Abstract
The amount of bone turnover in the skeleton is has been identified as a predictor of fracture risk independent of areal bone mineral density (aBMD) and is increasingly cited as an explanation for discrepancies between areal bone mineral density and fracture risk. A number of mechanisms have been proposed to explain how bone turnover influences bone biomechanics, including regulation of tissue degree of mineralization, the disconnection or fenestration of individual trabeculae by remodeling cavities, and the ability of cavities formed during the remodeling process to act as stress risers. While these mechanisms can influence bone biomechanics, they also modify bone mass. If bone turnover is to explain any of the observed discrepancies between fracture risk and areal bone mineral density, however, it must not only modify bone strength but modify bone strength in excess of what would be expected from the associated change in bone mass. This article summarizes biomechanical studies of how tissue mineralization, trabecular disconnection and the presence of remodeling cavities might have an effect on cancellous bone strength independent of bone mass. Existing data support the idea that all of these factors may have a disproportionate effect on bone stiffness and/or strength, with the exception of average tissue degree of mineralization, which is unlikely to have an effect on bone strength that is independent of aBMD. Disproportionate effects of mineral content on bone biomechanics may instead come from variation in tissue degree of mineralization at the micro-structural level. The biomechanical explanation for the relationship between bone turnover and fracture incidence remains to be determined but must be examined not in terms of bone strength but in terms of bone strength relative to bone mass.
INTRODUCTION
Bone turnover represents the total volume of bone that is both resorbed and formed over a period of time [1]. In adults, bone turnover occurs primarily through bone remodeling, a focal process that involves the coupled activity of osteoclasts and osteoblasts [2]. Changes in the amount of bone turnover cause local changes in bone volume and the average age of tissue in a bone, resulting in alterations in tissue degree of mineralization and trabecular microarchitecture. Clinical findings that biochemical markers of bone turnover can predict fracture risk independent of areal bone mineral density (aBMD) have led to the suggestion that the amount of bone turnover in the skeleton can have a biomechanical effect independent of bone mass1 [3–8] and that bone turnover may help to explain discrepancies between aBMD and fracture risk that have been observed in clinical studies [5, 6]. The biomechanical effects of alterations in bone turnover are commonly attributed to modifications in tissue degree of mineralization, the fenestration or disconnection of individual trabeculae and/or by remodeling cavities acting as stress risers [4, 6–9]. While these mechanisms can influence bone strength, they also modify bone mass. If one of these mechanisms is to explain any of the discrepancies between aBMD and fracture risk, however, it must have an effect on bone strength that is much larger than would be expected from the change in bone mass alone. The purpose of this article is to review the biomechanical effects of changes in bone that can be caused by bone turnover. The current article concentrates on the biomechanics of human cancellous bone specimens 3–5mm in smallest dimension, as that is the scale at which the mechanisms mentioned above all have biomechanical significance. Biomechanics of bone at this size scale is also important because a factor that does not have a disproportionate biomechanical effect at this scale could not have a disproportionate biomechanical effect at the scale of the whole bone and would therefore be unlikely to influence fracture risk [10]. The biomechanical effects of each of the aspects of bone turnover are summarized relative to a 6% difference in bone mass to allow comparisons in which bone mass is not a confounding factor (Table 1, a 6% decline in bone mass has been selected as that is the estimated size of the remodeling space in the spine [11]).
Table 1.
The reduction in human cancellous bone stiffness and strength associated with a 6% difference in bone volume or mass is shown. The 95% confidence interval is reported if available, otherwise the range across all cited studies is shown. A factor that causes a greater change in bone stiffness or strength than that caused by a 6% reduction in bone volume (the first row) has the potential to explain how bone turnover can influence fracture risk independent of bone quantity.
| Process through which bone remodeling modifies bone mass | Difference in Bone Mass (%) | Expected Reduction in Stiffness (%) | Expected Reduction in Strength (%) | Source |
|---|---|---|---|---|
| Reduction in bone volume | −6% (Volume) | 12–16% | 9–14% | Empirical power law models [14, 17–21] |
| Reduction in average tissue degree of mineralization from 65% to 62% ash by weight. | −6%(Bone Mineral Content) | 11–13% | 11–13% | 95% confidence interval from empirical power law models [41] |
| Intraspecimen variation in tissue degree of mineralization | 0% Reduction in bone mass; Increase in COV of tissue mineralization from 20% to 50% | 14–24% | Not Yet Evaluated | Micro-computed tomography based finite element models [45, 46] |
| Removal of Trabeculae | −6% (Volume) | 3–39% | 18–35% | 3D cellular solid finite element models [50, 51] |
| Addition of Remodeling Cavities | −6% (Volume) | 12–47% | 13–61% | Micro-computed tomography based finite element models [53, 55] |
Bone Volume
Because the first step in the remodeling process is bone resorption, each remodeling event is associated with the formation of a temporary cavity. The total volume of bone occupied by all remodeling cavities and unmineralized bone tissue (osteoid) is known collectively as the remodeling space [12]. Increases in bone turnover result in an increase in the volume occupied by the remodeling space and cause a corresponding reduction in mineralized bone volume.
