Abstract
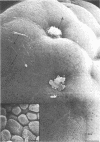
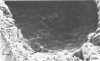
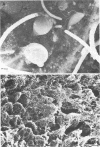
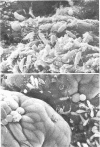
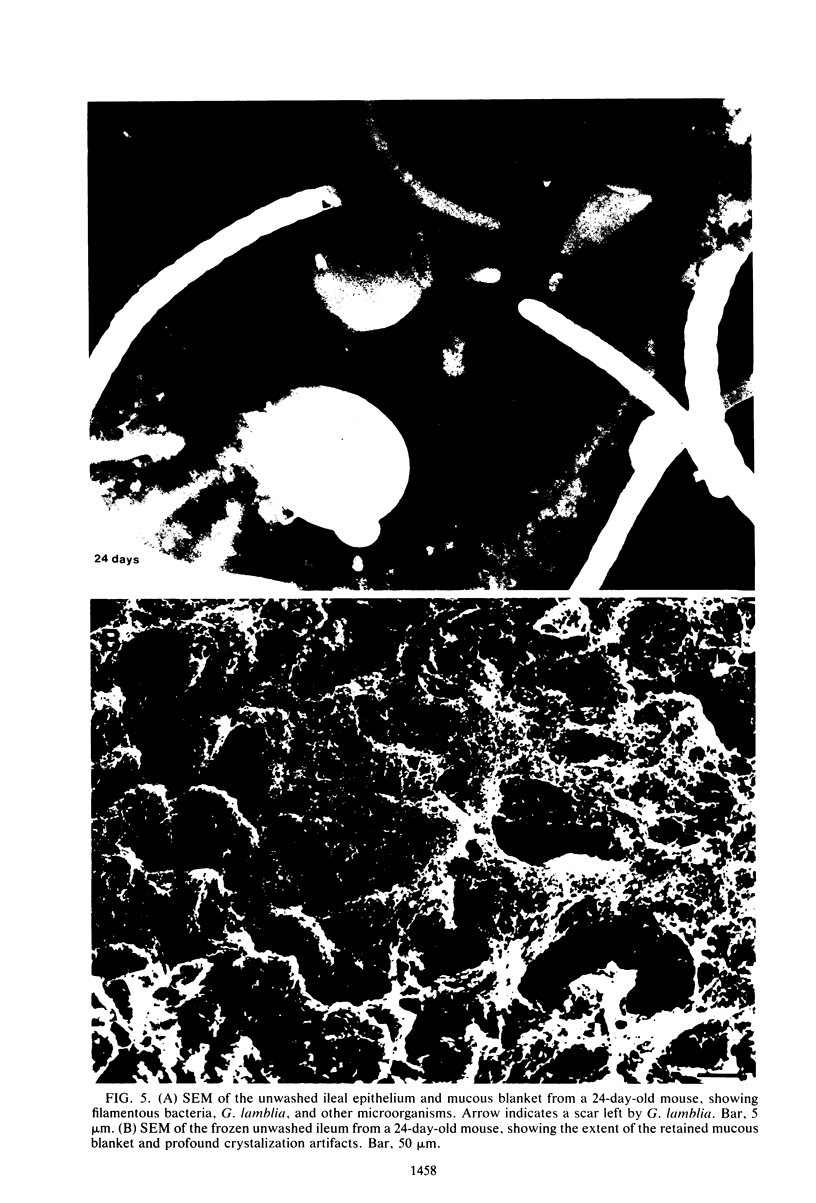
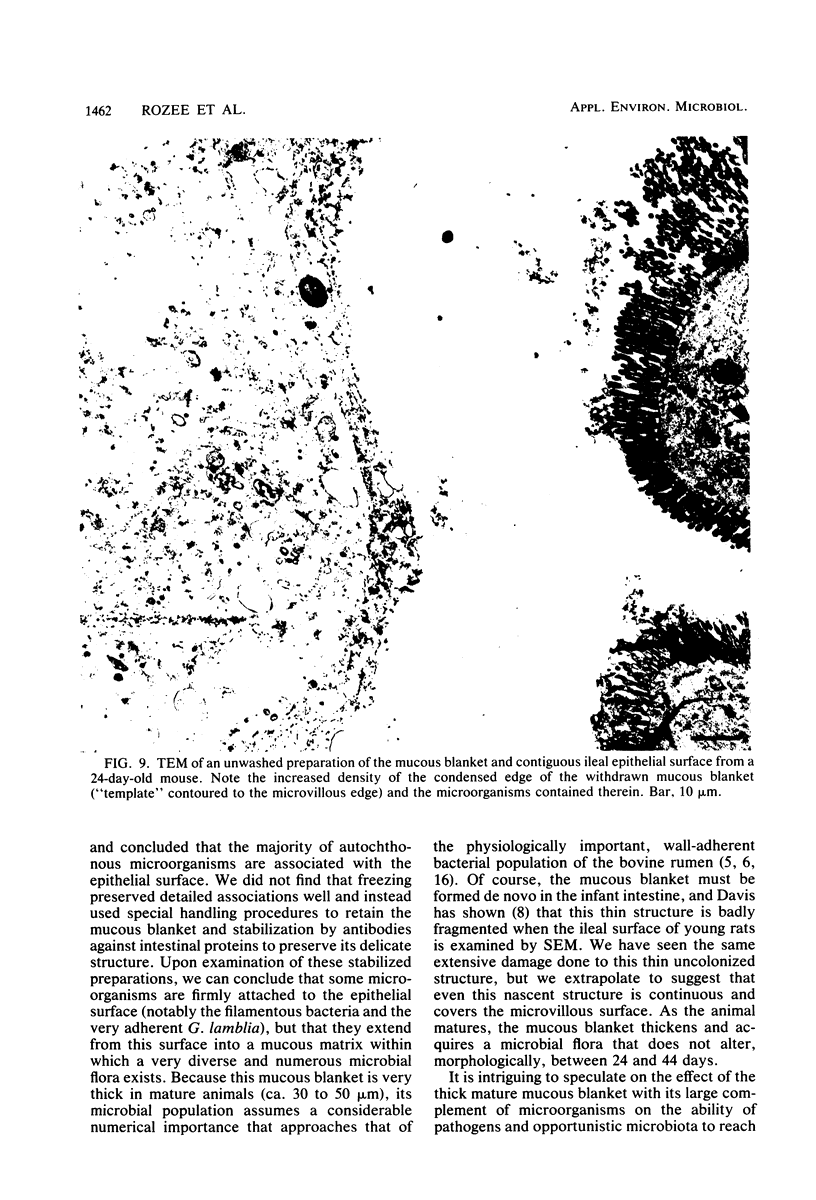
We have developed new methods to minimize fluid shear during preparation of specimens for electron microscopy and to retain the mucous blanket that covers the tissue surface of the ileum in mice. We also used general stabilization by nonspecific antibodies to minimize the collapse of the mucous layer during dehydration for electron microscopy. These methods allowed us to visualize the gradual progression of the mucous blanket from a thin diaphanous layer in newborn animals to a very thick (ca. 50 micrometers), coherent structure in older animals that contained a mixed population of bacteria and protozoa. Some bacteria, notably filamentous forms, were patently anchored to the epithelial tissue but projected into the mucous blanket, whereas others clearly existed within the mucous blanket and were unattached to the epithelial surface. Similarly, some protozoa were firmly attached to the tissue surface, whereas others were suspended in the viscous mucous blanket. In an adult animal, the mucous blanket was a very thick layer which actually occluded most of the tissue surface and contained a rich variety of bacteria and protozoa.
Full text
PDF










Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bayer M. E., Thurow H. Polysaccharide capsule of Escherichia coli: microscope study of its size, structure, and sites of synthesis. J Bacteriol. 1977 May;130(2):911–936. doi: 10.1128/jb.130.2.911-936.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg H. C. How spirochetes may swim. J Theor Biol. 1976 Feb;56(2):269–273. doi: 10.1016/s0022-5193(76)80074-4. [DOI] [PubMed] [Google Scholar]
- Chase D. G., Erlandsen S. L. Evidence for a complex life cycle and endospore formation in the attached, filamentous, segmented bacterium from murine ileum. J Bacteriol. 1976 Jul;127(1):572–583. doi: 10.1128/jb.127.1.572-583.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng K. J., McCowan R. P., Costerton J. W. Adherent epithelial bacteria in ruminants and their roles in digestive tract function. Am J Clin Nutr. 1979 Jan;32(1):139–148. doi: 10.1093/ajcn/32.1.139. [DOI] [PubMed] [Google Scholar]
