Abstract
The Antarctic biota is highly endemic, and the diversity and abundance of taxonomic groups differ from elsewhere in the world. Such characteristics have resulted from evolution in isolation in an increasingly extreme environment over the last 100 Myr. Studies on Antarctic species represent some of the best examples of natural selection at the molecular, structural and physiological levels. Analyses of molecular genetics data are consistent with the diversity and distribution of marine and terrestrial taxa having been strongly influenced by geological and climatic cooling events over the last 70 Myr. Such events have resulted in vicariance driven by continental drift and thermal isolation of the Antarctic, and in pulses of species range contraction into refugia and subsequent expansion and secondary contact of genetically distinct populations or sister species during cycles of glaciation. Limited habitat availability has played a major role in structuring populations of species both in the past and in the present day. For these reasons, despite the apparent simplicity or homogeneity of Antarctic terrestrial and marine environments, populations of species are often geographically structured into genetically distinct lineages. In some cases, genetic studies have revealed that species defined by morphological characters are complexes of cryptic or sibling species. Climate change will cause changes in the distribution of many Antarctic and sub-Antarctic species through affecting population-level processes such as life history and dispersal.
Keywords: Antarctic, phylogenetics, evolution, population genetics, glacial cycles
1. Introduction
There are strong contrasts in the species diversity of terrestrial versus marine ecosystems in the Antarctic. Terrestrial systems are species poor with a flora composed mainly of mosses, liverworts and lichens, with just two flowering plants, Deschampsia antarctica and Colobanthus quitensis. The terrestrial fauna is dominated by nematodes, tardigrades, rotifers and microarthropods, including springtails and mites with the addition of a few higher insects (Convey 2001). The marine biota, in contrast, is relatively diverse with the species richness of benthic communities, for example, comparable to those of other marine ecosystems with intermediate levels of diversity (Gutt et al. 2004). However, the distribution of marine species within higher taxa differs to that elsewhere in the world. Fishes and molluscs are poorly represented in the Southern Ocean, a factor that is at least partially responsible for the major latitudinal clines in species diversity of these groups from the tropics to the Antarctic (Clarke & Johnston 2003). Some marine groups are missing in the Antarctic including brachyuran crabs, balanomorph barnacles and sharks (Barnes et al. 2006). Other groups, especially the pycnogonids and peracarid crustaceans, are exceptionally diverse in the Antarctic compared to other parts of the world (Clarke & Johnston 2003).
Despite the obvious differences between the Antarctic terrestrial and marine biotas, common forces may have driven evolution in both ecosystems, including isolation, selection, extinction, immigration, vicariance and dispersal (e.g. Briggs 2003). More than 150 years ago, scientists recognized that the fragmented distribution of the Southern Hemisphere flora may have resulted from the previous existence of a southern supercontinent now known as Gondwana. It is accepted that the break-up of Gondwana over the last 165 Myr was a major factor in determining the distribution of species both in the Antarctic continent and in the Southern Ocean. This event led to the physical separation of the Antarctic continent and coastal seas from Australia, Africa and South America. However, the switching of the climate from ‘greenhouse’ conditions in the Cretaceous to ‘icehouse’ conditions during the Eocene (42 Myr BP) led to cycles of glaciation in the Antarctic with the establishment of a permanent ice sheet from approximately 34 Myr BP (Tripati et al. 2005). This led to a strong latitudinal gradient in temperature across the Southern Ocean associated with the polar frontal zone (PFZ) and strong selection in Antarctic species for tolerance to low temperatures and the presence of ice. The extent of the Antarctic ice sheet oscillated throughout the Cenozoic and is also likely to have resulted in repeated shifts in the distribution of species both in land and in the coastal seas of the Antarctic. Fragmentation of populations and isolation in refugia, during glacial maxima, are likely to have been an important mechanism of allopatric speciation in Antarctic taxa, potentially acting as taxonomic diversity pumps (Clarke & Crame 1989, 1997).
Many groups of Antarctic animals and plants are thought to exhibit a high degree of endemism (for marine taxa this ranges from 35 to 90% of species, Arntz et al. 1997; for some terrestrial groups endemism may reach 100%, e.g. nematodes, Andrássy 1998) although such conclusions are limited by knowledge of the biota of the surrounding continents, islands and oceans. Some species have ranges that extend outside the Antarctic providing evidence that dispersal into and colonization of the Antarctic ecosystem has occurred during the Cenozoic and even after the last glaciation (e.g. Wise 1967; Barnes et al. 2006). Such dispersal events may partially explain regional differences within both terrestrial and marine ecosystems in the Antarctic, although this implies limitations to dispersal following invasions either as a result of life history or the sparse distribution of suitable habitat separated by large geographical distances.
Much of our present knowledge of the diversity and biogeography of the Antarctic biota comes from conventional studies on the systematics and distribution of terrestrial and marine animals and plants. Such approaches are limited as apparent morphological similarity may not reflect the true evolutionary relationships between taxa. This is especially the case when there is a lack of fossil evidence, as in the Antarctic, resulting from limited exposure of rocks on the continent. Molecular approaches, allow the reconstruction of the evolutionary history of biological communities, including the identification of major events that have resulted from climate change or plate tectonics. They also provide a powerful way to assess the distribution of diversity at specific and sub-specific levels. This paper synthesizes studies that have been undertaken on the phylogenetics and molecular ecology of Antarctic taxa in order to understand patterns in the diversity of marine and terrestrial species and the evolutionary history of the Antarctic biota. The role of the Antarctic in the evolution of globally distributed taxa is also examined, necessarily including non-Antarctic species.
2. The extratropical Southern Hemisphere biota: vicariance or recent dispersal?
It is useful to compare the wider patterns of biogeography in the Southern Hemisphere to identify similarities with the history of the Antarctic biota. This is because two of the important evolutionary forces within the Antarctic, vicariance and dispersal have often been treated as competing explanations for the distribution of the Southern Hemisphere biota (Sanmartín & Ronquist 2004). The Gondwana supercontinent underwent a sequential fragmentation over approximately 165 Myr. This was initiated with rifting between India/Australia and East Antarctica and was completed with the separation of southern South America and the Antarctic about 41 Myr BP (Scher & Martin 2006), with the opening and subsequent deepening (Livermore et al. 2005) of the Drake Passage, and with the separation of the South Tasman Rise and East Antarctica about 32 Myr BP (Lawver & Gahagen 2003).
The disjunct distributions of many groups of plants and animals in the Southern Hemisphere have largely been explained by vicariance. That the ancient flora of South America, New Zealand, Antarctica and Australia was shared is supported by fossil evidence (Raven & Axelrod 1974). However, recent studies, especially those based on molecular phylogenetics, have suggested that in many cases post-Gondwanan dispersal must have played an important role in determination of present day distributions of animals and plants. For example, the distribution of Southern beeches (Nothofagus spp.) was thought to be a classic example of vicariance related to the break-up of Gondwana (e.g. Craw et al. 1999). However, recent phylogenetic analysis based on chloroplast DNA sequences indicates that while the relationships between some Nothofagus lineages are compatible with continental drift (e.g. comparisons between species in Australia and South America), others, for example, between species presently distributed in Australia and New Zealand, can only be explained by transoceanic dispersal (Knapp et al. 2005).
Similar relationships have been detected, by sequence analysis, between Gunnera species located in New Zealand and Tasmania (Wanntorp & Wanntorp 2003). Dispersal events have been detected over much longer distances, such as between New Zealand and Oceania (e.g. Metrosideros spp.; Wright et al. 2000), between New Zealand and New Guinea (e.g. Parahebe spp.; Wagstaff & Garnock-Jones 2000; Myosotis spp.; Winckworth et al. 2002a) and between South America and New Zealand (e.g. Sophora spp.; Hurr et al. 1999; Hebe spp.; Wagstaff & Garnock-Jones 2000; Tetrachondra spp.; Wagstaff et al. 2000). The timing of these dispersal events has been estimated at occurring in the Late Miocene to Pleistocene, depending on the species and geographical localities (Hurr et al. 1999; Wagstaff & Garnock-Jones 2000; Wagstaff et al. 2000; Winckworth et al. 2002b; Knapp et al. 2005). Late dispersal events are also supported by the fact that there is very limited sequence divergence in neutral loci between congeneric species, and in some cases, there has been insufficient time for speciation to occur (e.g. Chionohebe and Hebe; Wagstaff & Garnock-Jones 2000) or species are sufficiently closely related to allow hybridization to take place (e.g. Sophora; Hurr et al. 1999).
In contrast to plants, animals have generally been assumed to show distributions consistent with vicariance and phylogenetic analyses support this hypothesis (Sanmartín & Ronquist 2004). However, DNA-sequencing studies have shown that there are exceptions, such as the fish Galaxias maculatus (Waters et al. 2000) and the ratite birds (Cooper et al. 2001). For some insects and other groups of small aquatic invertebrates (e.g. triclad flatworms), present day distributions suggest that in many cases transoceanic dispersal in these groups has occurred (Briggs 1995).
Various mechanisms of transoceanic dispersal have been suggested (Barnes et al. 2006). In plants, the floristic affinities of extratropical islands in the Southern Hemisphere are best explained by direction-dependent processes, especially dispersal by prevailing winds (Muñoz et al. 2004). Direction-dependent processes rather than random dispersal, in which floristic affinity will reflect geographical distance between islands, provided the best explanation for the distribution of mosses, liverworts and lichens. For pteridophytes, associations are approximately equal between both types of dispersal process, reflecting a limitation on the maximum dispersal distance for these plants (Muñoz et al. 2004). Stronger relationships between floristic affinity and direction-dependent processes have been found when maximum annual wind speed figures are used for analyses, indicating the importance of extreme weather events in dispersal (Muñoz et al. 2004). The southern latitudes are dominated by the west wind drift, favouring dispersal in an easterly direction (Winckworth et al. 2002b). It has been suggested that within weather systems, cyclonic airflow may provide opportunities for wind dispersal in a westward direction (Winckworth et al. 2002b) and many of the recent dispersal events detected in plants are consistent with this (e.g. Metrosideros; Wright et al. 2000; Gunnera; Wanntorp & Wanntorp 2003). In particular, during present El Niño/Southern Oscillation (ENSO) events, weakened easterly winds in the tropics lead to westerly winds at lower latitudes. During the last glacial maximum (LGM), the west wind drift may have been stronger across New Zealand and subtropical/tropical easterlies may have been weaker. This coupled with increased frequencies of El Niño during cool periods over the last 15 000 years may have provided opportunities for dispersal in an eastward direction at lower latitudes followed by westward dispersal on the southeast trade wind (Wright et al. 2000).
Wind has a strong potential to disperse plants with light seeds or spores (Barnes et al. 2006). In other cases, plants have buoyant seeds that may remain viable for several years (e.g. Sophora; Hurr et al. 1999). However, some plants, such as Nothofagus, have dense, heavy seeds, reportedly intolerant of seawater (e.g. Craw et al. 1999). Current understanding of the ability of some of these species to disperse across water is poor (e.g. Knapp et al. 2005). Extreme weather, tectonic events or erosion of the land may cause entire plants to end up floating in the oceans and dispersing over large distances, potentially transporting seeds (e.g. Barber et al. 1959). Biotic vectors, such as birds (e.g. skuas), may be responsible for dispersal of some plants in the extratropical Southern Hemisphere (Winckworth et al. 2002b). For aquatic species able to tolerate saltwater, dispersal through the ocean is a probable explanation of transoceanic dispersal (e.g. Waters et al. 2000). For freshwater species, such as the triclad Cura pinguis, distributed in Australia, New Zealand and New Caledonia (Briggs 1995), passive dispersal on floating vegetation is likely although transport by biotic vectors is also possible (Barnes et al. 2006). All potential mechanisms of dispersal will be aided by the existence of stepping stones such as oceanic islands (Winckworth et al. 2002b). Such stepping stones, along the Norfolk Ridge or Lord-Howe Rise, were implicated in the post-Gondwanan dispersal of ratites (fossil evidence suggests that early palaeognathes could fly) between Australia, New Caledonia and New Zealand (Cooper et al. 2001).