Biomechanical testing of cancellous bone specimens has shown that bone stiffness (expressed as the elastic modulus, E) and strength are related to apparent density (ρ, g/cm3) through power law relationships. Because apparent density is directly related to bone volume fraction (BV/TV) [13], the same power law relationships are valid for bone volume fraction as well. These power law relationships can be expressed as follows:
| (1) |
| (2) |
where E is the stiffness of the specimen (elastic modulus), σUlt is the strength (ultimate stress) in compression and A and B are constants [14] (for a comprehensive review please see [15, 16]). Studies of human cancellous bone [14, 17–21] have reported the exponent A to be as small as 1.2 [20] or as large as 3.0 [14], suggesting that a bone specimen with 6% less bone mass due to having less bone volume is expected be 7–17% less stiffness. The exponent B used to predict bone strength has been reported to be as small as 1.48 [22] or as large as 2.47 [19], suggesting that a specimen with 6% less bone mass is expected to be 9–14% less strong (Table 1).
Tissue Degree of Mineralization
After a new volume of bone is formed, it begins to accumulate mineral in a process that can continue for years afterwards [23, 24]. As a result, the degree of mineralization of older bone tissue is greater than that of newly formed tissue, so that the amount of bone turnover can influence the average tissue degree of mineralization [25, 26].
Examination of mineralized tissues across a wide range of species (including deer and whales) have associated increased tissue degree of mineralization with increased bone stiffness and strength and, in some cases, increased brittleness (the term ‘brittle’ is used here in an engineering sense expressing a material property, and not the likelihood of clinical fracture) [27, 28]. While these studies demonstrate that tissue degree of mineralization can be biomechanically important, most do not include analyses of bone porosity and therefore cannot be used to examine the biomechanical effects of tissue degree of mineralization independent of bone volume. In addition, it is important to keep in mind that trends observed among species do not necessarily apply within a species. For example, consider the commonly held idea that “hypermineralized” bone tissue is more brittle, a concept frequently noted when discussing possible adverse effects of long-term inhibition of bone turnover. Although comparisons among animals suggest that more highly mineralized tissue is more brittle, only two studies of human bone specimens have shown increased tissue degree of mineralization to be associated with increased brittleness (evaluated as impact energy in cortical bone specimens) [29, 30]. Indeed, other studies of human bone specimens have found increased tissue degree of mineralization to be associated with reduced brittleness (measured as compressive toughness in cancellous bone [31], or fracture toughness evaluated in cortical bone [32]). Additionally, a number of studies of human bone specimens did not observe a relationship between tissue degree of mineralization and brittleness (measured as toughness or energy to failure in cortical or cancellous bone [33–36]). While comparisons among species suggest that highly mineralized bone specimens are more brittle, it is not at all clear that specimens of human bone can become brittle through an increase in average tissue degree of mineralization alone.
With regard to bone stiffness and strength, few studies have been designed to separate the biomechanical effects of tissue degree of mineralization from those of bone volume. Follet and colleagues found that average tissue degree of mineralization (measured through quantitative contact radiography) was positively correlated with cancellous bone stiffness, strength and brittleness (brittleness evaluated as toughness) [31], and that tissue degree of mineralization had a biomechanical effect independent of bone volume. Others have used power law models to predict the biomechanical effects of tissue degree of mineralization in cortical bone [18, 37–40], although only two of these studies accounted for variation in bone volume fraction (both using non-human tissue [38, 40]). Currey suggested that the separate effects of bone volume and tissue degree of mineralization could be expressed with a two-parameter power law model and applied the approach to non-human tissue [38]. Hernandez and colleagues applied this statistical approach to human bone and found the following relationships [41]:
| (3) |
| (4) |
where E, σUlt and BV/TV are as defined above, α is the degree of mineralization (measured as ash mass/dry bone mass), and the exponents are expressed as mean ± standard error. This analysis is the only study of cancellous bone to detect and quantify the independent effects of bone volume and average tissue degree of mineralization on bone biomechanics. With regard to bone strength (equation 4), the exponent applied to tissue degree of mineralization, 2.79, is much greater than the exponent applied to bone volume fraction, 1.92, suggesting that cancellous bone strength is much more sensitive to differences in tissue degree of mineralization. But could differences in average tissue degree of mineralization account for discrepancies between clinical measures of bone mass and bone strength? Clinical measures of bone mass evaluate the total amount of mineral present (both mineralized volume and degree of mineralization). Assuming clinical evaluation of bone mineral content (BMC) are directly related to the inorganic content in bone (the ash content), BMC can be expressed as:
| (5) |
where ρt is the density of the mineralized bone tissue (in grams), a parameter that is linearly related to tissue degree of mineralization [13, 41]. By combining equation (3), equation (4) and equation (5) we can estimate the differences in bone biomechanics associated with a 6% difference in BMC caused entirely by reductions in average tissue degree of mineralization (this change in BMC corresponds to a reduction in tissue degree of mineralization from 65% to 62% ash by weight). Such a difference in BMC is expected to be associated with a difference in specimen stiffness of 11–13% and a difference in bone strength of 11–13% (both of these ranges are expressed using the 95% confidence interval of the regression coefficients above).
