Summary
The arcuate nucleus of the hypothalamus (ARH) is a key component of hypothalamic pathways regulating energy balance and leptin is required for normal development of ARH projections. Diet-induced obesity (DIO) has a polygenic mode of inheritance and DIO individuals develop the metabolic syndrome when a moderate amount of fat is added to the diet. Here we demonstrate that rats selectively bred to develop DIO, which are known to be leptin resistant before they become obese, have defective ARH projections that persist into adulthood. Furthermore, the ability of leptin to activate intracellular signaling in ARH neurons in vivo, and to promote ARH neurite outgrowth in vitro, is significantly reduced in DIO neonates. Thus, animals that are genetically predisposed toward obesity display an abnormal organization of hypothalamic pathways involved in energy homeostasis that may be the result of diminished responsiveness of ARH neurons to the trophic actions of leptin during postnatal development.
Introduction
It is increasingly accepted that obesity results from a combination of genetic and environmental factors (Bouret and Simerly, 2006; Levin, 2006; Martin-Gronert and Ozanne, 2005; Plagemann, 2006). Rodent models of obesity are valuable tools for studying the underlying biological processes that contribute to the development of obesity in humans. The model of diet-induced obesity (DIO) in rodents is particularly suited to this task as DIO rats share several features with human obesity, including polygenic inheritance (Levin et al., 2003a). In outbred Sprague-Dawley rats fed a moderate-fat, high energy diet (HE), about one-half develop DIO, whereas the remaining rats are diet resistant (DR), gaining no more weight than chow-fed controls (Levin et al., 1989).
One of the particular traits of DIO rats is that they exhibit leptin resistance characterized by elevated serum leptin and a decreased anorectic and thermogenic response to exogenous leptin, even before the animals are exposed to HE diet (Gorski et al., 2007; Levin and Dunn-Meynell, 2002b; Levin et al., 2004). Interestingly, these metabolic abnormalities appear even before the animals are exposed to HE diet. Cumulative evidence suggests that the leptin resistance observed in DIO rats is mediated through central leptin insensitivity. DIO rats have decreased expression of mRNA for the long form of leptin receptor (LRb) associated with reduced 125I-leptin binding in hypothalamic nuclei known to mediate the anorectic actions of leptin, such as the arcuate (ARH), ventromedial (VMH) and dorsomedial (DMH) nuclei of the hypothalamus (Irani et al., 2007; Levin et al., 2004; Levin et al., 2003b). Furthermore, leptin-induced phosphorylation of STAT3, a key signaling pathway coupled to LRb, is reduced in the hypothalamus of DIO rats before they become obese (Levin et al., 2004).
During neonatal life, leptin plays a critical role in the development of hypothalamic circuits that regulate feeding (Bouret et al., 2004c). Development of projection pathways from the ARH is disrupted in leptin-deficient (Lepob/Lepob) neonates, and this defect appears to be persistent since a normal pattern of innevation is never achieved in adult Lepob/Lepob mice (Bouret et al., 2004c). Furthermore, both orexigenic AgRP/NPY and anorexigenic POMC projections are affected by leptin deficiency (Bouret et al., 2004c).
Although DIO is a widespread phenomenon, the neurobiological mechanisms that contribute to the phenotype are poorly understood. In the present study we tested the hypothesis that reduced central leptin sensitivity observed in DIO rats is associated with abnormal development of projection pathways from the ARH. To test this hypothesis, we compared development of ARH circuits in offspring of DIO rats to pups born from DR mothers. We also assessed the ability of leptin to induce neurite extension in vitro from ARH neurons derived from DIO or DR rats.
Results
Development of ARH Circuits is Altered in Offspring of DIO dams
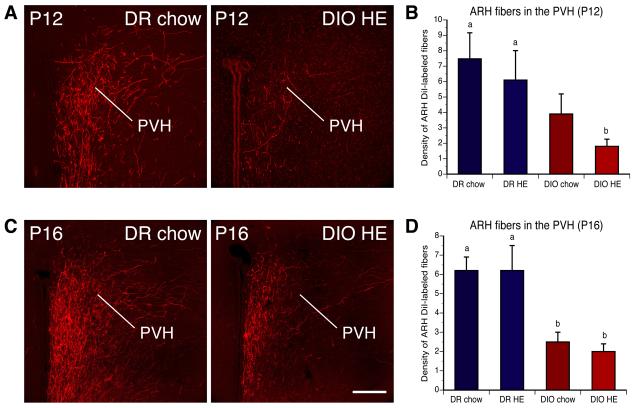
To investigate if development of ARH projections in DIO rats was different from that of DR rats, we placed crystals of 1,1'-dioctadecyl-3,3,3',3'-tetramethylindocarbocyanine perchlorate (DiI), a fluorescent tracer that labels axonal projections, into the ARH of brains derived from DR and DIO rats perfused at postnatal day 12 (P12) and P16. Discrete injection sites were obtained in 38 cases in which crystalline deposits of DiI were confined to the ARH (P12 DR chow rats, n = 4; P16 n = 5; P12 DR HE diet n = 5; P16, n = 4; P12 DIO chow, n = 4; P16, n = 5; P12 DIO HE diet, n = 6, P16, n = 5). In these cases, analysis of the implantation sites showed that the DiI extended throughout the ARH without significant spread of the tracer into adjacent areas (Fig. S1). Similar to findings reported previously in mice (Bouret et al., 2004b; Bouret et al., 2004c), the majority of labeled fibers emanating from the ARH were confined to the periventricular zone of the hypothalamus, which appears to be the major route for ascending ARH efferent connections. We paid particular attention to the development of projections from the ARH to the paraventricular nucleus of the hypothalamus (PVH), because of its well-established importance in the neural control of energy balance (Elmquist, 2005).