It is surprising that molecular evidence suggests that the majority of dispersal events detected in the extratropical Southern Hemisphere have been very recent, within 10 Myr (Winckworth et al. 2002b) with many in the last 2 Myr (Hurr et al. 1999; Wagstaff & Garnock-Jones 2000; Knapp et al. 2005). This may partially reflect limitations in molecular phylogeny reconstruction using extant taxa or limitations in the fossil record (Winckworth et al. 2002b). Extinctions, resulting from climatic and geological changes since the break-up of Gondwana, are likely to have eradicated many intermediate steps in dispersal events. However, recent dispersal may also reflect increased habitat availability in the Late Tertiary (Winckworth et al. 2002b). Given the evidence for recent dispersal events crossing large parts of the extratropical Southern Hemisphere, it would be surprising if recent immigrants did not form a significant portion of the sub-Antarctic and Antarctic biota.
3. The evolution and biodiversity of the terrestrial sub-Antarctic and Antarctic biota
(a) Plants
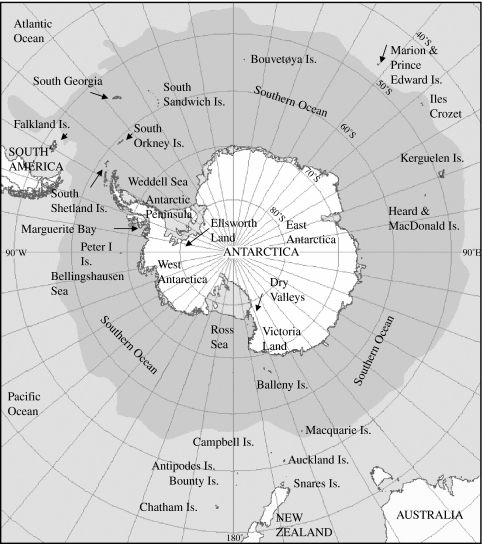
Terrestrial Antarctica has been divided into a number of zones based on climatic and biotic features (e.g. Lewis-Smith 1984; Longton 1988). Generally three zones have been described, the sub-Antarctic, the maritime Antarctic and the continental Antarctic. The sub-Antarctic consists of a ring of islands that surround the continent including Macquarie Island, Campbell Island, South Georgia, Marion Island, Îsles Crozet, Îsles Kerguelen and Heard and Macdonald Islands (figure 1). The maritime Antarctic includes the South Shetland Islands, South Sandwich Islands, Bouvetøya (Bouvet) Island, the South Orkney Islands and the western side of the Antarctic Peninsula down to 72° S (figure 1). The continental Antarctic includes the eastern side of the Antarctic Peninsula south of 63° S and the rest of the continent (Lewis-Smith 1984; Longton 1988; figure 1).
Figure 1.
Map of the Antarctic and sub-Antarctic showing sites and areas referred to in the text. Dark grey area is the Southern Ocean south of the average position of the Polar Frontal Zone. Adapted from the map of the Census of Antarctic Marine Life Sampling Area (http://www.caml.aq/data-maps/index.html).
A comprehensive analysis of the biodiversity of plants in the maritime and continental Antarctic has been carried out by Peat et al. (2007). This study concluded that continental Antarctica and the Antarctic Peninsula hosted distinct floras. Within the Antarctic Peninsula, there was evidence that the eastern Antarctic Peninsula had more similarities to the southwestern Antarctic Peninsula than to the continental Antarctic suggesting that there are three potential phytogeographical zones. Finer subdivisions of the maritime Antarctic were also indicated by the data on species presence. It was also found that there were almost no endemic mosses in the Antarctic and none from inland nunataks. In contrast, lichens showed a much higher level of endemicity, with many records of endemic species being located on inland nunataks. Many of the Antarctic moss species are known to have distributions outside the maritime and continental Antarctic, on the sub-Antarctic Islands and extratropical Southern Hemisphere landmasses, or are cosmopolitan (Skotnicki et al. 2000; McDaniel & Shaw 2005). This is consistent with post-glacial colonization of the Antarctic by these plants possibly through wind-borne dispersal (e.g. Muñoz et al. 2004; Barnes et al. 2006). In contrast, some lichen species are likely to have survived the Pleistocene glaciations in refugia. However, it is possible that the distribution of these species in the Antarctic has contracted, following climatic changes on the continent and immigration of other terrestrial plants after the last glaciation. It should also be noted that knowledge of the distribution of lichens in the Southern Hemisphere may be incomplete.
Mosses have been the subject of several genetic studies in the Antarctic and sub-Antarctic. These studies have been based on haplotype frequencies of allozymes (the dominant vegetative stage of mosses is haploid; e.g. Adam et al. 1997; Selkirk et al. 1997), random amplified polymorphic DNA markers (RAPDs; e.g. Seppelt et al. 1996 (1999); Adam et al. 1997; Selkirk et al. 1997, 1998; Skotnicki et al. 1997, 1998a,b, 1999, 2000, 2001, 2002, 2004) and DNA sequence studies, particularly of the internal transcribed spacer regions (ITS1 and ITS2) of the nuclear rRNA encoding multigene family (e.g. Skotnicki et al. 2002, 2004, 2005). Some of these studies have not sampled large numbers of individual moss colonies across study sites and RAPD analysis has been criticized generally for experimental artefacts, lack of reproducibility and the fact that the inheritance of loci is unknown (e.g. Hadrys et al. 1992; Grosberg et al. 1996). However, because mosses are haploid scoring RAPD loci is simplified and Antarctic studies have generally been optimized to increase reproducibility (e.g. Adam et al. 1997).
Genetic studies on Antarctic mosses have resulted in similar findings across a range of species. In almost all studies employing RAPDs, it has been found that the haplotypes of shoots from single moss clumps are variable (table 1). Genetic distance between shoots within clumps is lower than that estimated for comparisons of shoots in different clumps. Since sporophytes have not been observed for many of these species, at least in the Antarctic, it is assumed that variation in shoots within a single clump have arisen by somatic mutation (although sporophytes have been observed for B. pseudotriquetrum and Hennediella heimii in Victoria Land; Lewis-Smith & Convey 2002). As the branches of the vegetative phase of mosses grow from a single vegetative cell, somatic mutations will result in genetic variation among the branches of moss clumps (Skotnicki et al. 2000). Such intracolonial genetic mosaicism has not been reported commonly for mosses outside of the Antarctic. Explanations for this have included high mutation rates caused by exposure to UV-B radiation (e.g. Adam et al. 1997). However, observations and experiments on UV exposure in Antarctic mosses have shown that at least some species synthesize UV-protectant pigments and that DNA damage is repaired overnight (e.g. Lud et al. 2002; Newsham et al. 2002). Notwithstanding this, there is a possibility that mutation rates are increased by UV exposure and that the DNA repair machinery may operate at a lower efficiency at low temperatures. More significantly, Antarctic mosses grow very slowly and some of the colonies sampled may be very old. In such cases, somatic mutations may accumulate within a clump over time and the mutation rate may also increase owing to senescence or gradual degradation of the DNA repair machinery itself, especially as the plants are haploid (Gill et al. 1995).
Table 1.
Within colony variation and genetic structure of moss populations in the Antarctic (variation, multiple haplotypes detected within single moss clump; yes, genetic differentiation detected between populations at the geographical distance indicated. Data from, Seppelt et al. (1996 (1999)), Adam et al. 1997, Selkirk et al. 1997, 1998, Dale et al. 1999, Skotnicki et al. 1997, 1998a,b, 1999, 2000, 2001, 2002, 2004).
| geographical scale of separation | within clumps | less than 1 km | 10–100 km | 100–1000 km | 1000 km + | intercontinental |
|---|---|---|---|---|---|---|
| Bryum argenteum | variation | yes | yes | yes | ? | ? |
| Hennediella heimii | moderate variation | no | yes | yes | ? | ? |
| Campylopus pyriformis | variation | no | ? | ? | ? | yes |
| Sarconeurum glaciale | ? | no | no | no | yes | ? |
| Ceratodon purpureus | variation | yes | yes | yes | yes | yes |
| Bryum pseudotriquetum | variation | ? | yes | yes | ? | ? |
| Pohlia nutans | low–moderate variation | no | ? | ? | yes | ? |
Many studies on spatial genetic structure of moss populations in the Antarctic have detected significant differentiation over relatively small geographical distances (less than 1 km; table 1). A major contributory factor to the high levels of genetic structure detected in the continental Antarctic is that these mosses reproduce almost exclusively by asexual means and dispersal of propagules is limited. Evidence has been presented that mosses disperse along watercourses over relatively short distances (e.g. Skotnicki et al. 2000). Low availability of habitat inhibits successful colonization by limiting opportunities for stepping-stone dispersal over larger distances. Only 0.3% of the 14 million km2 area of the continent of Antarctica is ice free (British Antarctic Survey 2004; see also Peat et al. 2007). Particularly rich and genetically diverse moss populations tend to sporadically occur where habitat is more favourable owing to protection from harsh environmental conditions (e.g. Skotnicki et al. 1998a).
There is evidence for occasional longer distance wind-borne dispersal by Antarctic mosses that reproduce only through vegetative propagules, by identification of identical haplotypes in geographically separated sites (e.g. Bryum pseudotriquetrum colonies across sites separated by 45 km; Skotnicki et al. 2000; table 1). That such putative dispersal events can be detected suggests that dispersal processes in mosses in the continental Antarctic are taking place at a slow rate. However, the existence of exotic moss species at fumaroles is evidence that wind dispersal is the main agent for transoceanic dispersal of mosses into the Antarctic (e.g. Convey et al. 2000). These are likely to have become established through dispersal of sexually produced spores. Aerobiological studies also support such a means of long-distance dispersal (Marshall 1996). However, at present, there are no detailed phylogeographic studies on the genetic structure of mosses at transoceanic scales in this region that allow the timing of colonization of the Antarctic to be dated.
The moss H. heimii produces spores frequently in the maritime Antarctic (Lewis-Smith & Convey 2002; P. Convey 2006, personal communication British Antarctic Survey, Cambridge, UK) and shows relatively low genetic structure between populations at small spatial scales (Selkirk et al. 1998). Sarconeurum glaciale shows limited genetic differentiation between populations up to 75 km separation (Selkirk et al. 1997). This species is not supposed to reproduce sexually in the continental Antarctic and why it shows limited genetic structure compared to other species is unknown. Both Campylopus pyriformis and Pohlia nutans are found in geothermally active regions and probably represent recent colonists to the Antarctic. Genetic variability in such recent colonists may be limited owing to founder effects, as seems to be the case of P. nutans. It is therefore not surprising that studies of genetic structure over relatively small spatial scales, in such species, show limited differentiation (Skotnicki et al. 2001, 2002).
Mosses from outside of the Antarctic often show limited genetic structure among populations as a result of spore-mediated dispersal across large geographical distances (e.g. Polytrichum spp., van der Velde & Bijlsma 2003; Sphagnum spp., Thingsgaard 2001). However, non-Antarctic populations can also show high levels of genetic differentiation and this may be related to several different factors. Low frequency or a complete lack of sexual reproduction in some species (particularly those that are monoecious) means that long-distance dispersal is not possible for some mosses (Korpelainen et al. 2005). Historical processes, including population expansion from refugia following the LGM, can lead to significant genetic structure among moss populations (e.g. Thingsgaard 2001; van der Velde & Bijlsma 2003). Despite the evidence that many moss species are recent immigrants to the Antarctic, there is also the possibility that some may have survived the LGM in refugia and this may have contributed to the present genetic population structure (see below). Significant geographical barriers such as mountain ranges can also restrict gene flow between non-Antarctic populations (e.g. van der Velde & Bijlsma 2003).
