Abstract
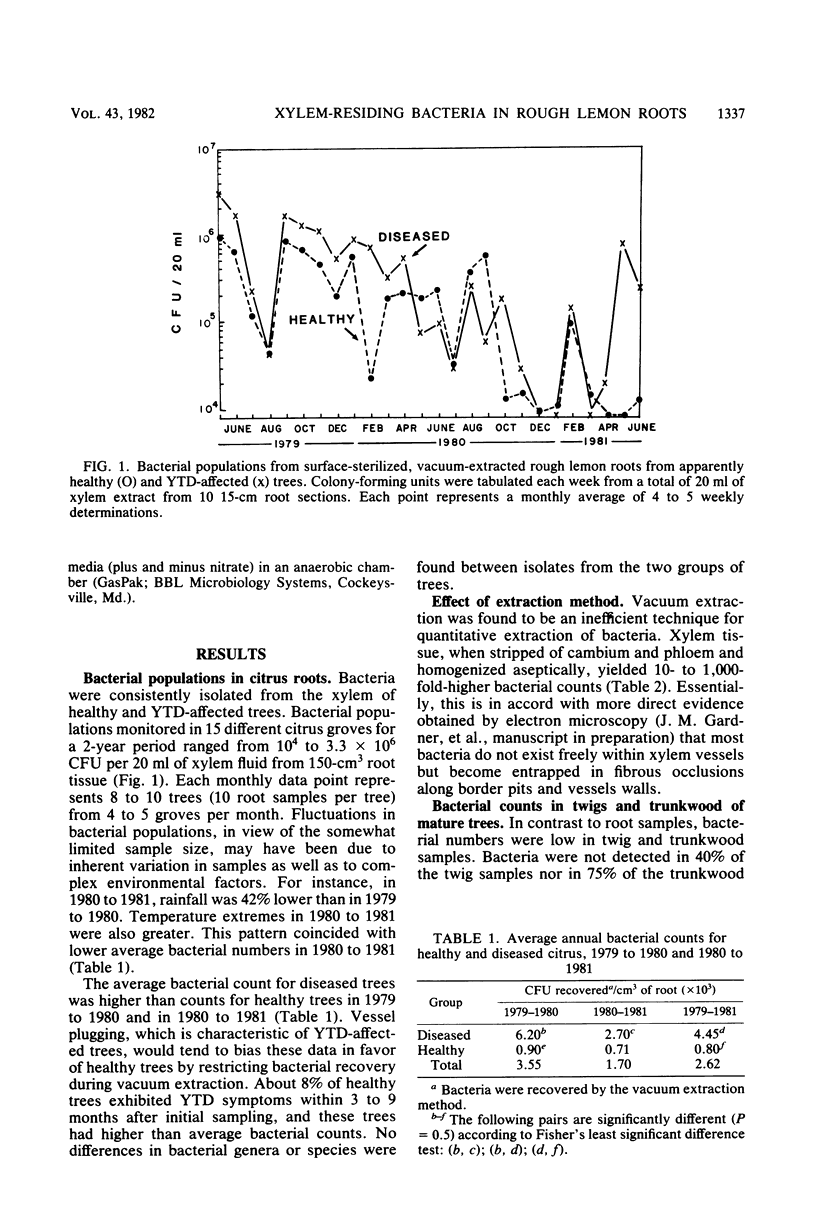
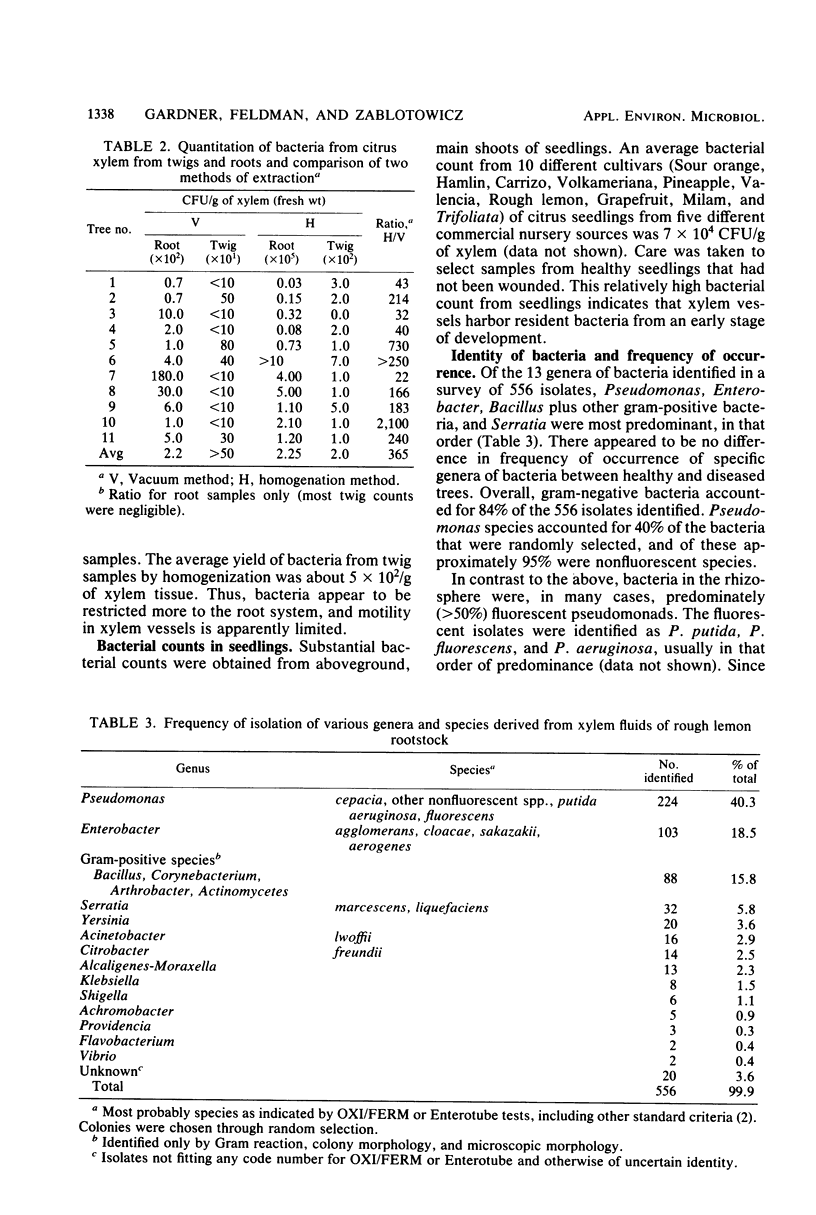
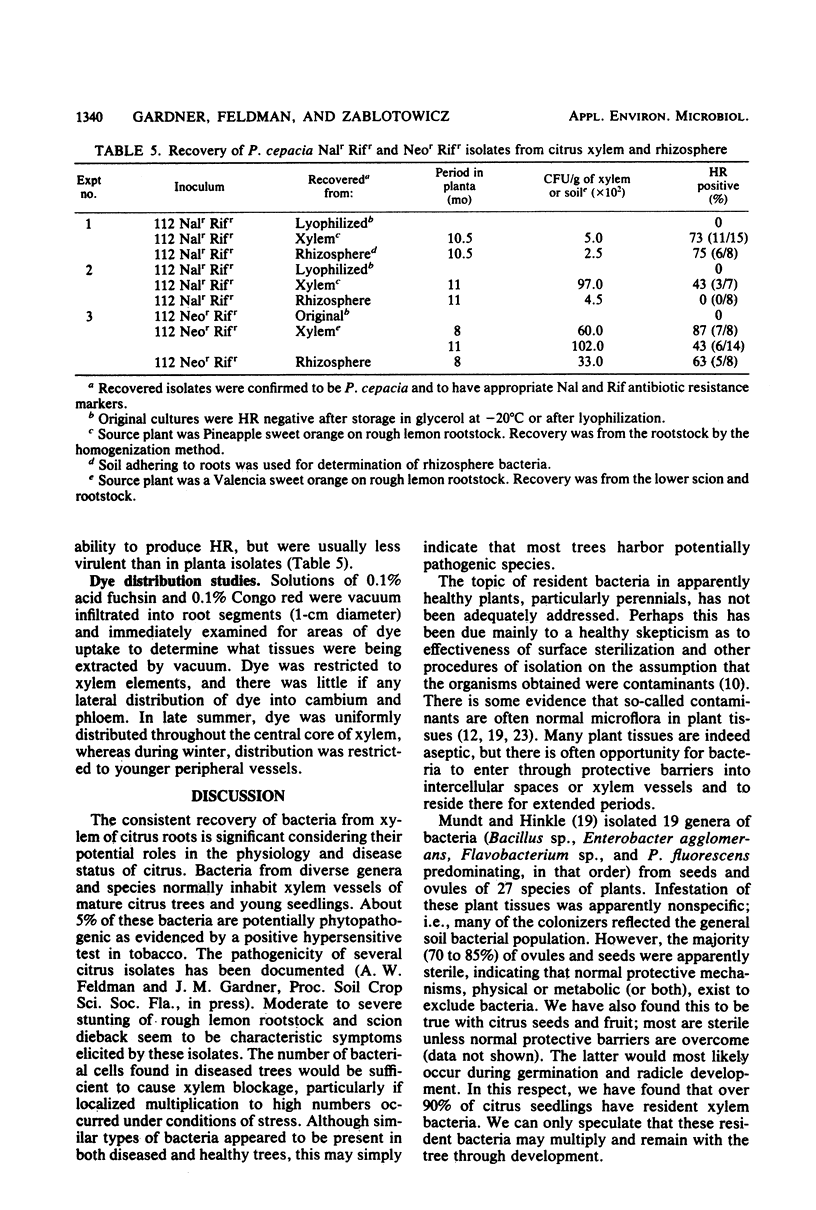
An aseptic vacuum extraction technique was used to obtain xylem fluid from the roots of rough lemon (Citrus jambhiri Lush.) rootstock of Florida citrus trees. Bacteria were consistently isolated from vascular fluid of both healthy and young tree decline-affected trees. Thirteen genera of bacteria were found, the most frequently occurring genera being Pseudomonas (40%), Enterobacter (18%), Bacillus, Corynebacterium, and other gram-positive bacteria (16%), and Serratia (6%). Xylem bacterial counts fluctuated seasonally. Bacterial populations ranged from 0.1 to 22 per mm3 of root tissue (about 102 to 2 × 104 bacteria per g of xylem) when bacterial counts were made on vascular fluid, but these numbers were 10- to 1,000-fold greater when aseptically homogenized xylem tissue was examined similarly. Some of the resident bacteria (4%) are potentially phytopathogenic. It is proposed that xylem bacteria have an important role in the physiology of citrus.