Analysis of DiI-labeled tissue revealed that by P12, projection pathways from the ARH to the PVH appear fully developed in the offspring of DR dams. In DR rats, labeled fibers extended rostrally through the periventricular pathway to provide dense innervation to the parvicellular parts of the PVH by P12 (Fig. 1A and B). However, fewer ARH-labeled axons were found laterally, in magnocellular parts of the nucleus. Although the overall distribution of labeled fibers was quite similar in DIO rats to that observed in DR rats, clear differences were apparent in the density of labeled fibers. In the PVH of DIO offspring, there were two to four times fewer labeled fibers compared with that observed in DR offspring on P12 (Fig. 1B). The average fiber densities in the PVH remained significantly lower in DIO rats on P16 compared to that of DR animals at the same age (Fig. 1D). A substantial disruption in the density of labeled fibers was observed in the dorsal zone of the medial parvicellular part of the PVH, and in the posterior magnocellular part of the PVH.
Figure 1. Disruption of projection pathways from ARH to the PVH in offspring of diet-induced obese dams.
A,C) Confocal images and B,D) quantitative comparisons of arcuate DiI-labeled fibers in the paraventricular nucleus of the hypothalamus (PVH) of offspring on P12 (A,B) and P16 (C,D) of diet-induced obese (DIO) and diet resistant (DR) dams fed with chow or high energy (HE) diet throughout pregnancy and lactation. Pups born to DIO dams showed a significant reduction in the density of DiI-labeled fibers innervating the PVH, relative to that of DR pups, regardless of maternal diet. Scale bar, 200 um. Data are presented as mean ± SEM. P < 0.05 between a and b.
To examine whether maternal diet influences development of ARH projections, we also evaluated the density of ARH projections to PVH of offspring derived from DIO and DR dams that were fed with either chow, or high energy (HE), diets throughout pregnancy and lactation. No significant differences in the density of DiI-labeled fibers were found between offspring of dams fed the chow or HE diet in either DIO or DR rats (Fig. 1B and D), suggesting that maternal diet was not a significant developmental determinant of ARH projections.
Permanent Disruption of Arcuate AgRP Fibers in DIO rats
To determine if the defects in ARH projections observed in neonatal DIO rats were permanent, we performed immunohistochemical labeling of AgRP in brain sections from DIO and DR rats. Because in adult animals neurons that express AgRP are restricted to NPY-containing neurons of the ARH, AgRP immunoreactive (IR) fibers serve as a marker for projections from ARH NPY neurons. As observed with the axonal labeling study in neonates, the density of AgRP-IR fibers was severely reduced in the PVH of adult DIO rats compared with that of DR rats (Fig. 2). In adults, the density of AgRP-IR fibers in the PVH remained two to four-fold lower in DIO animals relative to that of DR rats. Maternal and postnatal diets did not significantly affect the density of AgRP-IR fibers in DIO and DR rats. In contrast to what was observed with AgRP innervation, the density of aMSH-IR fibers in the PVH of DIO rats was not significantly different from that of DR rats. However, within the DIO group, the density of aMSH-IR fibers innervating the PVH was significantly reduced in offspring of chow-fed DIO dams compared to that of offspring of DIO dams fed with HE diet (Fig. 3).
Figure 2. Arcuate AgRP projections to the PVH are altered in adult DIO rats.
