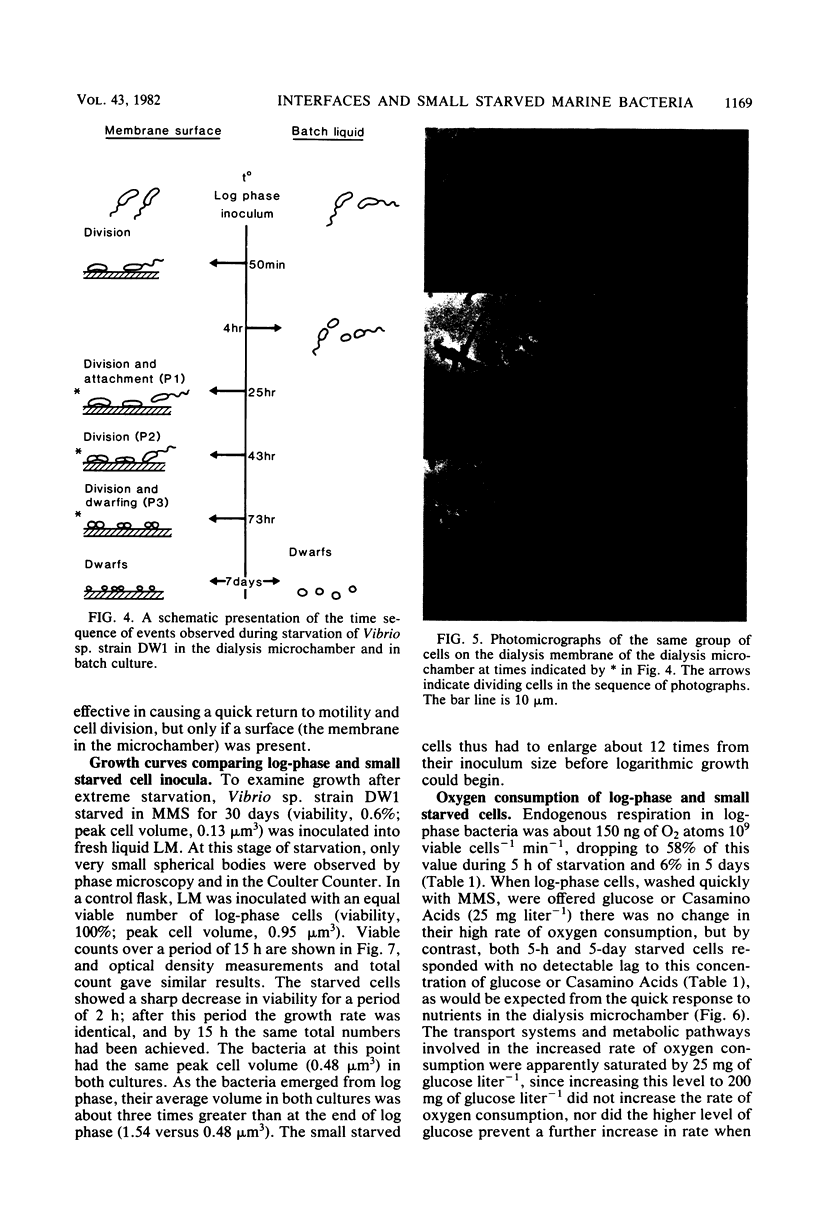
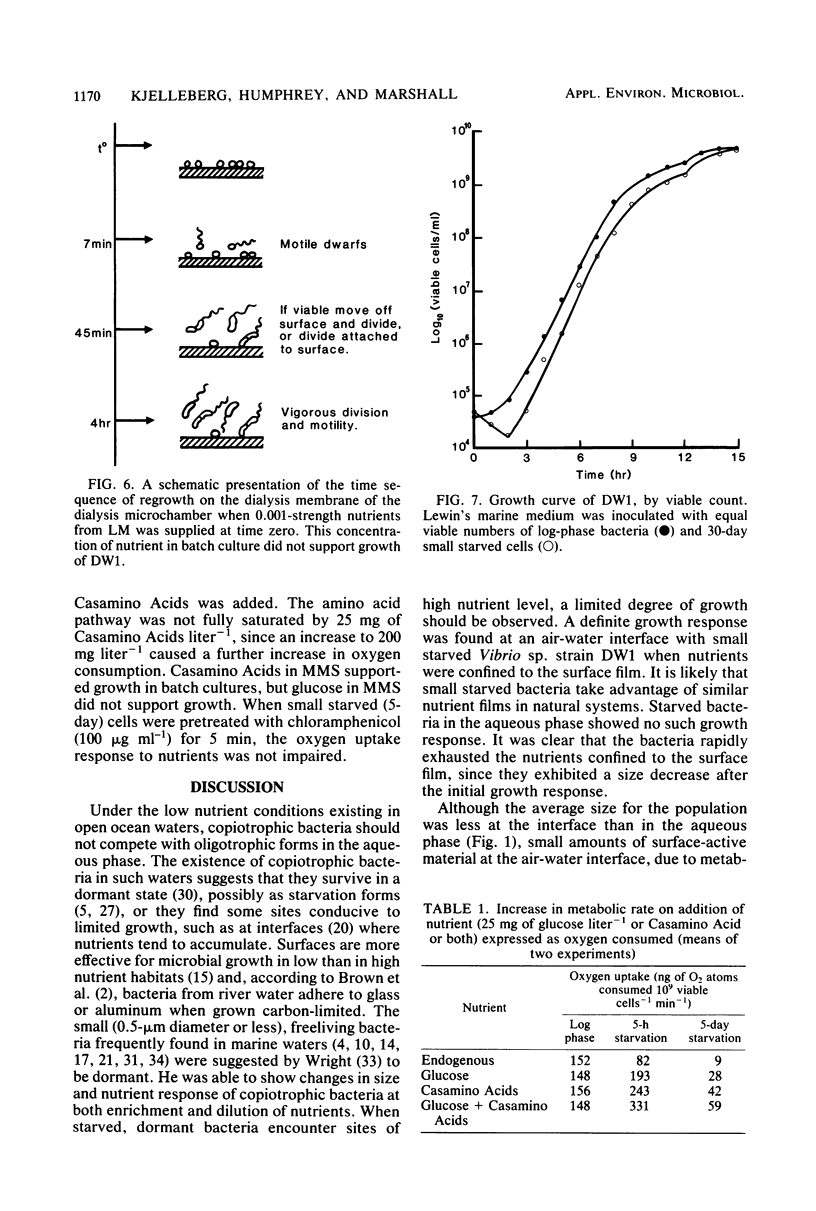
The copiotrophic marine Vibrio sp. strain DW1, shown previously in batch culture to increase in numbers at the onset of starvation and then to form viable small cells with low endogenous respiration, appears to have a survival advantage at interfaces. Vibrio sp. strain DW1 behaved differently at interfaces compared with the aqueous phase under starvation conditions: (i) small cells were observed at an air-water interface without nutrients, (ii) nutrients added to the air-water interface quickly produced larger cells at the surface, (iii) motility persisted many hours longer at the solid-water interface of a dialysis membrane in a microchamber at the onset of starvation, and (iv) regrowth and division at the solid-liquid interface occurred quickly and at nutrient concentrations too low to permit growth in the aqueous phase. It was concluded that, if small starved cells from copiotrophic bacteria can reach an interface, additional survival mechanisms become available to them: (i) interfaces constitute areas of favorable nutrient conditions, and (ii) interfaces lacking a sufficient amount of nutrient, nevertheless, trigger cells to become smaller, thus increasing their surface/volume ratio and the packing density.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blanchard D. C., Syzdek L. Mechanism for the water-to-air transfer and concentration of bacteria. Science. 1970 Nov 6;170(3958):626–628. doi: 10.1126/science.170.3958.626. [DOI] [PubMed] [Google Scholar]