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cox C. D., Rinehart K. L., Jr, Moore M. L., Cook J. C., Jr Pyochelin: novel structure of an iron-chelating growth promoter for Pseudomonas aeruginosa. Proc Natl Acad Sci U S A. 1981 Jul;78(7):4256–4260. doi: 10.1073/pnas.78.7.4256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis M. J., Purcell A. H., Thomson S. V. Pierce's disease of grapevines: isolation of the causal bacterium. Science. 1978 Jan 6;199(4324):75–77. doi: 10.1126/science.199.4324.75. [DOI] [PubMed] [Google Scholar]
- Gasson M. J. Indicator technique for antimetabolic toxin production by phytopathogenic species of pseudomonas. Appl Environ Microbiol. 1980 Jan;39(1):25–29. doi: 10.1128/aem.39.1.25-29.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isenberg H. D., Sampson-Scherer J. Clinical laboratory evaluation of a system approach to the recognition of nonfermentative or oxidase-producing gram-negative, rod-shaped bacteria. J Clin Microbiol. 1977 Mar;5(3):336–340. doi: 10.1128/jcm.5.3.336-340.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kado C. I., Heskett M. G. Selective media for isolation of Agrobacterium, Corynebacterium, Erwinia, Pseudomonas, and Xanthomonas. Phytopathology. 1970 Jun;60(6):969–976. doi: 10.1094/phyto-60-969. [DOI] [PubMed] [Google Scholar]