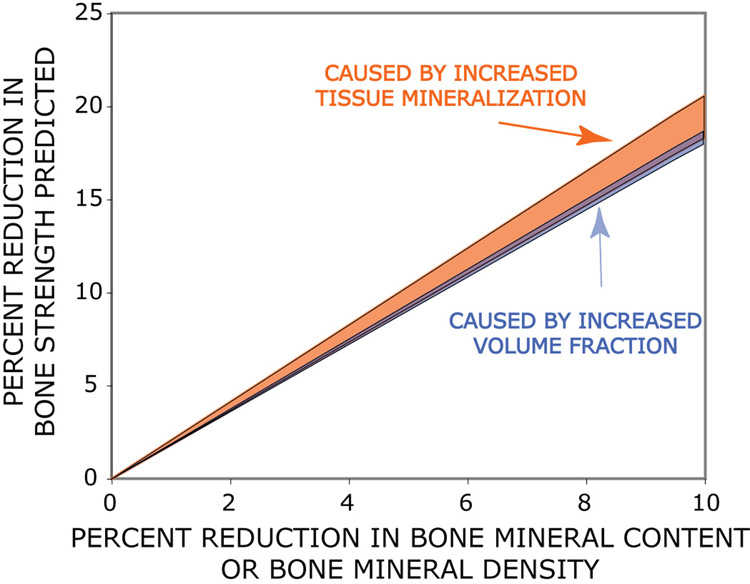
Another way of comparing the biomechanical effects of bone volume and tissue degree of mineralization is to examine the relationship between bone mineral content and bone strength [10]. Figure 1 shows the percent change in bone strength expected from a hypothetical reduction in bone mineral content under two different conditions: 1) when changes in bone mineral content are caused entirely by bone volume (lower region with dark blue shading); and 2) when changes in bone mineral content are caused entirely by tissue degree of mineralization (upper region with lighter orange shading). That these two confidence intervals overlap suggests that alterations in tissue degree of mineralization may not modify the relationship between bone strength and clinical measures of BMC. Hence, alterations in average tissue degree of mineralization may have little effect on the ability of BMC to predict bone strength and are therefore not expected to be responsible for discrepancies between fracture incidence and aBMD. Additional studies are needed to confirm this analysis (equation 3, equation 4) and to determine if the average degree of mineralization can have disproportionate effects on other mechanical properties (brittleness for example) or under different loading conditions (impact, shear, etc.).
Figure 1.
The predicted reduction in strength caused by a reduction in bone mineral content or bone mineral density (as would be measured by dual-energy x-ray absorptiometry) is shown 1) a hypothetical case where changes in bone mineral content are caused entirely by changes in volume fraction (dark blue region) and 2) a hypothetical case where changes in bone mineral content are caused entirely by reductions in tissue degree of mineralization (light orange region). The overlapping regions are based on the 95% confidence interval of the regression model reported by Hernandez and colleagues[41].
While most studies have concentrated on the biomechanical effects of average tissue degree of mineralization, variation of tissue degree of mineralization at the micro-scale, associated with variation in degree of mineralization among osteons/hemi-osteons, has also been implicated as a factor that can influence bone biomechanics. By altering the number and/or size of new remodeling events, bone turnover will not only modify the average tissue degree of mineralization, but also the variability of tissue degree of mineralization. Changes in variation of tissue degree of mineralization have been associated with alterations in bone turnover during bisphosphonate therapy and in metabolic bone disease [42, 43]. Variability of tissue degree of mineralization can also differ among regions of the skeleton (iliac crest v. calcaneous) [31].
Micro-computed tomography based finite element models have been useful for studying the biomechanical consequences of variation in tissue degree of mineralization because they make it possible to consider the biomechanical effects of tissue degree of mineralization independent of bone volume or microarchitecture. Finite element studies suggest that an increase in intraspecimen variation in tissue degree of mineralization (modeled as local variation in tissue stiffness) can cause a reduction in cancellous bone stiffness, even when trabecular microarchitecture and average tissue degree of mineralization are maintained constant [44–46]. Jaasma and colleagues determined that an increase in the coefficient of variation (standard deviation/mean) of the tissue stiffness from 20% to 50% would result in a reduction in elastic modulus of cancellous bone by 19–24%, even when average tissue degree of mineralization was maintained constant. More recently, Bourne and van der Meulen measured variation in mineral content directly using calibrated micro-computed tomography and found that an increase in the coefficient of variation of tissue stiffness from 20% to 50% is expected to cause a 14% reduction in the elastic modulus of cancellous bone.
Disconnection of Trabeculae
It is commonly stated that cavities formed during remodeling can disconnect or fenestrate trabeculae, modifying trabecular microarchitecture and potentially cause a disproportionate change in cancellous bone strength [7–9]. Quantifying the effect of trabecular disconnection experimentally is challenging because of technical difficulties in identifying and counting individual trabeculae (only recently have techniques for directly counting individual trabeculae in micro-CT images of cancellous bone been presented [47, 48]). Existing biomechanical analyses have therefore used cellular solid models with trabecular-like microarchitectures to mimic cancellous bone structure. Two- and three-dimensional cellular solid models indicate that removal of individual trabeculae can result in reductions in cancellous bone stiffness and strength that are greater than would be expected from the associated change in bone volume [49–51]. Three-dimensional models suggest that a 6% difference in bone volume caused by removal of trabeculae can reduce cancellous bone stiffness by 3% (only horizontal trabeculae removed) to 39% (only vertical/oblique trabeculae removed) and compressive strength by 18% (only horizontal trabeculae) to 35% (only vertical/oblique trabeculae)[51]. As these simulations represent extreme cases where only horizontal or only vertical/oblique trabeculae are removed, the actual changes in bone biomechanics resulting from trabecular disconnection are expected to be somewhere in between these values.