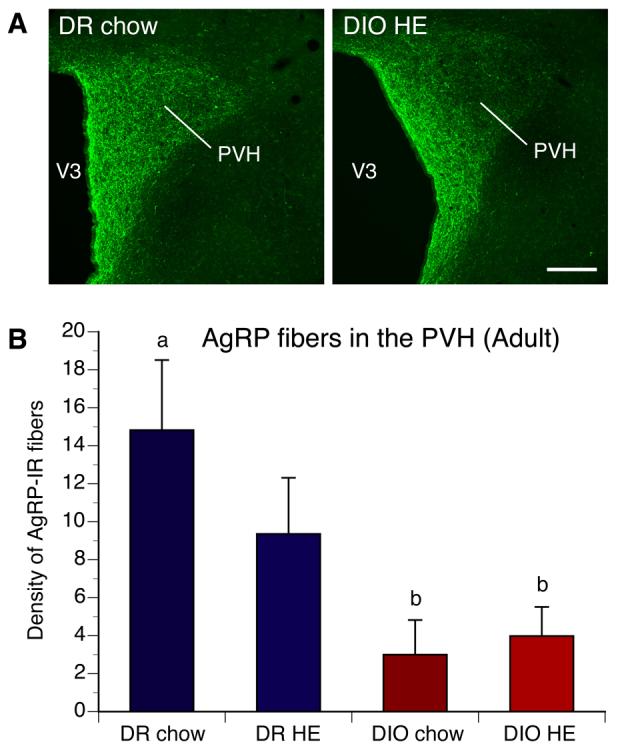
A) Confocal images and B) quantitative comparisons of AgRP-IR fibers innervating the paraventricular nucleus of the hypothalamus (PVH) of adult DIO and DR offspring of rats whose dams were fed chow or high energy (HE) diet during pregnancy and lactation. DIO animals showed a significant reduction in the density of AgRP-IR innervating the PVH, relative to that of DR rats, regardless of maternal diet. V3, third ventricle. Scale bar, 160 um. Data are presented as mean ± SEM. P < 0.05 between a and b.
Figure 3. aMSH projections to the PVH in adult DIO and DR rats.
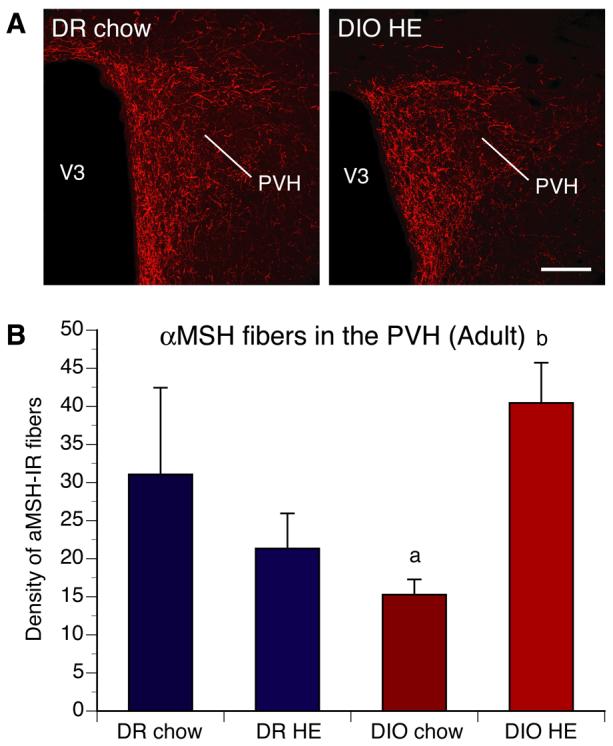
A) Confocal images and B) quantitative comparisons of aMSH-IR fibers innervating the paraventricular nucleus of the hypothalamus (PVH) of adult DIO and DR offspring of rats whose dams were fed chow or high energy (HE) diet during pregnancy and lactation. No differences in aMSH-IR fibers density in the PVH were found between DIO and DR rats overall. However, offspring of chow-fed DIO animals showed a significant reduction in the density of aMSH-IR innervating the PVH, relative to that of HE diet DIO offspring. V3, third ventricle. Scale bar, 160 um. Data are presented as mean ± SEM. P < 0.05 between a and b.
Decreased Leptin Signaling in the ARH of DIO rats During Postnatal Development
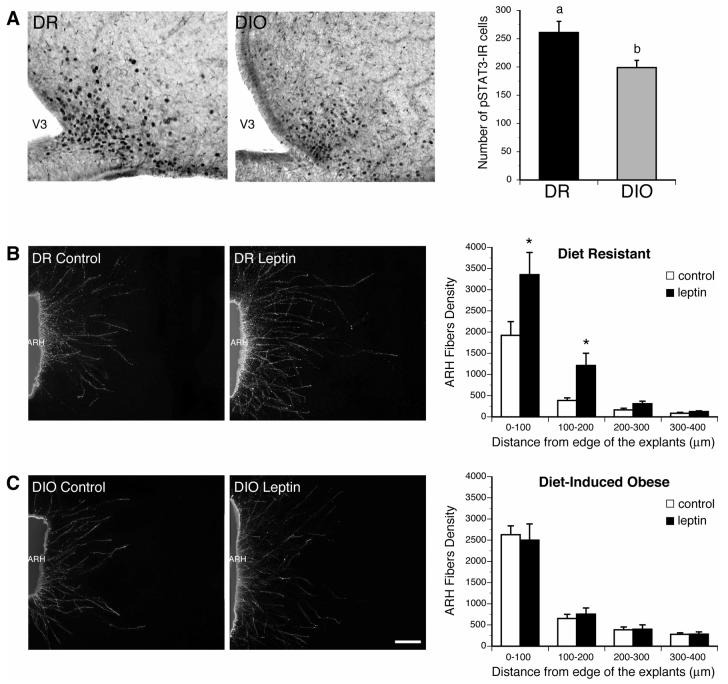
Adult DIO rats show evidence of central leptin resistance, but whether neonates are similarly affected is unknown. To determine if offspring of DIO dams display impaired leptin signaling in the ARH we evaluated the number of pSTAT3-IR neurons in the ARH of DR and DIO pups on P10, 45 min after injection with leptin. These pups were derived from chow-fed dams. Leptin treatment caused marked increases in pSTAT3 staining in the ARH of DR pups on P10 (Fig. 4A). However, the same leptin treatment resulted in significantly fewer pSTAT3-IR cells in the ARH of ten days old DIO rats. A quantitative analysis of this experimental material revealed that the number of pSTAT3-IR cells in the ARH of DIO rats was reduced by more than 24% compared with that of DR rats (Fig. 4A). These results suggest that leptin signaling in ARH neurons is impaired during postnatal development in DIO rats, relative to that of DR animals.