- DUBOS R., SCHAEDLER R. W., COSTELLO R., HOET P. INDIGENOUS, NORMAL, AND AUTOCHTHONOUS FLORA OF THE GASTROINTESTINAL TRACT. J Exp Med. 1965 Jul 1;122:67–76. doi: 10.1084/jem.122.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis C. P., McAllister J. S., Savage D. C. Microbial colonization of the intestinal epithelium in suckling mice. Infect Immun. 1973 Apr;7(4):666–672. doi: 10.1128/iai.7.4.666-672.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis C. P. Preservation of gastrointestinal bacteria and their microenvironmental associations in rats by freezing. Appl Environ Microbiol. 1976 Feb;31(2):304–312. doi: 10.1128/aem.31.2.304-312.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis C. P., Savage D. C. Habitat, succession, attachment, and morphology of segmented, filamentous microbes indigenous to the murine gastrointestinal tract. Infect Immun. 1974 Oct;10(4):948–956. doi: 10.1128/iai.10.4.948-956.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito S. The enteric surface coat on cat intestinal microvilli. J Cell Biol. 1965 Dec;27(3):475–491. doi: 10.1083/jcb.27.3.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Malick L. E., Wilson R. B. Modified thiocarbohydrazide procedure for scanning electron microscopy: routine use for normal, pathological, or experimental tissues. Stain Technol. 1975 Jul;50(4):265–269. doi: 10.3109/10520297509117069. [DOI] [PubMed] [Google Scholar]
- McCowan R. P., Cheng K. J., Costerton J. W. Adherent bacterial populations on the bovine rumen wall: distribution patterns of adherent bacteria. Appl Environ Microbiol. 1980 Jan;39(1):233–241. doi: 10.1128/aem.39.1.233-241.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller L. L., Jacks T. J. Rapid chemical dehydration of samples for electron microscopic examinations. J Histochem Cytochem. 1975 Feb;23(2):107–110. doi: 10.1177/23.2.1117127. [DOI] [PubMed] [Google Scholar]
- Savage D. C., Dubos R., Schaedler R. W. The gastrointestinal epithelium and its autochthonous bacterial flora. J Exp Med. 1968 Jan 1;127(1):67–76. doi: 10.1084/jem.127.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spurr A. R. A low-viscosity epoxy resin embedding medium for electron microscopy. J Ultrastruct Res. 1969 Jan;26(1):31–43. doi: 10.1016/s0022-5320(69)90033-1. [DOI] [PubMed] [Google Scholar]
- Takeuchi A., Jervis H. R., Nakazawa H., Robinson D. M. Spiral-shaped organisms on the surface colonic epithelium of the monkey and man. Am J Clin Nutr. 1974 Nov;27(11):1287–1296. doi: 10.1093/ajcn/27.11.1287. [DOI] [PubMed] [Google Scholar]