To date, there is a single study on the Antarctic hairgrass, D. antarctica, based on amplified fragment length polymorphism (AFLP) analysis of populations from Signy Island and from Anchorage, Léonie and Lagoon Islands in the southern maritime Antarctic (off the Antarctic Peninsula; Holdregger et al. 2003). This study found that D. antarctica displayed low genetic diversity and no genotypes were shared between the Signy Island and southern populations, indicating a complete lack of gene flow between these localities. Furthermore, there was significant genetic differentiation found between sub-populations within these two regions, separated by up to 4 km distance. In the southern populations, a single genotype did occur on all the three islands. D. antarctica can self-fertilize and is often cleistogamous (Moore 1983) and, like mosses, vegetative propagation is thought to be important in dispersal (Komárková et al. 1985). It is possible that transport of tillers by birds takes place over small distances as they use the grass as nesting material (Holdregger et al. 2003).
If climate change ameliorates environmental conditions sufficiently then at least some Antarctic plants may be able to shift from asexual to sexual modes of reproduction or increase the rates of sexual reproduction (Convey 1996, 2003). Warming in the Antarctic and sub-Antarctic has already led to increased habitat availability and plants have already been observed to expand their distribution by increasing both population numbers and size (Chown & Convey 2007). These factors are likely to have a dramatic impact on the genetic structure of populations, as a result of increased occurrence of genetic recombination, increased dispersal capacity, population size and the number and spatial distribution of populations.
(b) Animals
The terrestrial fauna of the Antarctic is depauperate and consists of microarthropods, nematodes, tardigrades, rotifers and protozoans (Convey 2001, 2003). In some cases, not all of these elements are present and animal communities in the Dry Valleys of Victoria Land and nunataks of Ellsworth Land are among the simplest known on the Earth (Freckman & Virginia 1997, 1998; Convey & McInnes 2005). As with the plants, the terrestrial fauna can be divided into three distinct biogeographic regions, the continental Antarctic, the maritime Antarctic and the sub-Antarctic. The continental Antarctic and maritime Antarctic host distinct faunas with an almost complete separation at species level (Chown & Convey 2007). The majority of continental species are endemic and are probably pre-Pleistocene in origin, in some cases representing Gondwanan relicts (Wise 1967; Wallwork 1973; Greenslade 1995; Marshall & Pugh 1996; Pugh & Convey 2000; Stevens et al. 2006). These species must have survived recent glaciations in refugia such as nunataks where ancient ‘chalikosystems’, bare gravels with scattered microphytes, remained ice free (Marshall & Pugh 1996). Thus, vicariance, extinction driven by the extremely harsh environmental conditions and habitat availability have been the strong forces in shaping the continental Antarctic fauna.
In contrast, the maritime Antarctic fauna shows a much lower degree overall of endemism than the continent (Wallwork 1973; Pugh & Convey 2000), although levels of endemicity within specific groups vary (e.g. families of Acari: Pugh & Convey 2000; nematodes: Andrássy 1998; Maslen & Convey 2006). Some species are shared with the sub-Antarctic and these almost certainly represent post-glacial colonists as much of the presently available habitat in the maritime Antarctic will have been obliterated during the Pleistocene glaciations. Such data should be treated with caution as, for example, the collembolan Cryptopygus antarcticus antarcticus was also thought to occur on the Antarctic Peninsula and sub-Antarctic islands, but is now recognized to be a complex of morphologically similar species (Stevens et al. 2006). Within the Acari, many groups are capable of surviving seawater inundation (Pugh & Convey 2000; Coulson et al. 2002) and hydrochory (dispersal by water) is the most probable route for species colonizing the maritime Antarctic (Pugh & Convey 2000; Barnes et al. 2006). As with extratropical plants, there is also evidence for colonization of the maritime Antarctic by sub-Antarctic and extratropical species against the prevailing westerly Antarctic Circumpolar Current (ACC) presumably as a result of storm events (Pugh & Convey 2000; see above and figure 2).
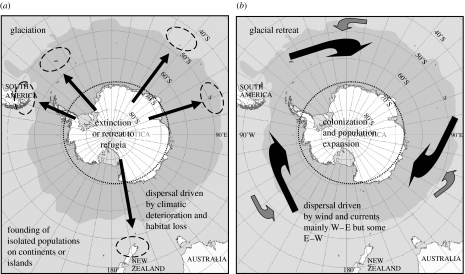
Figure 2.
Schematic diagram showing pulses of species range contraction and expansion during periods of (a) glaciation and (b) glacial retreat. Glaciation is characterized by extinction of taxa and range contraction into refugia on the Antarctic or on the sub-Antarctic islands or southern continents. Glacial retreat is characterized by range expansion, secondary contact of populations or sister species and radiation of clades.
Differences in the fauna of the northern and southern parts of the maritime Antarctic have been observed and different subspecies of Alaskozetes antarcticus and Halozetes belgicae are found in these areas (Wallwork 1973; although sometimes these subspecies are sympatric and the current systematic status is questionable). Some endemic maritime Antarctic species probably originated prior to the Pleistocene glaciations and are largely confined to the southern Maritime Antarctic (Wallwork 1973; Pugh & Convey 2000). For example, the acarids belonging to the Actenidida are mostly endemic. This group is not generally tolerant of prolonged exposure to seawater and must have survived in the maritime Antarctic in refugia, although these are currently unknown (Pugh & Convey 2000).
The sub-Antarctic fauna also shows high levels of endemism at the specific level but there are differences in the distribution of species in the western (South Georgia) and eastern (Macquarie Island to Crozet Islands) parts of the zone. Despite there being some species that occur throughout the sub-Antarctic zone, many eastern species do not occur in the western sub-Antarctic and vice versa. This picture changes at the generic level where more taxa occur across both the regions. Both Macquarie Island and South Georgia have continental origins and it is likely that vicariance has probably played a significant role in evolution of the fauna of this region (Wallwork 1973). This is supported by recent studies of the molecular phylogenetics of chironomid midges in the sub-Antarctic. Estimates of the divergence times of chironomid species, based on 28S DNA sequence data, on South Georgia, South America and the Antarctic Peninsula are consistent with geological estimates of the age of fragmentation of these continental blocks (Allegrucci et al. 2006). Divergence times of Cryptopygus spp. found on Îles Crozet and Îles Kerguelen are consistent with a mid- to late Miocene origin of populations/species suggesting that they are not recent but have survived in refugia over long time periods (Stevens et al. 2006). There is also some evidence of Pleistocene dispersal of Cryptopygus spp. among the sub-Antarctic islands based on mitochondrial cytochrome oxidase I sequence data (Stevens et al. 2006). Rafts of live individuals of the collembolan, Cryptopygus sp. (reported as C. antarcticus antarcticus), have been observed floating in the ocean, some distance from land (Gressitt 1967), again suggesting hydrochory as a likely route of transport. Post-glacial dispersal and immigration of many sub-Antarctic species remains a possibility. Elements of the sub-Antarctic fauna are certainly recent immigrants, originating from the temperate Southern Hemisphere or from the maritime Antarctic (Wallwork 1973; see Chown & Convey 2007).
Studies of genetic population structure in terrestrial Antarctic invertebrates based on allozyme electrophoresis and mtDNA sequence analyses reveal a consistent pattern of high levels of genetic differentiation between populations on spatial scales of 10–100 km and in one case less than 1 km (table 2). As with mosses, habitat availability for Antarctic invertebrates is a major factor in determining of their distribution and probably a significant limitation on effective gene flow between populations. The majority of studies on terrestrial invertebrates have been on Collembola from Victoria Land in the eastern Antarctic (Fanciulli et al. 2001; Frati et al. 2001; Stevens & Hogg 2003, 2006). Collembola are reliant on the availability of water, algae, fungal material or prey as food sources, and shelter (under stones) from winds, as they are sensitive to dehydration (Fanciulli et al. 2001). This combination of physical and biological factors exists in sparsely distributed patches and areas of non-suitable habitat between them may effectively limit dispersal even over relatively short distances. At larger scales, geographical features such as glaciers or tracts of sea ice act as major barriers to gene flow between populations (Fanciulli et al. 2001; Frati et al. 2001; Stevens & Hogg 2003). Genetic differentiation among such fragmented populations will be enhanced by genetic drift, especially where numbers of individuals within sub-populations are small. Founder events may also result in genetic differences between populations where the number of founding individuals is small when compared with the source population.
Table 2.
Genetic structure of terrestrial animals in Antarctica (yes, genetic differentiation detected between populations at the geographical distance indicated. Data from Courtright et al. (2000), Fanciulli et al. (2001), Frati et al. (2001), Stevens & Hogg (2003), Nolan et al. (2006) and Stevens & Hogg (2006)).
| geographical scale of separation | less than 1 km | 10–100 km | 100–1000 km | 1000 km+ |
|---|---|---|---|---|
| Nematoda | ||||
| Scottnema lindsayae | ? | yes | ? | ? |
| Acari | ||||
| Stereotydeus mollis | ? | yes | ? | ? |
| Collembola | ||||
| Gomphiocephalus hodgsoni | yes | yes | ? | ? |
| Gressittacantha terranova | yes | yes | ? | ? |
| Desoria klovstadi | ? | yes | ? | ? |
Observations of high levels of genetic structure in populations of the nematode, Scottnema lindsayae, are more difficult to explain (Courtright et al. 2000). Nematodes can form anhydrobiotic resistant stages that are potentially dispersed by wind (e.g. Carroll & Viglierchio 1981). In some species, this leads to a lack of genetic structure within geographical regions (e.g. Picard et al. 2004; Plantard & Porte 2004), the opposite to what is found with S. lindsayae. Alternatively, limited habitat availability or specific behavioural adaptations that have evolved owing to the former may act to prevent genetically effective dispersal in this species. Again, drift and founder effects may act to increase genetic differentiation among small populations.
High levels of genetic structure in populations of terrestrial invertebrates are not unusual. Many have low vagility as a result of limited adult mobility or a lack of dispersive resistant stages and this restricts gene flow between populations (Peterson & Denno 1998). In addition, such organisms often have highly specific environmental requirements and populations may occur in small patches of suitable habitat, often separated by large areas of unsuitable terrain. The occurrence of small populations means that genetic drift has a strong influence on genetic population structure. For the above reasons, historical impacts on the genetic population structure of terrestrial invertebrates tend to be important. In particular, the effects of the Pleistocene glaciations on fragmentation of populations into relatively small refugia and subsequent population expansion, as with terrestrial plants, has had a significant influence on the genetic population structure of terrestrial and freshwater invertebrates in temperate, sub-tropical and tropical latitudes (e.g. Hugall et al. 2002; Garrick et al. 2004; Hewitt 2004; Ribera & Vogler 2004).
Historical factors are highly significant in the determination of spatial genetic structure of Antarctic terrestrial invertebrates. The distribution of haplotypes among populations of the collembolan, Gomphiocephalus hodgsoni, in southern Victoria Land, is consistent with Pleistocene divergence of allopatric populations located in isolated refugia (Stevens & Hogg 2003; Nolan et al. 2006). Common nucleotides among haplotypes suggested limited dispersal from isolated populations along meltwater streams (Nolan et al. 2006), or over larger distances, by biological vectors such as birds (Stevens & Hogg 2003) since the LGM. There is also evidence of a hybridization zone between haplotype groups providing evidence of secondary contact during post-glacial population expansion (Nolan et al. 2006), although this occurs across smaller geographical scales than zones found in, e.g. northeastern Europe (Hewitt 2004). Some peripheral populations are characterized by low genetic variability suggesting founder events or the results of past bottlenecks (Stevens & Hogg 2003). Similar evidence of rare dispersal events were also detected in the collembolan Gressitacantha terranova where, again, low genetic variability suggests a relatively recent founder event (Fanciulli et al. 2001). Such events are infrequent suggesting that processes of dispersal and colonization from glacial refugia in the continental Antarctic are very slow probably owing to limited habitat availability. Studies on mitochondrial DNA sequence variation of the mite Stereotydeus mollis in Southern Victoria Land have revealed a similar pattern of population structure to G. hodgsoni, except the divergence between haplotypes in different localities is much greater suggesting the presence of cryptic species in some cases (Stevens & Hogg 2006). It was hypothesized that differences in the survival abilities of mites and collembolans over glaciations and differences in life history and physiology, leading to contrasting rates of molecular evolution may have contributed to differences in present genetic variation (Stevens & Hogg 2006). It is also possible that such biological differences mean that the mites have survived in refugia over more glacial cycles than the collembolans.