Remodeling Cavities
It has been proposed that cavities formed during bone remodeling (Howship’s lacunae) can act as stress risers, causing disproportionate reductions in the biomechanical performance of cancellous bone. Experimental evaluation of the effects of remodeling cavities on cancellous bone has been limited because a repeatable technique for making three-dimensional measures of remodeling cavities in cancellous bone has not yet been demonstrated. Existing data is therefore limited to predictions made from finite element models. A number of biomechanical analyses have illustrated how the presence of a cavity on the surface of a single trabecula may increase the stresses and strains in surrounding tissue [52–54]. Whether or not this biomechanical effect also occurs at a larger scale is necessary for remodeling cavity stress risers to be biomechanically relevant clinically. Two finite element analyses have suggested that remodeling cavities can have a disproportionate effect on cancellous bone stiffness and strength in specimens 3–5mm in smallest dimension [53, 55]. A reduction in bone volume of 6% caused by the addition of remodeling cavities was predicted to reduce the elastic modulus by 12–47% and the compressive strength by 13–61%. The ranges for these predictions are large because the biomechanical effects of remodeling cavities can be influenced by a number of factors including the initial bone volume fraction (more porous bone can be more sensitive to remodeling cavities) and the placement of remodeling cavities in the cancellous bone structure. When placed in regions of high strain within the cancellous bone structure (where tissue microdamage and mechanical stresses are expected to be greatest) remodeling cavities can have a large, disproportionate effect on cancellous bone biomechanics [55]. As a result, the degree to which remodeling cavities are targeted to tissue damage or tissue strain (two factors believed to stimulate bone remodeling) will modulate the effect of bone remodeling on bone biomechanics.
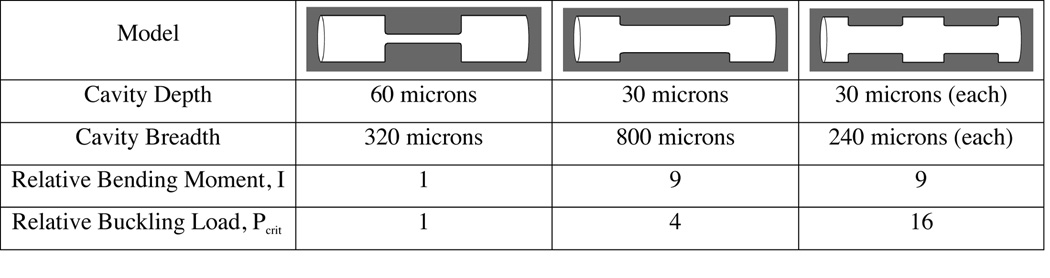
Additionally, the number and size (length, width, depth) of remodeling cavities may have biomechanical significance. Although an increase in bone turnover is commonly interpreted as an increase in the number of remodeling events, two-dimensional histomorphometry measurements cannot differentiate an increase in the number of remodeling events from an increase in the size of each individual event (width, length and depth) [56], a distinction that can result in very different stress distributions within cancellous bone. Simple mechanical analyses suggest that the number and size of remodeling cavities may influence the mechanical performance of a trabecula independent of bone volume or total amount of bone turnover (Figure 2). The number and size of remodeling cavities may also influence intraspecimen variation in tissue degree of mineralization by determining the size of each osteon or hemi-osteon and may also influence the rate at which trabeculae are disconnected by remodeling events (deeper cavities are more likely to disconnect trabeculae [57]). Unfortunately, little is known about the complete size and shape (length, width and depth) of remodeling cavities in human cancellous bone because two-dimensional techniques cannot obtain measure all three of these size dimensions at once [58, 59]. Micro-computed tomography is not as helpful as one would expect because few imaging systems can obtain the resolution needed to detect the scalloped surface of a remodeling cavity and those imaging systems with such high resolution can typically only observe one or two cavities per specimen, far too few to characterize the population of remodeling events in a region of the skeleton. Recently, serial block-face imaging using an automated microtome or milling machine has been used to image remodeling cavities in three dimensions, and may prove useful in determining the placement and size of remodeling cavities in human bone biopsies or cadaver tissue [54, 60].
Figure 2.
Three cylindrical trabeculae (150 microns in diameter) are shown with remodeling cavities wrapped around them circumferentially. The remodeling cavities in each image occupy the same volume (i.e. the same amount of bone turnover is depicted) but the number, surface size and depth of the cavities differs within the range observed histologically. The bending moment and critical load for Euler buckling (calculated within the tapered region only) is presented relative to that in the first image and is shown to vary by as much as an order of magnitude among the three possibilities.