- Lelliott R. A., Billing E., Hayward A. C. A determinative scheme for the fluorescent plant pathogenic pseudomonads. J Appl Bacteriol. 1966 Dec;29(3):470–489. doi: 10.1111/j.1365-2672.1966.tb03499.x. [DOI] [PubMed] [Google Scholar]
- Misaghi I., Grogan R. G. Nutritional and biochemical comparisons of plant-pathogenic and saprophytic fluorescent pseudomonads. Phytopathology. 1969 Oct;59(10):1436–1450. [PubMed] [Google Scholar]
- Mundt J. O., Hinkle N. F. Bacteria within ovules and seeds. Appl Environ Microbiol. 1976 Nov;32(5):694–698. doi: 10.1128/aem.32.5.694-698.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberhofer T. R. Comparison of the API 20E and Oxi/Ferm systems in identification of nonfermentative and oxidase-positive fermentative bacteria. J Clin Microbiol. 1979 Feb;9(2):220–226. doi: 10.1128/jcm.9.2.220-226.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen W. L., Chakrabarty K., Klucas R. V., Vidaver A. K. Nitrogen fixation (acetylene reduction) associated with roots of winter wheat and sorghum in Nebraska. Appl Environ Microbiol. 1978 Jan;35(1):129–135. doi: 10.1128/aem.35.1.129-135.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanier R. Y., Palleroni N. J., Doudoroff M. The aerobic pseudomonads: a taxonomic study. J Gen Microbiol. 1966 May;43(2):159–271. doi: 10.1099/00221287-43-2-159. [DOI] [PubMed] [Google Scholar]