Figure 4. Response of ARH neurons to leptin is reduced in DIO neonates.
A) Leptin-induced phosphoSTAT3-immunoreactivivity (pSTAT3-IR) in the ARH of DR and DIO pups on P10. DIO neonates show a 24% reduction in the number of pSTAT3-IR cells in the ARH following leptin administration as compared to that of DR pups. B,C) Isolated organotypic cultures of ARH from P6 DR (A) and DIO (B) rats were incubated for 36 h with leptin (5 ul/ml) or vehicle and then immunostained with beta III tubulin, a marker of neurites. Leptin induces an approximative 2-3 times increase in fiber density from ARH explants of DR rats. This effect was abolished if ARH explants were derived from DIO rats. Scale bar, 100 um. Data are presented as mean ± SEM. *, P < 0.001 between control and leptin-treated explants.
Trophic Action of Leptin on ARH Neurons is Reduced in DIO Neonates
Because DIO neonates exhibit central leptin resistance and because leptin is known to be critical for formation of ARH projections (Bouret et al., 2004c), we assessed whether alteration of projection pathways from the ARH in DIO rats was due to reduced ability of leptin to promote ARH neurite extension during development. Isolated organotypic explants of the ARH derived from DIO and DR pups were exposed to leptin in vitro and the trophic response was documented by measuring neurite outgrowth. By 36 hrs after addition of leptin (5 ug/ml) to the culture medium, there was a significant induction in the density of TuJ-IR neurites extending 100-200 um from the edge of ARH explants derived from DR pups, relative to explants maintained in medium lacking leptin (Fig. 4B). In contrast, this response was severely blunted if the explant was derived from a DIO animal (Fig. 4C). Neurite extension from ARH neurons treated with leptin did not differ from that of control explants, however, the overall density of neurites extending from explants from DIO pups appeared slightly greater than that or control DR explants. These observations indicate that ARH neurons derived from DIO rats are significantly less responsive to the neurotrophic action of leptin than are ARH neurons in explants derived from DR rats.
Discussion
It is generally accepted that projection pathways from the ARH to other parts of the hypothalamus play a key role in the neural control of food intake and body weight. However, an association between polygenic predisposition toward obesity and development of these critical pathways has never been examined. The axonal labeling experiments presented here indicate that pups born to dams that are predisposed to develop DIO display a significant reduction in the density of ARH axons innervating the PVH compared to offspring of DR dams. Notably, some of the most severely affected projections were those to subdivisions of the PVH that send projections to preganglionic neurons of the autonomic nervous system in the dorsal vagal complex and spinal cord (Swanson and Sawchenko, 1980). In addition, the defects in projections of arcuate AgRP/NPY neurons seem to be permanent in DIO animals since, in adults, the density of AgRP-IR fibers innervating the PVH remained lower, compared with that of DR rats. Since expression of AgRP/NPY mRNA is unaltered in adult DIO rats (Gorski et al., 2007; Ricci and Levin, 2003), low densities of AgRP-IR fibers seem to reflect a reduction in the density of NPY projections from the ARH. Similarly, the density of aMSH-IR fibers was reduced in the PVH of chow-fed DIO rats. However because these fibers may originate from neurons located in the ARH, or in the nucleus of the tractus solitarius (NTS) (Broberger et al., 1998; Sawchenko and Swanson, 1983), the decreased density of aMSH-containing fibers found in the PVH of chow-fed DIO may be due to altered projections from ARH POMC neurons, as well as possible changes in projections from POMC neurons located in the brain stem. Similarly, Enriori et al. found that leptin failed to modulate the secretion of melanocortin peptides in another animal model of diet-induced obesity (Enriori et al., 2007).