The impacts of glaciations on population size and distribution, limited vagility of many terrestrial Antarctic invertebrates and significant barriers to dispersal (mountain ranges or the ocean), may partially explain why the fauna of the continental and maritime Antarctic and sub-Antarctic are so distinct. The two areas also differ with respect to climate, hence environmental factors may prevent successful migration and colonization from one region to the other. One potential impact of climate change will be to increase habitat availability for many terrestrial species and thus to increase the rates of dispersal and colonization in both terrestrial plants and animals (Chown & Convey 2007). A potential test of this is the comparison of the genetic structure of terrestrial invertebrates and plants in the continental versus maritime Antarctic.
Finally, as with some mosses, it has been observed that Antarctic Collembola show high levels of genetic variability compared to those from other parts of the world. This has been attributed as possibly being a result of exposure to UV radiation (Frati et al. 2001). However, many of the species studied occur in cryptic habitats (e.g. in soil, under stones, flakes of weathered rock or snow; Wise 1967) or may be able move into shelter within or under clumps of vegetation if exposed to the Sun. Studies on micro-organisms have shown that even a thin covering of physical material such as soil, dust, snow or ice or biological material such as algal tissue can afford protection from the harmful affects of UV radiation (e.g. Cockell et al. 2003). Whether such cryptic habitats afford protection to metazoans is untested at present. Genetic variation in natural populations has been positively correlated with environmental variability and stress (e.g. Nevo 2001). The extreme physical conditions of Antarctic ecosystems may therefore promote genetic polymorphism in populations of Antarctic organisms, although the connection between genome and environment is unproven.
4. The marine environment
As with terrestrial plants, vicariance arising from the break-up of Gondwana played a significant role in determination of the biogeography of extant marine taxa in the Southern Hemisphere. The patellid limpet genera, Nacella and Cellana, are currently distributed in the Indo-West Pacific, Australia, South Africa and Antarctica (Koufopanou et al. 1999). Phylogenies based on mitochondrial DNA sequences, and fossil evidence, suggest that these groups probably arose 100 Myr BP (Koufopanou et al. 1999; Goldstein et al. 2006) from a common ancestor that originated in the Weddellian marine faunal province (e.g. Zinsmeister 1982). However, inconsistencies in the phylogenetic relationships of species within these genera suggest that other biogeographic origins are possible or that more recent dispersal also influenced distribution (Koufopanou et al. 1999; Goldstein et al. 2006).
Stronger evidence of the influence of vicariance in the biogeography of marine species has arisen from mitochondrial and nuclear DNA sequence studies of littorinid gastropods (Williams et al. 2003). In particular, the estimated time of divergence of the South American species, Austrolittorina araucana from other Austrolittorina spp. is consistent with the fragmentation of Gondwana or vicariance caused by climatic factors (Williams et al. 2003). However, as with other organisms, more recent transoceanic dispersal of littorinids has also played a significant role in determination of distribution and speciation of Southern Hemisphere littorinids, including species of Austrolittorina and Afrolittorina (Williams et al. 2003). This may have been promoted by strong current flows in the past, as with terrestrial plants (Oligocene to Early Miocene: Beu et al. 1997; Williams et al. 2003).
Hypotheses relating the biogeography of Southern Hemisphere marine invertebrates to vicariant events suggest that Antarctic taxa became extinct, probably as a result of climatic cooling (e.g. Williams et al. 2003). Analysis of the marine fauna has shown that many groups of marine invertebrates (e.g. brachyuran decapods, Stomatopoda, balaniform barnacles; Brandt et al. 2004) and fishes are poorly represented or missing from the Southern Ocean and continental waters of Antarctica. Other groups have undergone radiations in the waters of the Southern Ocean, and overall the fauna is highly endemic (Clarke & Johnston 2003). As with the terrestrial biota, species distributions for several groups of animals indicate that the fauna can be divided into a number of regions (Hedgepeth 1969; Dell 1972; White 1984; Barnes & De Grave 2000; see below). How and why some groups of animals have survived and radiated in the Southern Ocean while others have gone extinct is complex, but is probably best addressed by examination of the single group for which most data exist, the notothenioid fish.
(a) Evolution of notothenioid fishes
The fish fauna of the shelf and upper slope regions of the Antarctic comprise 222 species to date (Eastman 2005). Of these, about 101 species belong to the suborder Notothenioidei and these dominate the abundance (more than 90% in Ross Sea) and biomass (more than 90% in Ross Sea) of the fish fauna on the Antarctic shelf (Eastman 2000, 2005). Other groups of fishes are also found, including the liparid fishes, which are more important in the deep waters of the Antarctic and the zoarcids (Eastman 2000). Studies on the molecular phylogenetics of the notothenioid fishes have concluded that the initial diversification of non-Antarctic notothenioid groups, including the Bovichtidae, the Pseudaphritidae and the Eleginopidae, distributed in the coastal waters of South America, southern Australia and Tasmania, resulted from vicariance associated with the fragmentation of Gondwana (Near 2004). The remainder of the notothenioid fishes form a monophyletic group of species that are characterized by possession of antifreeze glycoproteins (AFGPs; Lecointre et al. 1997; Ritchie et al. 1997; Bargelloni et al. 2000; Near 2004; Near et al. 2004).
AFGPs evolved by the recruitment of a small section of the trypsinogen gene between the first intron and the second exon, the deletion of most of the rest of the gene (from the second exon to fifth intron) and the amplification of a section of DNA coding for Thr–Ala–Ala, from the second exon, to form the repetitive tripeptide backbone of the AFGP molecule (Chen et al. 1997). AFGPs work by preventing the growth of large ice crystals from seed crystals or from other ice nucleators and were a key evolutionary step that allowed the notothenioids to diversify in the icy waters of the Antarctic (Chen et al. 1997; Eastman 2000; Near 2004; Cheng & Detrich 2007). The timing of evolution of this gene was estimated at 5–14 Myr BP based on an estimation of divergence time between trypsinogen and AFGP using teleost mitochondrial DNA divergence rates (Chen et al. 1997). The more rapid evolution of the mitochondrial DNA compared to nuclear DNA means that this may be an underestimate of divergence times. More recently, the origin of the AFGP-bearing notothenioid families has been estimated at approximately 24 Myr BP from times of divergence derived from phylogenetic analysis of mitochondrial 12S and 16S rDNA. However, the divergence time estimates were constrained by a single fossil record, the identity of which is controversial (Near 2004). Both of these estimates are after the opening of Drake Passage and the establishment of permanent ice sheets in the Antarctic (Tripati et al. 2005) and indicate that the evolution of AFGPs must have been driven by climatic changes at the Oligocene–Miocene boundary or Middle-Miocene Climatic Transition. Following the evolution of AFGPs, there was a duplication of the genes creating a large multigene family (Hsiao et al. 1990; Fletcher et al. 2001). This may have been a response to the requirement for large quantities of AFGP in the body fluids of these fishes and may have helped to compensate for the general depression of rates of gene expression and protein synthesis at low temperatures (e.g. Fraser et al. 2002).
Notothenioids show many other adaptations, at the molecular level, to life at low temperatures. Several studies have revealed amino acid substitutions that confer greater flexibility to proteins (e.g. lactate dehydrogenase A4, Fields & Somero 1998; trypsins, Leiros et al. 1998; Sec61p a protein translocation channel in endoplasmic reticulum membrane, Römisch et al. 2003; α- and β-tubulins, Parker & Detrich 1998; Detrich et al. 2000). Duplication of genes other than those coding for AFGPs has also occurred (e.g. tubulins; Parker & Detrich 1998). Some groups, notably the icefish (family Channichthyidae), have also lost genes as a result of evolutionary adaptation to life in the Antarctic. Icefish are the only vertebrates that lack haemoglobin and red blood cells in the circulatory fluid. The loss of haemoglobin expression resulted from the deletion of the entire β-globin gene and parts of the α-globin gene (reviewed in di Priscu et al. 2002; Cheng & Detrich 2007) and may have occurred as a single event. These losses may reflect the high energetic costs of circulating a viscous corpuscular blood fluid at low temperature (di Priscu et al. 2002). This has been partially compensated for by the development of large gills, scaleless skin and large increases in heart size and blood volume (Hemmingsen 1991; Cheng & Detrich 2007). Some channichthyids also lack myoglobin in their cardiac muscles (Moylan & Sidell 2000). Other general physiological adaptations in the Notothenioidei include increased density of mitochondria in cardiac and red skeletal muscle tissue, thought to compensate for a reduced respiratory capacity at low temperatures. The mode of muscle development is also unusual in notothenioid fishes and they have evolved giant muscle fibres (Johnston 2003) made possible by decreased constraints exerted by diffusion of oxygen into tissues, as a result of high environmental oxygen concentration and reduced metabolic rates at low temperature (Clarke & Johnston 1999; Peck & Conway 2000; see Clark et al. 2004).
The Notothenioidei are monophyletic and comprise six families, five of which only occur in the high Antarctic including the Harpagiferidae, Artedidraconidae, Bathydraconidae and Channichthyidae and one, the Nototheniidae, which includes a number of non-Antarctic species (Near et al. 2004). While the prevalent lifestyle of Antarctic notothenioids is benthic, radiation of the suborder has involved evolution from a benthic lifestyle to pelagic or partially pelagic niches as zooplanktivores or piscivores occupying niches filled by taxonomically diverse groups of fishes at temperate and tropical latitudes (Eastman 2000). In some cases, this involved the reduction of buoyancy by reduced skeletal mineralization (through neoteny) and alterations in the lipid composition of the body. Other groups of fishes in the Antarctic, including the zoarcids and liparids, like the notothenioids, lack swim bladders, but these have failed to evolve into vacant niches as successfully. Exploitation of non-benthic food sources also forced evolutionary changes in the morphology of the head and mouthparts of some species (e.g. Channicthyidae; Iwami 1985). As a result of their extensive radiation, notothenioids are considered an example of a marine species flock (Johns & Avise 1998; Eastman 2000).
The radiation of notothenioids in the Antarctic was promoted by contraction of populations into refugia during glacial cycles and subsequent allopatric speciation (e.g. Channichthyidae; Near et al. 2003; Patarnello et al. 2003). This may have occurred as recently as over the last 100 000 years and refugia may have included the islands of the maritime or sub-Antarctic (Near et al. 2003) or ice-free areas of the continental shelf.