- Casida L. E., Jr Small cells in pure cultures of Agromyces ramosus and in natural soil. Can J Microbiol. 1977 Feb;23(2):214–216. doi: 10.1139/m77-032. [DOI] [PubMed] [Google Scholar]
- Donachie W. D. Relationship between cell size and time of initiation of DNA replication. Nature. 1968 Sep 7;219(5158):1077–1079. doi: 10.1038/2191077a0. [DOI] [PubMed] [Google Scholar]
- Druilhet R. E., Sobek J. M. Starvation survival of Salmonella enteritidis. J Bacteriol. 1976 Jan;125(1):119–124. doi: 10.1128/jb.125.1.119-124.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duxbury T. A microperfusion chamber for studying the growth of bacterial cells. J Appl Bacteriol. 1977 Oct;43(2):247–251. doi: 10.1111/j.1365-2672.1977.tb00748.x. [DOI] [PubMed] [Google Scholar]
- HARRISON A. P., Jr, LAWRENCE F. R. PHENOTYPIC, GENOTYPIC, AND CHEMICAL CHANGES IN STARVING POPULATIONS OF AEROBACTER AEROGENES. J Bacteriol. 1963 Apr;85:742–750. doi: 10.1128/jb.85.4.742-750.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobbie J. E., Daley R. J., Jasper S. Use of nuclepore filters for counting bacteria by fluorescence microscopy. Appl Environ Microbiol. 1977 May;33(5):1225–1228. doi: 10.1128/aem.33.5.1225-1228.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kogure K., Simidu U., Taga N. A tentative direct microscopic method for counting living marine bacteria. Can J Microbiol. 1979 Mar;25(3):415–420. doi: 10.1139/m79-063. [DOI] [PubMed] [Google Scholar]
- Lewin R. A., Lounsbery D. M. Isolation, cultivation and characterization of flexibacteria. J Gen Microbiol. 1969 Oct;58(2):145–170. doi: 10.1099/00221287-58-2-145. [DOI] [PubMed] [Google Scholar]