CONCLUSIONS
Table 1 provides a unique way of comparing the biomechanical effects of the factors that have been discussed by reporting the expected differences in biomechanics associated with the same bone mass (in this case a 6% difference in bone mass). Because bone mass is no longer a confounding factor when using this table it is possible to infer which of the aspects of bone remodeling have the potential to provide a biomechanical explanation for discrepancies between aBMD and fracture risk. Because bone volume is the most common cause of variation in bone mass, we can consider bone volume (the first row in Table 1) to express the standard biomechanical effect of bone mass. An aspect of bone that has the potential to explain discrepancies between aBMD and fracture incidence will have a biomechanical effect that is greater than would be expected from that caused by bone volume alone. For example, a 6% difference in bone mass caused by the average tissue degree of mineralization (second row in Table 1) is associated with an 11–13% difference in bone strength, a range that is well within that expected for the same reduction in bone mass caused by bone volume (9–14%, first row of Table 1). As a result, this analysis suggests that it is unlikely that differences in average tissue degree of mineralization can have a disproportionate effect on cancellous bone compressive strength and and average tissue degree of mineralization would be unlikely to contribute to a biomechanical explanation for discrepancies between aBMD and fracture incidence.
Two conclusions can be made from comparing the remaining factors in Table 1 to the effect of bone volume. First, existing experimental and computational data suggest that, while average tissue degree of mineralization can influence bone strength, it may not be able to explain discrepancies between bone biomechanics and clinical measures of bone mass. Local variability of tissue degree of mineralization is a more likely explanation. Secondly, while existing biomechanical analyses support the idea that trabecular disconnection and remodeling cavities may have a disproportionate effect on bone biomechanics (i.e. the biomechanical effects can exceed those expected from difference in bone volume), there is considerable overlap between the biomechanical effects of these factors and that of bone volume, so that it is not yet completely clear that these factors can have a disproportionate biomechanical effect. Whether or not these factors have a biomechanically relevant effect independent of bone mass will depend on characteristics of bone remodeling that we currently know little about, such as the number and size of remodeling events and how well remodeling cavities are targeted to mechanical stress/strain and microscopic tissue damage. Additionally there is growing evidence that aspects of collagen such as the concentration of naturally occurring non-enzymatic cross-links, can be modified by bone turnover and can influence the biomechanical performance of bone specimens [36, 61–65]. Unfortunately regression models accounting for these relationships in cancellous bone specimens are not available so the potential effect is not listed in Table 1. As it is unlikely that collagen cross-linking can be detected by aBMD any biomechanical effect of collagen in bone biomechanics would be likely to have a disproportionate effect on bone biomechanics. Lastly, it is not clear whether all of these biomechanical effects are independent of one another or if they can interact to have synergistic effects. Further study of the biomechanical effects of bone remodeling and the degree to which those effects are explained by bone mass is necessary to truly understand the relationships between bone turnover and fracture risk.
ACKNOWLEDGEMENTS
This work was supported by NIH/NIAMS R21 AR054448.
Funding Sources: NIH/NIAMS R21AR054448
Footnotes
The term “bone mass” will be used here to describe the total mass of mineralized tissue in a bone specimen (grams) and should not be confused with aBMD, a measure of bone density performed using dual-energy x-ray absorptiometry that is expressed in the units g/cm2.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Parfitt AM. Misconceptions (2): turnover is always higher in cancellous than in cortical bone. Bone. 2002;30:807–809. doi: 10.1016/s8756-3282(02)00735-4. [DOI] [PubMed] [Google Scholar]
- 2.Parfitt AM, Mundy GR, Roodman GD, Hughes DE, Boyce BF. A new model for the regulation of bone resorption, with particular reference to the effects of bisphosphonates. J Bone Miner Res. 1996;11:150–159. doi: 10.1002/jbmr.5650110203. [DOI] [PubMed] [Google Scholar]