Despite a reduction in ARH projections, no differences have been reported in the size of the ARH, PVH, and DMH between DIO and DR rats (Levin and Dunn-Meynell, 2002a), suggesting that cell number in the ARH was the same in offspring of DIO and DR rats. Thus, the DIO genotype may specifically affect axonal extension of ARH neurons, as opposed to other neurodevelopmental processes specifying neuronal cell number such as neurogenesis, apoptosis, or neuronal migration. In addition, the DIO genotype seems to alter the density, but not the pattern of innervation, since the overall distribution of labeled fibers in the PVH was similar in DIO and DR rats.
The retarded and diminished development of ARH projections may be due to a reduction of hypothalamic leptin signaling in DIO rats that is present during the critical postnatal period when ARH projections develop. Adult DIO rats have reduced expression of LRb mRNA and of 125I-leptin binding, as well as altered leptin-induced levels of pSTAT3, in several hypothalamic nuclei, including the ARH (Irani et al., 2007; Levin et al., 2004; Levin et al., 2003b). Here we demonstrate that DIO neonates show diminished leptin signaling as evidenced by a significant reduction in the number of pSTAT3-IR cells present in the ARH following leptin administration compared to the significant induction in pSTAT3-IR seen in DR pups. This finding is consistent with recent data suggesting that leptin signaling is required for the normal development of ARH projections in mice (Bouret et al., 2004a). Leptin deficiency in mice causes a permanent reduction in the density of axons that project from the ARH to the PVH (Bouret et al., 2004c) and similar disruptions were observed in leptin receptor-deficient animals such as Leprdb/Leprdb mice (Bouret et al., 2004a) and Zucker rats (Bouret and Simerly, 2007). Furthermore, data in mice indicate that the LRb-STAT3 signaling pathway is specifically required for normal development of ARH projections (Bouret et al., 2004a). Therefore, suppression of leptin signaling in ARH neurons likely contributes to the relative leptin insensitivity observed in the ARH of DIO rats, which in turn may be responsible for abnormal development of ARH projections. Consistent with this hypothesis, our in vitro experiments revealed that ARH neurons of DIO rats are not responsive to the trophic action of leptin, whereas leptin caused robust extension of neurite outgrowth from ARH explants derived from DR pups. The neurodevelopmental abnormalities observed in DIO rats therefore seem to be caused, at least in part, by altered hypothalamic leptin sensitivity at the level of the ARH neurons, rather than by alterations in leptin levels, which appear to be similar in DIO and DR rats until the 8th week of life (Ricci and Levin, 2003). Whether this apparent alteration in leptin sensitivity is due to leptin receptor mediated events, or changes in the neurodevelopmental mechanisms influenced by leptin signaling, remains to be determined.
Attenuated insulin signaling may also have deleterious consequences for development of ARH circuits in DIO rats. Insulin levels are elevated in offspring of DIO mothers fed with a HE diet during the critical period of hypothalamic development (Gorski et al., 2007). In addition, the milk of DIO dams contains high levels of insulin (Gorski et al., 2006), and 125I-insulin binding is reduced in the ARH of DIO, compared with DR rats, regardless of diet (Irani et al., 2007). When considered together with the overall importance of insulin signaling for other aspects of brain development (Heidenreich KA and SP., 1989; Puro DG and E., 1984; Recio-Pinto et al., 1984), these observations suggest that altered insulin signaling in DIO rats during development may contribute to abnormal development of ARH projections. That direct injection of insulin to the region of the mediobasal hypothalamus in the immediate postnatal period --a critical period for development of projections from the ARH to the PVH— induces lasting effects on body weight and energy balance later in life, is consistent with this hypothesis (Plagemann A et al., 1992).
In addition to genetic factors, a variety of studies in humans and rodents suggests that maternal nutrition and the presence of maternal obesity or diabetes during gestation can have long-lasting effects on the development of obesity and diabetes in offspring. Thus, pups born to obese mothers have an enhanced risk of becoming obese and developing metabolic syndrome, especially if they have a genetic predisposition to develop DIO (Guo and Jen, 1995; Levin and Govek, 1998). Furthermore, exposure of pregnant dams to a high-fat diet results in permanent changes in gene expression of hypothalamic neuropeptides regulating energy balance in the offspring (Gorski et al., 2007). However, in our animal model, maternal diet did not have a significant influence on development of ARH projections. These results suggest that in offspring of dams selectively bred to express the DIO or DR genotype, genetic background prevails over environmental factors to influence formation of ARH projection pathways. However, maternal diet did appear to influence development of projection pathways containing aMSH in DIO rats. Since these projections may originate from either ARH or NTS neurons, it remains to be determined whether maternal nutrition impacts the development of one of these populations of POMC neurons. Furthermore, we cannot exclude the possibility that maternal or post-weaning diet may induce more subtle changes in ARH circuitry, such as changes in synapse density or peptide release. It is also possible that dietary factors may influence development of other components of hypothalamic feeding circuits yet to be identified. Nevertheless, the results of the present study strongly suggest that one of the underlying causes of leptin resistance in DIO animals may be immature formation of axonal projections that normally distribute leptin signals throughout the hypothalamus.