- Marshall K. C., Stout R., Mitchell R. Selective sorption of bacteria from seawater. Can J Microbiol. 1971 Nov;17(11):1413–1416. doi: 10.1139/m71-225. [DOI] [PubMed] [Google Scholar]
- Novitsky J. A., Morita R. Y. Morphological characterization of small cells resulting from nutrient starvation of a psychrophilic marine vibrio. Appl Environ Microbiol. 1976 Oct;32(4):617–622. doi: 10.1128/aem.32.4.617-622.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novitsky J. A., Morita R. Y. Survival of a psychrophilic marine Vibrio under long-term nutrient starvation. Appl Environ Microbiol. 1977 Mar;33(3):635–641. doi: 10.1128/aem.33.3.635-641.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- POSTGATE J. R., HUNTER J. R. The survival of starved bacteria. J Gen Microbiol. 1962 Oct;29:233–263. doi: 10.1099/00221287-29-2-233. [DOI] [PubMed] [Google Scholar]
- Poindexter J. S. The caulobacters: ubiquitous unusual bacteria. Microbiol Rev. 1981 Mar;45(1):123–179. doi: 10.1128/mr.45.1.123-179.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torrella F., Morita R. Y. Microcultural study of bacterial size changes and microcolony and ultramicrocolony formation by heterotrophic bacteria in seawater. Appl Environ Microbiol. 1981 Feb;41(2):518–527. doi: 10.1128/aem.41.2.518-527.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright R. T. Measurement and significance of specific activity in the heterotrophic bacteria of natural waters. Appl Environ Microbiol. 1978 Aug;36(2):297–305. doi: 10.1128/aem.36.2.297-305.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]