Can similarities be drawn between the radiation of the Notothenioidei and other groups of marine animals? Penguins (Spheniscidae) have evolved a number of novel features including the transformation of wing morphology for swimming, skeletal modifications for locomotion on land, modifications of plumage for insulation at low temperatures and adaptations in breeding systems (Giannini & Bertelli 2004; Bertelli & Giannini 2005). Fossil records place the earliest penguins on the Antarctic Peninsula 55 Myr BP, prior to the final separation of the Antarctic and South America (Tambussi et al. 2005). Analysis of the molecular phylogeny of penguins based on combined nuclear and mitochondrial sequence data (5851 bp) suggests that the penguins diverged from albatrosses about 70 Myr BP and originated in Gondwana (Baker et al. 2006). Evidence of a diverse penguin fauna exists for the Antarctic Peninsula to the Late Eocene (34–36 Myr BP) including both large and small species (Tambussi et al. 2005). The climatic cooling associated with the Eocene–Oligocene transition (34 Myr BP) and the opening of Drake Passage, led to the extinction of the large-bodied penguin taxa. During this period, the genera Spheniscus, Eudyptes and Eudyptula diverged from the basal ‘Antarctic’ penguins of the Aptenodytes and Pygoscelis lineages (Baker et al. 2006). Subsequently, these groups dispersed northwards into warmer waters, probably aided by the ACC, mainly in a west to east direction, followed by allopatric speciation. Approximately 12–14 Myr BP, a further cooling event occurred in the Antarctic accompanied by intensification of the ACC (Middle Miocene Climatic Transition), leading to a further period of cladogenesis in sub-Antarctic and cool-temperate penguins (Baker et al. 2006). Further dispersal and speciation events have occurred up to the last 2 Myr BP (Baker et al. 2006) as with other Southern Hemisphere extratropical species. This group therefore originated in the Antarctic but differs from the Notothenioidei in that it is most diverse and has undergone most radiation outside of the Antarctic. Likewise, the albatrosses are predominantly distributed in the sub-Antarctic and southern Atlantic, Indian and Pacific Oceans with one monophyletic clade having radiated in the North Pacific (cytochrome b sequence data; Nunn et al. 1996; see also Page et al. 2004). Several species are distributed in the Southern Ocean although they breed in the sub-Antarctic (e.g. Diomedea exulans, Thalassarche melanophris, T. cauta, T. chrysostoma and Phoebetria palpebrata).
For Antarctic marine invertebrates, it is difficult to decide whether radiations have occurred in the Antarctic owing to the lack of research on most groups. Molecular phylogenies based on mitochondrial DNA sequences indicate that the thermal isolation of the Antarctic resulting from the opening of Drake Passage was followed by vicariant speciation in several marine invertebrate groups including krill (separation of Euphausia superba and E. crystallorophias from the sub-Antarctic E. vallentini; Patarnello et al. 1996) and sea urchins (Sterechinus neumayeri; Lee et al. 2004). These groups have not undergone the same kind of radiation as the notothenioid fishes. However, the peracarid crustaceans, especially the isopods and amphipods have undergone a significant species radiation in the Antarctic. Following geographical and thermal isolation, as with the notothenioids, it is thought that extinction events in the Antarctic gave rise to many vacant niches providing opportunities for speciation in the peracarids (Brandt 2000). Data from DNA-sequencing studies are largely consistent with radiations in amphipod families following the geographical isolation of the Antarctic (Lörz & Held 2004). In some cases, speciation involved shifts up and down the continental slope and on or off the continental shelf, aided by the narrow temperature range in the water column and the tendency towards eurybathy for many taxa (Brandt et al. 2004; but see below). This was aided by the retreat and advance of ice shelves which provided opportunities for allopatric speciation followed by secondary contact of species (e.g. for the Serolidae Brandt et al. 2004; see below). Radiations of Peracarida were also accompanied by changes in the morphology of the feeding appendages of many taxa, indicating that shifts in feeding strategies may have been an important aspect of expansion into new niches (e.g. Watling & Thurston 1989 for the Iphimediidae; De Broyer et al. 2004 bathyal & abyssal peracarids). Gigantism, made possible by the high oxygen concentrations in cold seawater (Chapelle & Peck 1999), probably also played a role in evolution of some of these groups (Brandt 2000).
Other groups of invertebrates are highly speciose in the Southern Ocean and shallow seas of the Antarctic compared to elsewhere in the world, including the pycnogonids and the ascidians (Clarke & Johnston 2003). The octopuses of the family Octopodidae have also been found to be more speciose and taxonomically more complex than previously realized on the basis of both molecular and morphological studies (e.g. Allcock & Piertney 2002; Allcock et al. 2003, 2004). This group may also have undergone a significant radiation in Antarctic waters in response to vacant niches or a lack of predators.
It is likely that, like the notothenioids, the evolution of several invertebrate groups in the Antarctic was a response to vacant niches and has also involved specific adaptations to life at low temperatures. The absence of durophagous (shell-breaking) predators in the Antarctic (Clarke et al. 2004) or of direct competitors may have aided the invasion of such niches. The Antarctic may also have acted as a centre of origin for Peracarida and other taxa found in the deep sea of the world's oceans. Colonizations of the deep sea are likely to have occurred following switches from greenhouse to icehouse conditions and were made possible by the thermohaline circulation (Rogers 2000). Further research is required on the evolution of Antarctic marine invertebrates, particularly on high-diversity groups such as peracarids, pycnogonids and ascidians.
(b) Distribution, dispersal and ecology of Antarctic marine species
The strong, eastwards flowing ACC and the nearshore East Wind Drift have been considered as promoting the dispersal of marine organisms throughout the waters of the Southern Ocean (e.g. Bargelloni et al. 2000). In addition, the low range of temperature variation with depth and the fact that nearshore waters of the Antarctic are deep compared with elsewhere in the world are thought to have promoted eurybathy in many taxa (Gutt 1991; Brey et al. 1996). These hypotheses have been supported by observations of wide geographical distribution of many taxa in the Southern Ocean. However, in terms of biogeography, the marine fauna is divisible into several distinct zones, namely, the high Antarctic, around the continent and Southern Ocean south of the PFZ and the sub-Antarctic, north of the PFZ (Hedgepeth 1969; Dell 1972). These regions have been divided into sub-regions on the bases of faunal composition. For example, Barnes & De Grave (2000) identified four zones, based on the distribution of bryozoans within the Antarctic, including the East Antarctic, the West Antarctic, the Antarctic Peninsula and the Scotia Arc (see also White 1984; Clarke & Johnston 2003). Such faunal differences reflect different geological, geographical and climatic histories of the regions of the Antarctic (e.g. Barnes & De Grave 2000), more severe environmental conditions in higher latitudes and differences in habitat availability (e.g. Raguá-Gil et al. 2004). However, molecular studies are demonstrating that species distributions are more limited than previously considered and that even where species ranges are apparently circumpolar, populations are not often genetically homogeneous.
For example, the genetic structure of several protozoans has been analysed in the Southern Ocean. In the pelagic realm, significant genetic heterogeneity in the SSU rRNA gene has been detected between samples of the planktonic foraminiferan, Neogloboquadrina pachyderma (Darling et al. 2004) and in the nuclear internal transcribed spacer region (ITS1) in Phaeocystis antarctica (Medlin et al. 2000). In the case of N. pachyderma, estimates of times of divergence based on the molecular clock indicated that differentiation among genotypes occurred during the last 1 Myr during the Mid-Pleistocene Transition, the period when orbitally forced glacial cycles switched in periodicity from 41 to 100 kyr cycle. This and other studies have identified close genetic relationships to Arctic taxa as strains or sister species suggesting recent but discontinuous gene flow between the poles (Medlin et al. 1994, 2000; Darling et al. 2000, 2004; Montresor et al. 2003). The single study on a benthic foraminiferan, Bathyallogromia weddellensis, failed to detect significant genetic differentiation between samples within the Weddell Sea, even where these came from localities separated by several thousand metres depth (Gooday et al. 2004).
Among Antarctic marine invertebrates, krill, E. superba, have been subjected to the most studies based on a variety of genetic markers including allozymes and mtDNA sequencing. Genetic differentiation among krill populations was expected because population density is concentrated into a number of different geographical areas within large gyres (Lubimova et al. 1985; Mackintosh 1972, 1973). These studies have produced contradictory results with the majority indicating a lack of genetic differentiation (Schneppenheim & MacDonald 1984; Fevolden 1986, 1988; Kühl & Schneppenheim 1986; MacDonald et al. 1986; Fevolden & Schneppenheim 1988, 1989). However, analyses of sequence variation of mitochondrial genes of Antarctic krill and the ice krill, Euphausia crystallorophias, have indicated genetic differentiation in geographically separated samples (e.g. Fevolden & Ayala 1981; Zane et al. 1998; Zane & Patarnello 2000; Jarman & Nicol 2002; Jarman et al. 2002). These observations indicate that genetic structure is not related to geographical separation of populations and may represent local genetic variance among krill populations arising from differences between krill swarms (Jarman et al. 2002). Resolution of genetic differentiation between krill populations at circum-Antarctic scales will require careful design of sampling programmes to account for local variance possibly arising from differences among swarms (Jarman et al. 2002), complex migratory behaviour over different spatial and temporal scales (Murphy et al. 2007) and the enormous overall population size in this species.
Slight but significant genetic differentiation has been detected using RAPDs, in populations of the squid, Moroteuthis ingens, sampled from the Falkland Islands and Macquarie Island (Sands et al. 2003). While this species is regarded as pelagic, it is associated with waters overlying continental/island shelves to depths of 1450 m (Jackson 1997) and deep water may act as a barrier to gene flow in this species. The populations sampled also lay either side of the PFZ and this may also act as a barrier to dispersal of larvae (see below for toothfish). The small levels of differentiation between these populations may have resulted from the recent diversification of these populations or a founder event (Sands et al. 2003). Genetic differentiation among populations of squid on the Patagonian Shelf in the South Atlantic has also been detected using allozymes (Martialia hyadesi; Brierley et al. 1993) and microsatellites (Illex argentinus; Adcock et al. 1999), although in the latter case, genetic differences between samples were not regarded as conclusive evidence of differentiation between populations.
Analysis of the genetic population structure of benthic invertebrates has consistently detected higher levels of genetic differentiation compared with pelagic invertebrate species within geographical regions (scales of tens to hundreds of kilometres; Beaumont & Wei 1991; Allcock et al. 1997; Rogers et al. 1998; Held & Wägele 2005) and between regions (Page & Linse 2002). In some cases, differentiation was sufficiently high that it may represent the occurrence of species complexes in Antarctic taxa (e.g. Rogers et al. 1998; Page & Linse 2002; Held & Wägele 2005), a phenomenon that has been observed globally for many groups of marine invertebrates (Knowlton 1993, 2000). In the case of the giant isopod, Glyptonotus antarcticus, distinct haplotypes occur sympatrically in the eastern Weddell Sea separated by depth (one haplotype coming from more than 600 m depth, the other from shallow water). This challenges the concept of extended eurybathy in Antarctic species. Analysis of genetic differentiation in vertically separated populations of bivalves in the Scotia Sea also indicates the existence of cryptic species with different bathymetric ranges (K. Linse 2006, personal communication British Antarctic Survey, Cambridge, UK).
Higher levels of genetic structure in benthic invertebrates probably reflect lower capacity for dispersal than pelagic species at larval and adult stages (some groups such as peracarids brood larvae). The benthic environment is also more complex than the pelagic, offering a larger variety of habitats and greater opportunity for habitat specialization. The spatial fragmentation of benthic habitats will force genetic structuring of populations unable to cross intervening areas of unfavourable habitat (Wiens 2004). Such a situation becomes extreme where populations were historically separated during cycles of glaciation (see below).