Experimental Procedures
Animals and diet
Animal usage was in compliance with and approved by the Institutional Animal Care and Use Committee of the East Orange Veterans Affairs Medical Center (East Orange, NJ) and of the Saban Research Institute of Childrens Hospital of Los Angeles. The breeding pairs were derived from rats bred selectively for their propensity to genotypically develop DIO or DR (Levin et al., 1997, Levin et al., 2003a). DIO and glucose-intolerant phenotypes appear inherited as a polygenic trait in this model, since breeding these rats against obesity resistant Fisher F344 rats transmits the phenotype completely (Levin et al., 2003a). Rats were housed at 23-24°C on a 12:12-h light-dark cycle. At weaning, 14 DIO and 14 DR females were divided into one of four groups of 7 dams each: Group 1) DR chow dams were maintained on Purina rat chow (#5001). Group 2) DR Ensure dams were fed a high energy (HE) diet composed of 8% corn oil, 44% sweetened condensed milk, and 48% Purina rat chow (Research Diets #D12266B) ad libitum. They were also given access to chocolate Ensure (Ross Products) (Levin and Govek, 1998). Group 3) DIO chow dams were fed chow. Group 4) DIO HE-diet rats were fed the HE diet (Levin and Govek, 1998). After 8 weeks on diet they were mated with males of the same genotype and then were kept on their respective diets through gestation and lactation. At birth, all litters were culled to 10 pups. At weaning (P21), male pups (n = 6-7 per group) were fed with powdered chow (pups from DR- and DIO- chow dams) or with HE diet (pups from DR- and DIO- HE diets) until 12 weeks of age.
DiI implants
Male rat pups from each group (n = 8-10 per group) of animals were anesthetized and perfused on P12 and P16, with 4% paraformaldehyde. The brains were removed, numerically coded to insure unbiased processing and analysis, and crystals of DiI were implanted as described previously (Bouret et al., 2004b; Bouret et al., 2004c). Briefly, an insect pin was used to place a crystal of DiI (1,1'-dioctadecyl-3,3,3',3'-tetramethylindocarbocyanine perchlorate; Molecular Probes) (35 um in diameter) into the ARH of each brain under visual guidance. After incubation in the dark for 5 weeks at 37°C, sections were collected through the hypothalamus from each brain, and evaluated with both conventional fluorescence and confocal microscopy.
AgRP and aMSH Immunohistochemistry
Because the efficacy of DiI labeling decreases in adult animals, we used AgRP immunolabeling as a marker for ARH projections (Bouret et al., 2004c). Anesthetized male rats were perfused transcardially at 12 weeks of age with 4% paraformaldehyde, the brains frozen and sectioned at 30 um, and then processed for immunofluoresence as described elsewhere (Bouret et al., 2004c).
Quantitative analysis
For quantification, 4-6 animals with implant sites that were closely matched in terms of location and the diffusion of the tracer were selected for analysis. A series of 20 adjacent optical sections were collected at 1 um intervals through the PVH (2 sections/animal) by using a Leica SP-confocal microscope equipped with a 10X objective (numerical aperture, 0.40; working distance, 360 um). Image analysis was performed by using Metamorph image analysis software (Universal Imaging) (Bouret et al., 2004b; Bouret et al., 2004c). Briefly, each image plane was binarized and skeletonized. The integrated intensity was then calculated for each image, which was proportional to the total length of labeled fibers in the image. This procedure was carried out on each image plane in the stack, and the values for all image planes in a stack summed.
pSTAT3 immunostaining and analysis
DIO and DR rats on P10 were injected intraperitoneally with leptin (National Hormone Research Institute, 5 mg/kg, n = 6 per group) and were perfused 45 min later with a solution of 2% paraformaldehyde. We previously reported that vehicle-injected animals had a low density of pSTAT3-IR cells and displayed no variation between DIO and DR rats (Levin et al., 2004). Frozen coronal sections were cut at 35 um coronal sections and then processed for pSTAT3 immunostaining as described previously (Levin et al., 2004). pSTAT3 immunopositive cells were counted manually by using a Bioquant image analysis system (Nashville, TN). The average number of cells counted in three ARH sections in each rat was taken for statistical comparisons.
Isolated ARH explant cultures
Brains were collected from DIO and DR rats on P6 and sectioned at 200 um with a vibroslicer. The ARH was then carefully dissected out of each section under a stereomicroscope. Explants were cultured onto a rat tail collagen matrix (Upstate) in EOL-1 serum-free medium as described (Bouret et al., 2004c; Ibanez et al., 2001). Beginning on the first day in vitro (DIV) each explant was transferred to fresh medium containing either leptin (Peprotech, 5 ug/ml) or vehicle alone (5 mM Na Citrate buffer) (n = 5-7 cases per group). After 36 h the explants were fixed in paraformaldehyde and neurites were stained with beta III tubulin (TuJ monoclonal antibody, Babco). Digital images were then collected with a Zeiss Z1 motorized microscope. The resulting image stack was analyzed by using Metamorph image analysis software (Universal Imaging Corp.). Briefly, each image was deconvolved, binarized according to user defined threshold criteria, and then skeletonized. The integrated intensity was calculated in 4 different regions of interest (100×100 um) spaced at 100, 200, 300, and 400 um extending radially from the edge of the explants.