- 3.Garnero P, Hausherr E, Chapuy MC, Marcelli C, Grandjean H, Muller C, Cormier C, Breart G, Meunier PJ, Delmas PD. Markers of bone resorption predict hip fracture in elderly women: the EPIDOS Prospective Study. J Bone Miner Res. 1996;11:1531–1538. doi: 10.1002/jbmr.5650111021. [DOI] [PubMed] [Google Scholar]
- 4.Garnero P. Markers of bone turnover for the prediction of fracture risk. Osteoporos Int. 2000;11 Suppl 6:S55–S65. doi: 10.1007/s001980070006. [DOI] [PubMed] [Google Scholar]
- 5.Cummings SR, Karpf DB, Harris F, Genant HK, Ensrud K, LaCroix AZ, Black DM. Improvement in spine bone density and reduction in risk of vertebral fractures during treatment with antiresorptive drugs. Am J Med. 2002;112:281–289. doi: 10.1016/s0002-9343(01)01124-x. [DOI] [PubMed] [Google Scholar]
- 6.Delmas PD, Li Z, Cooper C. Relationship between changes in bone mineral density and fracture risk reduction with antiresorptive drugs: some issues with meta-analyses. J Bone Miner Res. 2004;19:330–337. doi: 10.1359/JBMR.0301228. [DOI] [PubMed] [Google Scholar]
- 7.Riggs BL, Melton LJ., 3rd Bone turnover matters: the raloxifene treatment paradox of dramatic decreases in vertebral fractures without commensurate increases in bone density. J Bone Miner Res. 2002;17:11–14. doi: 10.1359/jbmr.2002.17.1.11. [DOI] [PubMed] [Google Scholar]
- 8.Heaney RP. Is the paradigm shifting? Bone. 2003;33:457–465. doi: 10.1016/s8756-3282(03)00236-9. [DOI] [PubMed] [Google Scholar]
- 9.Parfitt AM. What is the normal rate of bone remodeling? Bone. 2004;35:1–3. doi: 10.1016/j.bone.2004.03.022. [DOI] [PubMed] [Google Scholar]
- 10.Hernandez CJ, Keaveny TM. A biomechanical perspective on bone quality. Bone. 2006;39:1173–1181. doi: 10.1016/j.bone.2006.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Heaney RP. The bone-remodeling transient: implications for the interpretation of clinical studies of bone mass change. J Bone Miner Res. 1994;9:1515–1523. doi: 10.1002/jbmr.5650091003. [DOI] [PubMed] [Google Scholar]
- 12.Jaworski ZFG. Parameters and indices of bone resorption. In: Meunier PJ, editor. Bone histomorphometry, Second International Workshop. Lyon, France: Armour Montague; 1976. [Google Scholar]
- 13.Martin RB, Burr DB, Sharkey NA. Skeletal Tissue Mechanics. New York: Spinger-Verlag; 1998. [Google Scholar]
- 14.Carter DR, Hayes WC. The compressive behavior of bone as a two-phase porous structure. J Bone Joint Surg. 1997;59-A:954–962. [PubMed] [Google Scholar]
- 15.Guo XE. Mechanical properties of cortical bone and cancellous tissue. In: Cowin SC, editor. Bone Mechanics Handbook. Boca Raton: CRC Press; 2001. pp. 10.1–10.23. [Google Scholar]
- 16.Keaveny TM, Morgan EF, Niebur GL, Yeh OC. Biomechanics of trabecular bone. Annu Rev Biomed Eng. 2001;3:307–333. doi: 10.1146/annurev.bioeng.3.1.307. [DOI] [PubMed] [Google Scholar]
- 17.Gibson LJ. The mechanical behavior of cancellous bone. J Biomech. 1985;18:317–328. doi: 10.1016/0021-9290(85)90287-8. [DOI] [PubMed] [Google Scholar]
- 18.Keller TS. Predicting the compressive mechanical-behavior of bone. J Biomech. 1994;27:1159–1168. doi: 10.1016/0021-9290(94)90056-6. [DOI] [PubMed] [Google Scholar]
- 19.Goulet RW, Goldstein SA, Ciarelli MJ, Kuhn JL, Brown MB, Feldkamp LA. The relationship between the structural and orthogonal compressive properties of trabecular bone. J Biomech. 1994;27:375–389. doi: 10.1016/0021-9290(94)90014-0. [DOI] [PubMed] [Google Scholar]
- 20.Kopperdahl DL, Keaveny TM. Yield strain behavior of trabecular bone. J Biomech. 1998;31:601–608. doi: 10.1016/s0021-9290(98)00057-8. [DOI] [PubMed] [Google Scholar]
- 21.Morgan EF, Bayraktar HH, Keaveny TM. Trabecular bone modulus-density relationships depend on anatomic site. J Biomech. 2003;36:897–904. doi: 10.1016/s0021-9290(03)00071-x. [DOI] [PubMed] [Google Scholar]
- 22.Morgan EF, Keaveny TM. Dependence of yield strain of human trabecular bone on anatomic site. J Biomech. 2001;34:569–577. doi: 10.1016/s0021-9290(01)00011-2. [DOI] [PubMed] [Google Scholar]
- 23.Parfitt AM. The physiologic and clinical significance of bone histomorphometric data. In: Recker RR, editor. Bone histomorphometry: techniques and interpretation. Boca Raton, FL: CRC Press; 1983. pp. 143–223. [Google Scholar]
- 24.Akkus O, Polyakova-Akkus A, Adar F, Schaffler MB. Aging of microstructural compartments in human compact bone. J Bone Miner Res. 2003;18:1012–1019. doi: 10.1359/jbmr.2003.18.6.1012. [DOI] [PubMed] [Google Scholar]
- 25.Meunier PJ, Boivin G. Bone mineral density reflects bone mass but also the degree of mineralization of bone: therapeutic implications. Bone. 1997;21:373–377. doi: 10.1016/s8756-3282(97)00170-1. [DOI] [PubMed] [Google Scholar]