Studies on the genetic structure of Antarctic fish populations have also detected significant levels of genetic differentiation at the regional and sub-regional scale in the Antarctic and sub-Antarctic (e.g. Smith & McVeagh 2000; Appleyard et al. 2002; Shaw et al. 2004; Kuhn & Gaffney 2006; Rogers et al. 2006). In some cases, genetic differentiation in populations separated by only small geographical distances has been detected. In Patagonian toothfish, Dissostichus eleginoides, almost fixed differences in mitochondrial haplotype frequencies have also been detected across a relatively small geographical range between the Patagonian Shelf and South Georgia (Shaw et al. 2004; Rogers et al. 2006). These localities are separated by a channel that is thought to be deeper than the maximum depth of distribution of this species (more than 2000 m). In addition, these areas are separated by two oceanic fronts, the PFZ and the sub-Antarctic Front, that probably prevent larval dispersal between them (Rogers et al. 2006). Marked differences in levels of genetic differentiation detected with mitochondrial and nuclear (microsatellite) markers were also detected for toothfish leading to the suggestion of sex-biased dispersal in this species (Shaw et al. 2004; Rogers et al. 2006). In the mackerel icefish, Champsocephalus gunneri, analysis of haplotypes and genotype frequencies has detected significant genetic differences between populations on South Georgia and Shag Rocks, separated by approximately 150 km distance (Kuhn & Gaffney 2006; see also Allcock et al. 1997 for Pareledone turqueti). Evidence from analyses of mismatch distributions of mitochondrial haplotypes suggests that significant genetic differentiation within the Scotia Sea is linked to historical events leading to population expansions in the region between 37 and 137 kyr BP (Shag Rocks) or more recently (South Shetland Islands). These events are probably linked to the LGM and suggest similar mechanisms of lineage generation to those that led to speciation in the Channichthyidae (see above).
Analysis of the genetic structure of pinniped populations based on allozymes, mtDNA (control-region or d-loop) haplotype variation and nuclear sequences and microsatellites have also indicated significant genetic differentiation between populations of the southern elephant seal (Mirounga leonine), sub-Antarctic and Antarctic fur seals (Arctocephalus tropicalis, Arctocephalus gazella; Gales et al. 1989; Slade et al. 1998; Wynen et al. 2000). For the southern elephant seal, levels of divergence between populations suggested that they separated at periods corresponding to the end of the LGM (20 kyr BP), or at the start of the LGM (200 kyr BP). Historical factors combined with high levels of natal philopatry, especially in females, are thought to account for the marked structure found in fur seal populations. This suggests a pattern whereby seal populations become isolated in refugia during glacial periods, probably located around the islands of the sub-Antarctic or on the southern tips of South Africa, South America and along the southern coast of Australia (Slade et al. 1998). During periods of post-glacial warming, population expansion and colonization of areas further south takes place. Arctocephalus gazella has been subject to a number of investigations of male reproductive success and other aspects of reproductive behaviour using microsatellites as markers to track parentage (e.g. Hoffman et al. 2003, 2004; Hoffman & Amos 2005).
Perhaps the most striking evidence of the historical impacts of the LGM has come from analysis of variation in sequences of the mitochondrial control region in Adelie penguins (Pygoscelis adeliae). Both extant penguins and bones excavated from old nesting sites more than 6 kyr BP revealed two ancient haplotype lineages (Lambert et al. 2002; Ritchie et al. 2004), one confined to the Ross Sea and the other occurring all around the Antarctic. The time to common ancestor for these two lineages was between 30 and 308 kyr BP, depending on method of estimation, with most estimates falling within the LGM (Ritchie et al. 2004). Median networks of relationships between haplotypes for the two lineages show star-shaped phylogenetic trees typical of population expansions. The data are explained as resulting from Adelie penguins becoming confined to two geographically separated refugia during the LGM, one geographically close or adjacent to the Ross Sea, the other of unknown location. At the end of the LGM, the populations expanded and came into secondary contact (Ritchie et al. 2004). Why one lineage is much more widespread than the other is unclear but may be related to the precise location of the refugium for the Antarctic-wide lineage. This may have been closer to more localities suitable as nesting colonies once the ice retreated or there may have been differential timing of retreat of ice near the two refugia. There is no evidence that penguins from the two lineages form separate biological species so the time over which the populations were isolated was insufficient for speciation to take place.
Interestingly, analysis of microsatellites in the Adelie penguin, P. adeliae, revealed little genetic structure around the Antarctic, although there were some significant genetic differences detected among populations in pairwise comparisons with a site on the Antarctic Peninsula (Roeder et al. 2001). There is observational evidence of movement of Adelie penguins among colonies, especially in pre-breeding individuals (Ainley & DeMaster 1980). Movement between colonies has also been reported as a result of blockage of migratory routes to nesting sites by grounded icebergs and sea ice (Shepherd et al. 2005).
Analysis of genetic variation among populations of the largest vertebrates, cetaceans, is complicated by the occurrence of social groups (pods) with strong matrilineal relatedness (Hoelzel 1998). This can bias estimates of genetic differentiation at the regional and oceanic scales, especially if sample size for geographical areas is limited. Evidence to date suggests that for some cetaceans, there is little or no genetic structure among populations at interoceanic scales including comparisons between the Southern Ocean, North Pacific and North Atlantic (e.g. fin whale, Balaenoptera physalus, Wada & Numachi 1991; see also Hoelzel 1998; Pastene et al. 2005a). However, genetic differentiation has been detected among populations of whales sampled from different regions in the Southern Ocean including the humpback whale, Megaptera novaeangliae, (Palsbøll et al. 1995; Pastene et al. 2005a) and the Antarctic minke whale, Balaenoptera bonaerensis (Pastene et al. 2005b). In both of these cases, the whales feed in the Southern Ocean but migrate annually to breeding grounds in the southern Pacific, Atlantic and Indian Oceans. Genetic differentiation is maintained by reproductive isolation of populations in the breeding areas, although in minke whales, genetic structure is less pronounced in male animals. This may arise from sex-biased dispersal which has been detected in several cetacean species with females often maintaining long-term social bonds within matrilineal-related pods while males may disperse from natal groups (e.g. humpback whale, Palumbi & Baker 1994; sperm whale, Physeter macrocephalus, Lyrholm et al. 1999)
Converse to expectations of homogeneity among populations of marine organisms around the Southern Ocean, there is evidence of significant genetic structure at the regional level between all but a few pelagic and one semi-pelagic species (table 3). Genetic structure at the sub-regional scale is detected among all benthic and some semi-pelagic species and the former have demonstrated genetic differentiation over less than 200 km (table 3). Historical factors have clearly played a significant role in structuring populations especially the Quaternary glaciations. During glacial periods, populations have been driven into refugia around the periphery of the Antarctic or in unknown localities (see figure 2). Resultant population fragmentation has led to the establishment of genetically distinct lineages which have diverged sometimes to the point of speciation. This has been followed by range expansion and sometimes secondary contact of populations of Antarctic marine taxa (see figure 2). It is remarkable that genetically distinct regional populations have undergone so little effective mixing. What has prevented genetically effective migration across open marine environments since the LGM (Wiens 2004)?
Table 3.
Patterns of genetic differentiation among species of marine animals (scales of genetic differentiation: inter-regional, between ocean sectors of the Southern Ocean (Atlantic, Indian and Pacific); sub-regional, within ocean sectors of the Southern Ocean; local, across a distance of less than 200 km. Asterisk, one or more pairwise comparisons are across the Antarctic Polar Front. Dagger, differentiation may involve inter-species comparisons (cryptic species). Data from Fevolden & Ayala (1981), Schneppenheim & MacDonald (1984), Fevolden (1986, 1988), Kühl & Schneppenheim (1986), MacDonald et al. (1986), Fevolden & Schneppenheim (1988, 1989), Zane et al. (1998), Gales et al. (1989), Beaumont & Wei (1991), Wada & Numachi (1991), Palsbøll et al. (1995), Allcock et al. (1997), Slade et al. (1998), Rogers et al. (1998), Medlin et al. (2000), Smith & McVeagh (2000), Wynen et al. (2000), Zane & Patarnello (2000), Roeder et al. (2001), Appleyard et al. (2002), Jarman & Nicol (2002), Jarman et al. (2002), Page & Linse (2002), Sands et al. (2003), Darling et al. (2004), Gooday et al. (2004), Held & Wägele (2005), Pastene et al. (2005a,b), Shaw et al. (2004), Kuhn & Gaffney (2006) and Rogers et al. (2006)).
| geographical scale of separation | |||||
|---|---|---|---|---|---|
| species | phylum | habitat | inter-regional | sub-regional | local |
| Neogloboquadrina pachyderma | Protista | pelagic | yes* | no | n.a. |
| Phaeocystis antarctica | Protista | pelagic | no | no | n.a. |
| Bathyallogromia wedellensis | Protista | benthic | n.a. | no | no |
| Euphausia superba | Arthropoda | pelagic | no | no (?) | no |
| Euphausia crystallorophias | Arthropoda | pelagic | yes (?) | yes (?) | n.a. |
| Moroteuthis ingens | Mollusca | pelagic | yes* | no | n.a. |
| Parborlasia corrugatus | Nemertea | benthic | n.a. | n.a. | yes† |
| Nacella concinna | Mollusca | benthic | n.a. | Yes | no |
| Limatula pygmaea | Mollusca | benthic | yes*,† | n.a. | n.a. |
| Pareledone turqueti | Mollusca | benthic | n.a. | yes* | yes |
| Glyptonotus antarcticus | Arthropoda | benthic | yes† | yes† | n.a. |
| Dissostichus eleginoides | Chordata | demersal | yes* | yes | no |
| Champsocephalus gunneri | Chordata | demersal | yes | yes | yes |
| Mirounga leonine | Chordata | semi-pelagic | yes | yes | no |
| Arctocephalus tropicalis | Chordata | semi-pelagic | yes | yes | no |
| Arctocephalus gazella | Chordata | semi-pelagic | yes | yes | no |
| Pygoscelis adeliae | Chordata | semi-pelagic | no (?) | no | no |
| Balaenoptera physalus | Chordata | pelagic | no | no | no |
| Balaenoptera bonaerensis | Chordata | pelagic | yes | yes | n.a. |
| Megaptera novaeangliae | Chordata | pelagic | yes | yes | n.a. |
In pelagic species, lower levels of genetic structure do reflect the open nature of the pelagic environment. Here, barriers to migration and gene flow occur over large spatial scales and are related to environmental adaptation to abiotic factors such as temperature and biotic factors such as competition. This means that shifts in environmental conditions, resulting from global climate change, in the pelagic environment are likely to cause large-scale shifts in the distribution of dominant pelagic species (e.g. salps and krill; Atkinson et al. 2004). For benthic or semi-pelagic species, barriers to migration and gene flow occur despite pelagic larval phases or a strong swimming ability in adults. Outside of the Antarctic, marine species with planktotrophic development often demonstrate a poor-fit of larval duration to species ranges (e.g. Paulay & Meyer 2006) or levels of gene flow between populations (e.g. Barber et al. 2002). Reasons for this are often unclear, but in some cases, inferences regarding dispersal ability of species based on larval development are not realized in natural populations. Oceanographic phenomena at a range of scales may also limit larval dispersal. For example, physical modelling approaches have revealed that larvae may often be retained near source habitat, especially when data related to mortality or behaviour are incorporated into studies (reviewed in Levin 2006; see Cowen et al. 2006). Explanations for genetic differentiation in populations of Patagonian toothfish have suggested that the presence of fronts associated with the northern boundary of the ACC may act to limit gene flow between the Falkland Islands and South Georgia (Rogers et al. 2006). Physical modelling studies coupled with data on larval ecology may help to explain the genetic structure of some Antarctic marine species especially given the detailed oceanographic models and long-term datasets for the region (e.g. for Scotia Sea reviewed in Murphy et al. 2007). In other cases, the lack of suitable habitat lying between populations prevents genetically effective migration by stepping-stone dispersal between them (e.g. toothfish; Rogers et al. 2006; see Barber et al. 2002 for tropical example). The isolation of populations in allopatry also raises the possibility of ecological divergence between them driven by adaptation to local conditions (Wiens 2004). Evidence for rapid local adaptive evolution associated with post-glacial colonization has been seen in non-Antarctic terrestrial taxa (e.g. Clarke et al. 2001).