Statistical analysis
Data sets were analyzed for statistical significance using Statview (SAS Institute) for a Kruskal-Wallis ANOVA, with a Fisher's least significant difference post hoc comparison.
Supplementary Material
Acknowledgments
We wish to thank Ms. Melissa Kirigiti (Oregon National Primate Research Center) for technical assistance. This work was supported by NIH Grants DK65900 (RBS), DK30066 (JNG, BEL) and F31 NS050903 (CMP) and the Research Service of the Veterans Administration (BEL).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bouret SG, Simerly RB. Development of Leptin-Sensitive Circuits. J. Neuroendocrinol. 2007;19:575–582. doi: 10.1111/j.1365-2826.2007.01563.x. [DOI] [PubMed] [Google Scholar]
- Bouret SG, Draper SJ, Bates SH, Kirigiti MA, Chen S, Bjornholm M, Myers MG, Jr., Simerly RB. Leptin promotes formation of projection pathways from the arcuate nucleus of the hypothalamus through activation of ObRb signaling pathways; Proc of the 34nd Annual Meeting The Society For Neuroscience; San Diego, CA. 2004a. [Google Scholar]
- Bouret SG, Draper SJ, Simerly RB. Formation of Projection Pathways from the Arcuate Nucleus of the Hypothalamus to Hypothalamic Regions Implicated in the Neural Control of Feeding Behavior in Mice. J. Neurosci. 2004b;24:2797–2805. doi: 10.1523/JNEUROSCI.5369-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouret SG, Draper SJ, Simerly RB. Trophic Action of Leptin on Hypothalamic Neurons That Regulate Feeding. Science. 2004c;304:108–110. doi: 10.1126/science.1095004. [DOI] [PubMed] [Google Scholar]
- Bouret SG, Simerly RB. Developmental programming of hypothalamic feeding circuits. Clinical Genetics. 2006;70:295–301. doi: 10.1111/j.1399-0004.2006.00684.x. [DOI] [PubMed] [Google Scholar]
- Broberger C, Johansen J, Johansson C, Schalling M, Hokfelt T. The neuropeptide Y/agouti gene-related protein (AGRP) brain circuitry in normal, anorectic, and monosodium glutamate-treated mice. Proc. Natl. Acad. Sci. USA. 1998;95:15043–15048. doi: 10.1073/pnas.95.25.15043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enriori PJ, Evans AE, Sinnayah P, Jobst EE, Tonelli-Lemos L, Billes SK, Glavas MM, Grayson BE, Perello M, Nillni EA, et al. Diet-Induced Obesity Causes Severe but Reversible Leptin Resistance in Arcuate Melanocortin Neurons. Cell Metabolism. 2007;5:181–194. doi: 10.1016/j.cmet.2007.02.004. [DOI] [PubMed] [Google Scholar]
- Gorski JN, Dunn-Meynell AA, Hartman TG, Levin BE. Postnatal environment overrides genetic and prenatal factors influencing offspring obesity and insulin resistance. Am. J. Physiol. 2006;291:R768–778. doi: 10.1152/ajpregu.00138.2006. [DOI] [PubMed] [Google Scholar]
- Gorski JN, Dunn-Meynell AA, Levin BE. Maternal obesity increases hypothalamic leptin receptor expression and sensitivity in juvenile obesity-prone rats. Am. J. Physiol. 2007;292:R1782–1791. doi: 10.1152/ajpregu.00749.2006. [DOI] [PubMed] [Google Scholar]
- Guo F, Jen KLC. High-fat feeding during pregnancy and lactation affects offspring metabolism in rats. Physiology & Behavior. 1995;57:681–686. doi: 10.1016/0031-9384(94)00342-4. [DOI] [PubMed] [Google Scholar]
- Heidenreich KA, SP T. Insulin receptors mediate growth effects in cultured fetal neurons. I. Rapid stimulation of protein synthesis. Endocrinology. 1989;125:1451–1457. doi: 10.1210/endo-125-3-1451. [DOI] [PubMed] [Google Scholar]
- Ibanez MA, Gu G, Simerly RB. Target-dependent sexual differentiation of a limbic-hypothalamic neural pathway. J. Neurosci. 2001;21:5652–5659. doi: 10.1523/JNEUROSCI.21-15-05652.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irani BG, Dunn-Meynell AA, Levin BE. Altered Hypothalamic Leptin, Insulin, and Melanocortin Binding Associated with Moderate-Fat Diet and Predisposition to Obesity. Endocrinology. 2007;148:310–316. doi: 10.1210/en.2006-1126. [DOI] [PubMed] [Google Scholar]