- 26.Hernandez CJ, Beaupré GS, Marcus R, Carter DR. A theoretical analysis of the contributions of remodeling space, mineralization and bone balance to changes in bone mineral density during alendronate treatment. Bone. 2001;29:511–516. doi: 10.1016/s8756-3282(01)00613-5. [DOI] [PubMed] [Google Scholar]
- 27.Currey JD. Physical characteristics affecting the tensile failure properties of compact bone. J Biomech. 1990;23:837–844. doi: 10.1016/0021-9290(90)90030-7. [DOI] [PubMed] [Google Scholar]
- 28.Currey JD. Bones: Structure and Mechanics. Princeton, NJ, USA: Princeton University Press; 2002. [Google Scholar]
- 29.Currey JD. Changes in the impact energy absorption of bone with age. J Biomech. 1979;12:459–469. doi: 10.1016/0021-9290(79)90031-9. [DOI] [PubMed] [Google Scholar]
- 30.Currey JD, Brear K, Zioupos P. The effects of ageing and changes in mineral content in degrading the toughness of human femora. J Biomech. 1996;29:257–260. doi: 10.1016/0021-9290(95)00048-8. [DOI] [PubMed] [Google Scholar]
- 31.Follet H, Boivin G, Rumelhart C, Meunier PJ. The degree of mineralization is a determinant of bone strength: a study on human calcanei. Bone. 2004;34:783–789. doi: 10.1016/j.bone.2003.12.012. [DOI] [PubMed] [Google Scholar]
- 32.Yeni YN, Brown CU, Norman TL. Influence of bone composition and apparent density on fracture toughness of the human femur and tibia. Bone. 1998;22:79–84. doi: 10.1016/s8756-3282(97)00227-5. [DOI] [PubMed] [Google Scholar]
- 33.McCalden RW, McGeough JA, Barker MB, Court-Brown CM. Age-related changes in the tensile properties of cortical bone. The relative importance of changes in porosity, mineralization, and microstructure. J Bone Joint Surg Am. 1993;75:1193–1205. doi: 10.2106/00004623-199308000-00009. [DOI] [PubMed] [Google Scholar]
- 34.Ding M, Dalstra M, Danielsen CC, Kabel J, Hvid I, Linde F. Age variations in the properties of human tibial trabecular bone. J Bone Joint Surg Br. 1997;79:995–1002. doi: 10.1302/0301-620x.79b6.7538. [DOI] [PubMed] [Google Scholar]
- 35.Zioupos P, Kaffy C, Currey JD. Tissue heterogeneity, composite architecture and fractal dimension effects in the fracture of ageing human bone. International Journal of Fracture. 2006;139:407–424. [Google Scholar]
- 36.Nyman JS, Roy A, Tyler JH, Acuna RL, Gayle HJ, Wang X. Age-related factors affecting the postyield energy dissipation of human cortical bone. J Orthop Res. 2007;25:646–655. doi: 10.1002/jor.20337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vose GP, Kubala AL. Bone strength - its relationship to x-ray determined ash content. Human Biology. 1959;31:261–270. [Google Scholar]
- 38.Currey JD. The effect of porosity and mineral content on the Young's modulus of elasticity of compact bone. J Biomech. 1988;21:131–139. doi: 10.1016/0021-9290(88)90006-1. [DOI] [PubMed] [Google Scholar]
- 39.Currey JD. Strain rate and mineral content in fracture models of bone. J Orthop Res. 1988;6:32–38. doi: 10.1002/jor.1100060105. [DOI] [PubMed] [Google Scholar]
- 40.Schaffler MB, Burr DB. Stiffness of compact bone: effects of porosity and density. J Biomech. 1988;21:13–16. doi: 10.1016/0021-9290(88)90186-8. [DOI] [PubMed] [Google Scholar]
- 41.Hernandez CJ, Beaupre GS, Keller TS, Carter DR. The influence of bone volume fraction and ash fraction on bone strength and modulus. Bone. 2001;29:74–78. doi: 10.1016/s8756-3282(01)00467-7. [DOI] [PubMed] [Google Scholar]
- 42.Roschger P, Rinnerthaler S, Yates J, Rodan GA, Fratzl P, Klaushofer K. Alendronate increases degree and uniformity of mineralization in cancellous bone and decreases the porosity in cortical bone of osteoporotic women. Bone. 2001;29:185–191. doi: 10.1016/s8756-3282(01)00485-9. [DOI] [PubMed] [Google Scholar]
- 43.Roschger P, Paschalis EP, Fratzl P, Klaushofer K. Bone mineralization density distribution in health and disease. Bone. 2007 doi: 10.1016/j.bone.2007.10.021. [DOI] [PubMed] [Google Scholar]
- 44.van der Linden JC, Birkenhager-Frenkel DH, Verhaar JA, Weinans H. Trabecular bone's mechanical properties are affected by its non-uniform mineral distribution. J Biomech. 2001;34:1573–1580. doi: 10.1016/s0021-9290(01)00146-4. [DOI] [PubMed] [Google Scholar]
- 45.Jaasma MJ, Bayraktar HH, Niebur GL, Keaveny TM. Biomechanical effects of intraspecimen variations in tissue modulus for trabecular bone. J Biomech. 2002;35:237–246. doi: 10.1016/s0021-9290(01)00193-2. [DOI] [PubMed] [Google Scholar]
- 46.Bourne BC, van der Meulen MC. Finite element models predict cancellous apparent modulus when tissue modulus is scaled from specimen CT-attenuation. J Biomech. 2004;37:613–621. doi: 10.1016/j.jbiomech.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 47.van Lenthe GH, Stauber M, Muller R. Specimen-specific beam models for fast and accurate prediction of human trabecular bone mechanical properties. Bone. 2006;39:1182–1189. doi: 10.1016/j.bone.2006.06.033. [DOI] [PubMed] [Google Scholar]