For many Antarctic taxa, the contrast in genetic structure revealed by mitochondrial and nuclear markers is striking. Differences in genetic differentiation between mitochondrial and nuclear markers should be expected at equilibrium as a result of differences in effective population size between nuclear and mitochondrial genes (Crochet et al. 2003). Additionally, if populations are reproductively isolated in refugia, a lower effective population size in mitochondrial genes will lead to increased rates of divergence among lineages than for nuclear genes. For several Antarctic taxa (e.g. Patagonian toothfish; Adelie penguins), populations exhibit near complete fixation for different mitochondrial haplotypes (Shaw et al. 2004; Rogers et al. 2006) or lineages with distinct haplotypes have different geographical distributions (Ritchie et al. 2004), while genetic structure estimated using microsatellites is significant but weak (Shaw et al. 2004; Rogers et al. 2006) or non-existent (Roeder et al. 2001). This is often cited as being a result of sex-biased dispersal with males showing a greater tendency to disperse than females. However, such large differences in mitochondrial and nuclear markers raise the possibility that selection has played a part in divergence of lineages originating in different refugia. Different haplotype lineages of Patagonian toothfish lie on either side of the PFZ across which there is a strong latitudinal gradient in sea temperatures. During the LGM, strong divergence in mitochondrial genes may have been driven by contrasting environmental conditions in different refugia causing directional selection and selective sweeps for specific haplotypes, a strong mechanism for lineage sorting (e.g. Crochet et al. 2003). The importance of mitochondrial gene products as metabolic enzymes (e.g. cytochrome oxidases) and as structural molecules (e.g. ribosomal RNA) may render them sensitive to selection by environmental temperature or physical factors that are correlated with it (Pörtner et al. 2007). Selection may still operate to maintain separate mitochondrial lineages even in the face of some degree of gene flow between populations. Potential mechanisms may include selective mating or through a selective disadvantage against female hybrids (Crochet et al. 2003). Selection hybrids may also occur through problems associated with co-adaptation of mitochondrial and nuclear genes within mitochondrial lineages (Edmands & Burton 1999; Willett & Burton 2001), although these would be expected to disadvantage both male and female hybrids. Testing whether such mechanisms may be in operation will require the use of neutral genetic models to compare genetic differentiation between populations based on nuclear and mitochondrial genes taking into account differences in effective population size of the markers (e.g. Crochet et al. 2003). Cases where mitochondrial differentiation was much higher than expected merit further investigation given the implications for understanding evolution and biodiversity of the Antarctic marine biota.
5. Conclusions
The Antarctic has been a cradle of evolution both within the Southern Hemisphere and globally. The break-up of Gondwana consisted of a series of events that drove vicariance-dominated evolution in many terrestrial and marine groups. However, the evolution of the Antarctic biota in both marine and terrestrial ecosystems has been strongly driven by climate. Estimating which major climatic and geographical events led to the origination of new lineages in the Antarctic biota is subject to error in estimating times of divergence of Antarctic taxa and results based on molecular, biogeographic and fossil evidence may be controversial (e.g. timing of origin of AFGP and the radiation of the notothenioids, see above). However, summarizing the current data on times of divergence of various taxa shows that they tend to be clustered at significant climatic and geological events since the Cretaceous (table 4). Timings of the extinctions of Antarctic taxa are less clear as these are not represented in phylogenies constructed from extant species, although the Eocene/Oligocene boundary was certainly significant. Many of these climatic events were associated with cooling, the growth of ice sheets and increased strength of the ACC (e.g. Beu et al. 1997; Zachos et al. 2001). Extreme low temperature, the presence of ice during these periods, coupled with marked seasonality eliminated many elements of the terrestrial and marine biota found elsewhere on the planet. This occurred sequentially over the last 40 Myr in successive events, hence the lack of evidence for a single major extinction event and evidence that some taxa, no longer present in the Antarctic, occurred as recently as the Pliocene (Clarke et al. 2004). Strong natural selection exerted by these environmental factors led to adaptation of the Antarctic biota to extreme but often, especially in the case of marine organisms, very narrow physical parameters. The elimination of taxa commonly found elsewhere on Earth stimulated the radiation of groups that have adapted to the Antarctic environment either owing to competitive release, removal of predation pressure or the existence of vacant niches.
Table 4.
Significant periods in the evolution of the Antarctic biota from the Jurassic onwards (time periods were identified from dates to common ancestors of lineages from molecular phylogenetic data constrained in some cases by fossil data. Cladogenesis is defined as the time of origin of a lineage that later radiated into multiple taxa. Speciation is an event leading to the separation of two extant sister species. Data from Patarnello et al. (1996), Beu et al. (1997), Bargelloni et al. (2000), Wynen et al. (2000), Zachos et al. (2001), Patarnello et al. (2003), Clarke et al. (2004), Darling et al. 2004, Lörz & Held (2004), Near et al. (2004), Ritchie et al. (2004), Knapp et al. (2005), Baker et al. (2006), Scher & Martin (2006) and Kuhn & Gaffney 2006).
| time | 165–41 Myr ago | 41–34 Myr ago | 20–24 Myr ago | 14–12 Myr ago | 8–5 Myr ago | 1 Myr ago | 100 kyr ago |
|---|---|---|---|---|---|---|---|
| geological period | Jurassic–Cretaceous–Eocene | Eocene/Oligocene boundary | Oligocene/Miocene boundary | Middle-Miocene | Late Miocene/Early Pliocene | Mid-Pleistocene | Neogene |
| events | break-up of Gondwana | opening of Drake's passage and commencement of Antarctic circumpolar current onset of continental glaciation | major transient glaciation at epoch boundary | Middle-Miocene climatic transition onset of rapid climatic cooling | Initiation of glaciation of West Antarctica | Mid-Pleistocene revolution shift to 100 kyr glacial cycles | Last glacial maximum |
| evolutionary response | cladogenesis vicariance (continental separation) | vicariance (thermal) extinction dispersal cladogenesis | cladogenesis molecular innovation (AFGP) | dispersal radiation | radiation speciation | cycles of population contraction, isolation in refugia and expansion. Speciation. Transoceanic dispersal. | population contraction, isolation in refugia and expansion. Dispersal into Antarctic following LGM. |
| taxa | penguins patellids? Littorinids Notothenioidei flowering plant groups | extinction and cladogenesis in penguins. Extinctions in marine invertebrates and fish radiation in Antarctic amphipoda (?) Eleginops and Antarctic notothenioids diverge | cladogenesis in sub-Antarctic penguins Antarctic krill diverge from sub-Antarctic krill origin and initial radiation of AFGP-bearing notothenioids | dispersal and speciation in Antarctic and sub-Antarctic/temperate penguins Dissostichus eleginoides and D. mawsoni diverge origin of high-Antarctic notothenioids, trematomines, lepidonotothens | speciation in sub-Antarctic/temperate penguins Radiation of channichthyids and Trematomus divergence of Euphausia vallentini and E. frigida | differentiation in Neogloboquadrina genotypes | divergence of lineages in Pygoscelis adeliae, Champsocephalus gunneri, Arctocephalus tropicalis and A. gazella. Speciation in Chionodraco Transoceanic migrations of terrestrial biota |
The onset of icehouse conditions and, over more recent times, millennial-scale cyclic glaciations has driven cycles of population fragmentation, reproductive isolation and allopatric speciation or intraspecific lineage divergence in Antarctic marine and terrestrial ecosystems (the taxonomic diversity pump; Clarke & Crame 1989, 1997; see figure 2). In the terrestrial environment, species were eliminated or driven into refugia, as occurred elsewhere in the world (e.g. Hewitt 2003, 2004). However, after the LGM, unlike many non-Antarctic regions, such as northern Europe, where subsequent population expansion from glacial refugia was geographically extensive and rapid, in the Antarctic this has been extremely limited. This is because the harsh Antarctic environment limits habitat availability, and species inherently have a low-dispersal capacity or are restricted to asexual reproduction preventing the production of dispersive spores or seeds. As with non-Antarctic terrestrial taxa, subject to range contraction and expansion as a result of Quaternary glaciations, in the Antarctic, lineages that have diverged during cold periods in refugia remain distinct in the present day. The difference is that the geographical range of distinct lineages and contact zones are over much smaller spatial scales (tens to hundreds of kilometres). It is perhaps more remarkable that genetic structure resulting from cyclic glaciations has remained so predominant in many marine species. Again, this probably results from a lack of habitat suitable for stepping-stone dispersal across large areas of the ocean, coupled with hydrographic barriers to dispersal although selection may play a role. Biology of individual species in terms of behaviour, life history and patterns of mortality all contribute to the lack of genetically effective migration between geographical areas.
Lineage splitting resulting from isolation of populations in glacial refugia in Antarctic terrestrial and marine ecosystems has resulted in different levels of divergence among lineages depending on the taxa involved (e.g. Wynen et al. 2000; Stevens & Hogg 2006). Genetic divergence between allopatric populations occurs as a result of changes in population size (bottlenecks), founder events, genetic drift and divergent environmental selection in different refugia (Hewitt 2004). Many of these effects will be magnified in small populations and they may interact with aspects of the biology of individual species, especially life history, population dynamics and the ability to tolerate environmental change (e.g. Stevens & Hogg 2006). However, reconstruction of phylogenetic histories from molecular data remains an approximation as it is difficult to account for populations that become extinct. Therefore, elucidating the reasons for contrasting levels of divergence in lineages among taxa will remain extremely difficult.
A problem related to this is how to define species in Antarctic (and other) ecosystems given the range of divergence among refugial lineages. In many cases, molecular or DNA barcoding approaches (Hebert et al. 2003) can help to identify cryptic species and have been successfully applied to Antarctic marine and terrestrial taxa (Held & Wägele 2005; Stevens & Hogg 2006). Molecular barcoding approaches infer the application of phylogenetic or evolutionary species concepts of species definition. In some cases, emergent species can show limited genetic divergence, obscuring diagnosis using such methods (Hebert et al. 2004). Genetic divergence between lineages and even speciation may also occur without reproductive isolation leading to problems in defining species under the biological species concept (Wiens 2004). Therefore, a broader species concept may be appropriate when considering such lineages (e.g. metapopulation lineage concept; De Queiroz 2005a,b) and may require consideration of data related to other aspects of biology of the taxa concerned (Wiens 2004).
The Antarctic Peninsula and Bellingshausen Sea are subject to the most rapid regional warming on the Earth (Hansen et al. 1999; Vaughan et al. 2001). Marked ecological changes have already been seen in parts of the Antarctic Peninsula and in the sub-Antarctic with significant changes in species abundance and range having been observed (e.g. Quayle et al. 2002; reviewed in Chown & Convey 2007). Predicting the outcomes of global climate change on Antarctic and sub-Antarctic species is complex. Marine species in particular may be constrained as a result of their evolutionary history in their ability to respond to temperature increase. Exposure to temperatures above 5°C causes the most stenothermal species to die and various important biological functions fail below these temperatures (reviewed in Peck et al. 2005; Pörtner et al. 2007). Recent experiments have demonstrated that, at least in some cases, Antarctic marine species lack the capacity to upregulate heat shock proteins (HSP70), usually associated with responses to thermal stress in non-Antarctic organisms (Hofmann et al. 2000). Environmental or ecogenomic approaches will provide new insights into how Antarctic species have evolved to adapt to their extreme environment. They will also allow a more detailed and thorough understanding of physiological effects of environmental stress arising from climate change in Antarctic species (Peck et al. 2005).
For Antarctic, sub-Antarctic and temperate species unable to adapt to changes in physical conditions the geographical range of populations will change. Species will retreat south, although this may be hampered by large oceanic barriers to dispersal especially given the rapid onset of global warming. In addition, changes in habitat availability and life-history parameters that result from environmental warming will also affect the distribution of populations and genetic exchange between them. This is especially true of terrestrial species where organisms may alter the numbers of generations per unit time or even shift from predominantly asexual to sexual means of reproduction and alternative mechanisms of dispersal (spores versus vegetative fragments). The result will be significant changes in the relative abundance of the species within entire communities accompanied by changes in ecological processes (Chown & Convey 2007). In addition, the amelioration of climate will make it more likely that invasive species arriving as a result of range expansion southwards or as introductions by humans will become established with significant consequences for the native biota (Barnes et al. 2006).