- Joel K. Elmquist RCNBMIBBL. Identifying hypothalamic pathways controlling food intake, body weight, and glucose homeostasis. J. Comp. Neurol. 2005;493:63–71. doi: 10.1002/cne.20786. [DOI] [PubMed] [Google Scholar]
- Levin B. Metabolic imprinting: critical impact of the perinatal environment on the regulation of energy homeostasis. Phil Trans R Soc Lond B. 2006;361:1107–1121. doi: 10.1098/rstb.2006.1851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin BE, Dunn-Meynell AA. Maternal obesity alters adiposity and monoamine function in genetically predisposed offspring. Am. J. Physiol. 2002a;283:R1087–1093. doi: 10.1152/ajpregu.00402.2002. [DOI] [PubMed] [Google Scholar]
- Levin BE, Dunn-Meynell AA. Reduced central leptin sensitivity in rats with diet-induced obesity. Am. J. Physiol. 2002b;283:R941–948. doi: 10.1152/ajpregu.00245.2002. [DOI] [PubMed] [Google Scholar]
- Levin BE, Dunn-Meynell AA, Balkan B, Keesey RE. Selective breeding for diet-induced obesity and resistance in Sprague-Dawley rats. Am. J. Physiol. 1997;273:R725–730. doi: 10.1152/ajpregu.1997.273.2.R725. [DOI] [PubMed] [Google Scholar]
- Levin BE, Dunn-Meynell AA, Banks WA. Obesity-prone rats have normal blood-brain barrier transport but defective central leptin signaling before obesity onset. Am. J. Physiol. 2004;286:R143–150. doi: 10.1152/ajpregu.00393.2003. [DOI] [PubMed] [Google Scholar]
- Levin BE, Dunn-Meynell AA, McMinn JE, Alperovich M, Cunningham-Bussel A, Chua SC., Jr. A new obesity-prone, glucose-intolerant rat strain (F.DIO) Am. J. Physiol. 2003a;285:R1184–1191. doi: 10.1152/ajpregu.00267.2003. [DOI] [PubMed] [Google Scholar]
- Levin BE, Dunn-Meynell AA, Ricci MR, Cummings DE. Abnormalities of leptin and ghrelin regulation in obesity-prone juvenile rats. Am. J. Physiol. 2003b;285:E949–957. doi: 10.1152/ajpendo.00186.2003. [DOI] [PubMed] [Google Scholar]
- Levin BE, Govek E. Gestational obesity accentuates obesity in obesity-prone progeny. Am J Physiol Regul Integr Comp Physiol. 1998;275:R1374–1379. doi: 10.1152/ajpregu.1998.275.4.R1374. [DOI] [PubMed] [Google Scholar]
- Levin BE, Hogan S, Sullivan AC. Initiation and perpetuation of obesity and obesity resistance in rats. Am. J. Physiol. 1989;256:R766–771. doi: 10.1152/ajpregu.1989.256.3.R766. [DOI] [PubMed] [Google Scholar]
- Martin-Gronert MS, Ozanne SE. Programming of appetite and type 2 diabetes. Early Human Development. 2005;81:981–988. doi: 10.1016/j.earlhumdev.2005.10.006. [DOI] [PubMed] [Google Scholar]
- Plagemann A. Perinatal Nutrition and Hormone-Dependent Programming of Food Intake. Hormone Research. 2006;65:83–89. doi: 10.1159/000091511. [DOI] [PubMed] [Google Scholar]
- Plagemann A, Heidrich I, Götz F, Rohde W, G. D. Lifelong enhanced diabetes susceptibility and obesity after temporary intrahypothalamic hyperinsulinism during brain organization. Exp. Clin. Endocrinol. 1992;99:91–95. doi: 10.1055/s-0029-1211143. [DOI] [PubMed] [Google Scholar]
- Puro DG, E. A. Insulin-mediated regulation of neuronal maturation. Science. 1984;225:1170–1172. doi: 10.1126/science.6089343. [DOI] [PubMed] [Google Scholar]
- Recio-Pinto E, Lang FF, Ishii DN. Insulin and Insulin-Like Growth Factor II Permit Nerve Growth Factor Binding and the Neurite Formation Response in Cultured Human Neuroblastoma Cells. Proc. Natl. Acad. Sci. USA. 1984;81:2562–2566. doi: 10.1073/pnas.81.8.2562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricci MR, Levin BE. Ontogeny of diet-induced obesity in selectively bred Sprague-Dawley rats. Am. J. Physiol. 2003;285:R610–618. doi: 10.1152/ajpregu.00235.2003. [DOI] [PubMed] [Google Scholar]
- Sawchenko PE, Swanson LW. The organization of forebrain afferents to the paraventricular and supraoptic nuclei of the rat. J. Comp. Neurol. 1983;218:121–144. doi: 10.1002/cne.902180202. [DOI] [PubMed] [Google Scholar]
- Swanson LW, Sawchenko PE. Paraventricular nucleus: a site for the integration of neuroendocrine and autonomic mechanisms. Neuroendocrinology. 1980;31:410–417. doi: 10.1159/000123111. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.