- 48.Liu XS, Sajda P, Saha PK, Wehrli FW, Bevill G, Keaveny TM, Guo XE. Complete Volumetric Decomposition of Individual Trabecular Plates and Rods and Its Morphological Correlations with Anisotropic Elastic Moduli in Human Trabecular Bone. J Bone Miner Res. 2007 doi: 10.1359/JBMR.071009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Silva MJ, Gibson LJ. Modeling the mechanical behavior of vertebral trabecular bone: Effects of age-related changes in microstructure. Bone. 1997;21:191–199. doi: 10.1016/s8756-3282(97)00100-2. [DOI] [PubMed] [Google Scholar]
- 50.Vajjhala S, Kraynik AM, Gibson LJ. A cellular solid model for modulus reduction due to resorption of trabeculae in bone. J Biomech Eng. 2000;122:511–515. doi: 10.1115/1.1289996. [DOI] [PubMed] [Google Scholar]
- 51.Guo XE, Kim CH. Mechanical consequence of trabecular bone loss and its treatment: a three-dimensional model simulation. Bone. 2002;30:404–411. doi: 10.1016/s8756-3282(01)00673-1. [DOI] [PubMed] [Google Scholar]
- 52.Smit TH, Burger EH. Is BMU-coupling a strain-regulated phenomenon? A finite element analysis. J Bone Miner Res. 2000;15:301–307. doi: 10.1359/jbmr.2000.15.2.301. [DOI] [PubMed] [Google Scholar]
- 53.van der Linden JC, Homminga J, Verhaar JA, Weinans H. Mechanical consequences of bone loss in cancellous bone. J Bone Miner Res. 2001;16:457–465. doi: 10.1359/jbmr.2001.16.3.457. [DOI] [PubMed] [Google Scholar]
- 54.McNamara LM, Van der Linden JC, Weinans H, Prendergast PJ. Stress-concentrating effect of resorption lacunae in trabecular bone. J Biomech. 2006;39:734–741. doi: 10.1016/j.jbiomech.2004.12.027. [DOI] [PubMed] [Google Scholar]
- 55.Hernandez CJ, Gupta A, Keaveny TM. A biomechanical analysis of the effects of resorption cavities on cancellous bone strength. J Bone Miner Res. 2006;21:1248–1255. doi: 10.1359/jbmr.060514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Parfitt AM. Targeted and nontargeted bone remodeling: relationship to basic multicellular unit origination and progression. Bone. 2002;30:5–7. doi: 10.1016/s8756-3282(01)00642-1. [DOI] [PubMed] [Google Scholar]
- 57.Reeve J. A stochastic analysis of iliac trabecular bone dynamics. Clin Orthop. 1986;261:264–278. [PubMed] [Google Scholar]
- 58.Mosekilde L. Consequences of the remodelling process for vertebral trabecular bone structure: a scanning electron microscopy study (uncoupling of unloaded structures) Bone Miner. 1990;10:13–35. doi: 10.1016/0169-6009(90)90046-i. [DOI] [PubMed] [Google Scholar]
- 59.Eriksen EF. Normal and pathological remodeling of human trabecular bone: three dimensional reconstruction of the remodeling sequence in normals and in metabolic bone disease. Endocr Rev. 1986;7:379–408. doi: 10.1210/edrv-7-4-379. [DOI] [PubMed] [Google Scholar]
- 60.Slyfield CR, Tomlinson RE, Tkachenko EV, Niemeyer KE, Pattanacharoenphon CG, Steyer G, Kazakia GJ, Wilson DL, Hernandez CJ. 3D visualization and measurement of resorption cavities in cancellous bone. 54th Annual Meeting of the Orthopaedic Research Society; San Francisco, CA, USA: 2008. p. 956. [Google Scholar]
- 61.Wang X, Shen X, Li X, Agrawal CM. Age-related changes in the collagen network and toughness of bone. Bone. 2002;31:1–7. doi: 10.1016/s8756-3282(01)00697-4. [DOI] [PubMed] [Google Scholar]
- 62.Hernandez CJ, Tang SY, Baumbach BM, Hwu PB, Sakkee AN, van der Ham F, Degroot J, Bank RA, Keaveny TM. Trabecular microfracture and the influence of pyridinium and non-enzymatic glycation-mediated collagen cross-links. Bone. 2005;37:825–832. doi: 10.1016/j.bone.2005.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Viguet-Carrin S, Roux JP, Arlot ME, Merabet Z, Leeming DJ, Byrjalsen I, Delmas PD, Bouxsein ML. Contribution of the advanced glycation end product pentosidine and of maturation of type I collagen to compressive biomechanical properties of human lumbar vertebrae. Bone. 2006;39:1073–1079. doi: 10.1016/j.bone.2006.05.013. [DOI] [PubMed] [Google Scholar]
- 64.Nyman JS, Roy A, Acuna RL, Gayle HJ, Reyes MJ, Tyler JH, Dean DD, Wang X. Age-related effect on the concentration of collagen crosslinks in human osteonal and interstitial bone tissue. Bone. 2006 doi: 10.1016/j.bone.2006.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Allen MR, Gineyts E, Leeming DJ, Burr DB, Delmas PD. Bisphosphonates alter trabecular bone collagen cross-linking and isomerization in beagle dog vertebra. Osteoporos Int. 2007 doi: 10.1007/s00198-007-0533-7. [DOI] [PubMed] [Google Scholar]