Footnotes
One contribution of 10 to a Theme Issue ‘Antarctic ecology: from genes to ecosystems. Part 2: evolution, diversity and function’.
References
- Adam K.D, Selkirk P.M, Connett M.B, Walsh S.M. Genetic variation in populations of the moss Bryum argenteum in East Antarctica. In: Battaglia B, Valencia J, Walton D.W.H, editors. Antarctic communities. Cambridge University Press; Cambridge, UK: 1997. pp. 33–38. [Google Scholar]
- Adcock G.J, Shaw P.W, Rodhouse P.G, Carvalho G.R. Microsatellite analysis of genetic diversity in the squid Illex argentinus during a period of intensive fishing. Mar. Ecol. Prog. Ser. 1999;187:171–178. [Google Scholar]
- Ainley D.G, DeMaster D.P. Survival and mortality in a population of Adélie penguins. Ecology. 1980;61:522–530. doi:10.2307/1937418 [Google Scholar]
- Allcock A.L, Piertney S.B. Evolutionary relationships of Southern Ocean Octopodidae (Cephalopoda: Octopoda) and a new diagnosis of Pareledone. Mar. Biol. 2002;140:129–135. doi:10.1007/s002270100687 [Google Scholar]
- Allcock A.L, Brierley A.S, Thorpe J.P, Rodhouse P.G. Restricted gene flow and evolutionary divergence between geographically separated populations of the Antarctic octopus Pareledone turqueti. Mar. Biol. 1997;129:97–102. doi:10.1007/s002270050150 [Google Scholar]
- Allcock A.L, Hochberg F.G, Rodhouse P.G.K, Thorpe J.P. Adelieledone, a new genus of octopodid from the Southern Ocean. Antarct. Sci. 2003;15:415–424. doi:10.1017/S0954102003001512 [Google Scholar]
- Allcock A.L, Collins M.A, Piatkowski U, Vecchione M. Thaumeledone and other deep water octopodids from the Southern Ocean. Deep-Sea Res. II. 2004;51:1883–1901. doi:10.1016/j.dsr2.2004.07.019 [Google Scholar]
- Allegrucci G, Carchini G, Todisco V, Convey P, Sbordoni V. Molecular phylogeny of Antarctic Chironomidae and its implications for biogeographical history. Polar Biol. 2006;29:320–326. doi:10.1007/s00300-005-0056-7 [Google Scholar]
- Andrássy I. Nematodes in the sixth continent. J. Nematode Morph. Syst. 1998;1:107–186. [Google Scholar]
- Appleyard S.A, Ward R.D, Williams R. Population structure of the Patagonian toothfish around Heard, McDonald and Macquarie Islands. Antarct. Sci. 2002;14:364–373. doi:10.1017/S0954102002000238 [Google Scholar]
- Arntz W.E, Brey T, Gallardo V.A. Antarctic marine biodiversity: an overview. In: Battaglia B, Valencia J, Walton D.W.H, editors. Antarctic communities: species, structure and survival. Cambridge University Press; Cambridge, UK: 1997. pp. 3–14. [Google Scholar]
- Atkinson A, Siegel V, Pakhomov E, Rothery P. Long-term decline in krill stock and increase in salps within the Southern Ocean. Nature. 2004;432:100–103. doi: 10.1038/nature02996. doi:10.1038/nature02996 [DOI] [PubMed] [Google Scholar]
- Baker A.J, Pereira S.L, Haddrath O.P, Edge K.-E. Multiple gene evidence for expansion of extant penguins out of Antarctica due to global cooling. Proc. R. Soc. B. 2006;273:11–17. doi: 10.1098/rspb.2005.3260. doi:10.1098/rspb.2005.3260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barber H.N, Dadswell H.E, Ingle H.D. Transport of driftwood from South America to Tasmania and Macquarie Island. Nature. 1959;184:203–204. doi:10.1038/184203a0 [Google Scholar]
- Barber P.H, Palumbi S.R, Erdmann M.V, Moosa M.K. Sharp genetic breaks among populations of Haptosquilla pulchella (Stomatopoda) indicate limits to larval transport: patterns, causes and consequences. Mol. Ecol. 2002;11:659–674. doi: 10.1046/j.1365-294x.2002.01468.x. doi:10.1046/j.1365-294X.2002.01468.x [DOI] [PubMed] [Google Scholar]
- Bargelloni L, Marcato S, Zane L, Patarnello T. Mitochondrial phylogeny of notothenioids: a molecular approach to Antarctic fish evolution and biogeography. Syst. Biol. 2000;49:114–129. doi: 10.1080/10635150050207429. doi:10.1080/10635150050207429 [DOI] [PubMed] [Google Scholar]
- Barnes D.K.A, De Grave S. Biogeography of southern polar bryozoans. Vie et Milieu. 2000;50:261–273. [Google Scholar]
- Barnes D.K.A, Hodgson D.A, Convey P, Allen C.S, Clarke A. Incursion and excursion of Antarctic biota: past present and future. Global Ecol. Biogeogr. 2006;15:121–142. doi:10.1111/j.1466-822X.2006.00216.x [Google Scholar]
- Beaumont A.R, Wei J.H.C. Morphological and genetic variation in the Antarctic limpet Nacella concinna (Strebel, 1908) J. Mollusc. Stud. 1991;57:443–450. doi:10.1093/mollus/57.4.443 [Google Scholar]
- Bertelli S, Giannini N.P. A phylogeny of extant penguins (Aves: Sphenisciformes) combining morphology and mitochondrial sequences. Cladistics. 2005;21:209–239. doi:10.1111/j.1096-0031.2005.00065.x [Google Scholar]
- Beu A.G, Griffin M, Maxwell P.A. Opening of Drake Passage gateway and Late Miocene to Pliocene cooling reflected in Southern Ocean molluscan dispersal: evidence from New Zealand and Argentina. Tectonophysics. 1997;281:83–97. doi:10.1016/S0040-1951(97)00160-1 [Google Scholar]
- Brandt A. Hypotheses on Southern Ocean peracarid evolution and radiation (Crustacea, Malacostraca) Antarct. Sci. 2000;12:269–275. [Google Scholar]
- Brandt A, Brökeland W, Brix S, Malyutina M. Diversity of Southern Ocean deep-sea Isopoda (Crustacea, Malacostraca)—a comparison with shelf data. Deep Sea Res. II. 2004;51:1753–1768. doi:10.1016/j.dsr2.2004.06.033 [Google Scholar]
- Brey T, Dahm C, Gorny M, Klages M, Stiller M, Arntz W.E. Do Antarctic benthic invertebrates show an extended level of eurybathy? Antarct. Sci. 1996;8:3–6. [Google Scholar]
- Brierley A.S, Rodhouse P.G, Thorpe J.P, Clarke M.R. Genetic evidence of population heterogeneity and cryptic speciation in the ommastrephid squid Martialia hyadesi from the Patagonian Shelf and Antarctic Polar Frontal Zone. Mar. Biol. 1993;116:593–602. doi:10.1007/BF00355478 [Google Scholar]
- Briggs J.C. Developments in palaeontology and stratigraphy. vol. 14. Elsevier; Oxford, UK: 1995. Global biogeography. [Google Scholar]
- Briggs J.C. Marine centres of origin as evolutionary engines. J. Biogeogr. 2003;30:1–18. doi:10.1046/j.1365-2699.2003.00810.x [Google Scholar]
- British Antarctic Survey 2004 Antarctica, 1 : 10 000 000 scale map. BAS (Misc.) 11 (http://www.antarctica.ac.uk/Resources/schoolzone/resources/Factsheets/index.html). British Antarctic Survey, Cambridge, UK.
- Carroll J.J, Viglierchio D.R. On the transport of nematodes by the wind. J. Nematol. 1981;13:476–482. [PMC free article] [PubMed] [Google Scholar]
- Chapelle G, Peck L.S. Polar gigantism dictated by oxygen availability. Nature. 1999;399:114–115. doi:10.1038/20099 [Google Scholar]
- Chen L, DeVries A.L, Cheng C.-H.C. Evolution of antifreeze glycoprotein gene from a trypsinogen gene in Antarctic notothenioid fish. Proc. Natl Acad. Sci. USA. 1997;94:3811–3816. doi: 10.1073/pnas.94.8.3811. doi:10.1073/pnas.94.8.3811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng C.-H, Detrich H.W., III Molecular ecophysiology of Antarctic notothenioid fishes. Phil. Trans. R. Soc. B. 2007;362:2215–2232. doi: 10.1098/rstb.2006.1946. doi:10.1098/rstb.2006.1946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chown S.L, Convey P. Spatial and temporal variability across life's hierarchies in the terrestrial Antarctic. Phil. Trans. R. Soc. B. 2007;362:2307–2331. doi: 10.1098/rstb.2006.1949. doi:10.1098/rstb.2006.1949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark M, et al. Antarctic genomics. Comp. Funct. Genomics. 2004;5:230–238. doi: 10.1002/cfg.398. doi:10.1002/cfg.398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke A, Crame J.A. The origin of the Southern Ocean marine fauna. In: Crame J.A, editor. Origins and evolution of the Antarctic biota. Geological Society; London, UK: 1989. pp. 253–268. [Google Scholar]
- Clarke A, Crame J.A. Diversity, latitude and time: patterns in the shallow sea. In: Ormond R.F.G, Gage J.D, Angel M.V, editors. Marine biodiversity: causes and consequences. Cambridge University Press; Cambridge, UK: 1997. pp. 122–147. [Google Scholar]
- Clarke A, Johnston N.M. Scaling of metabolic rate with body mass and temperature in teleost fish. J. Anim. Ecol. 1999;68:893–905. doi:10.1046/j.1365-2656.1999.00337.x [Google Scholar]
- Clarke A, Johnston N.M. Antarctic marine benthic diversity. Oceanogr. Mar. Biol. Annu. Rev. 2003;41:47–114. [Google Scholar]
- Clarke T.E, Levin D.B, Kavanaugh D.H, Reimchen T.E. Rapid evolution in the Nebria gregaria group (Coleoptera: Carabidae) and the palaeogeography of the Queen Charlotte Islands. Evolution. 2001;55:1408–1418. doi: 10.1111/j.0014-3820.2001.tb00662.x. doi:10.1554/0014-3820(2001)055[1408:REITNG]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Clarke A, Aronson R.B, Crame J.A, Gili J.-M, Blake D.B. Evolution and diversity of benthic the fauna of the Southern Ocean continental shelf. Antarct. Sci. 2004;16:559–568. doi:10.1017/S0954102004002329 [Google Scholar]
- Cockell C, Rettberg P, Horneck G, Scherer K, Dale-Stokes M. Measurements of microbial protection from ultraviolet radiation in polar terrestrial habitats. Polar Biol. 2003;26:62–69. [Google Scholar]
- Convey P. Reproduction of Antarctic flowering plants. Antarct. Sci. 1996;8:127–134. [Google Scholar]
- Convey P. Antarctic ecosystem. In: Levin S.A, editor. Encyclopedia of biodiversity. vol. 1. Academic Press; San Diego, CA: 2001. pp. 171–184. [Google Scholar]
- Convey P. Maritime Antarctic climate change: signals from terrestrial biology. Antarct. Res. Ser. 2003;79:145–158. [Google Scholar]
- Convey P, McInnes S.J. Exceptional tardigrade-dominated ecosystems in Ellsworth Land. Antarctica. Ecol. 2005;86:519–527. [Google Scholar]
- Convey P, Smith R.I, Hodgson D.A, Peat H.J. The flora of the South Sandwich Islands, with particular reference to the influence of geothermal heating. J. Biogeogr. 2000;27:1279–1295. doi:10.1046/j.1365-2699.2000.00512.x [Google Scholar]
- Cooper A, Lalueza-Fox C, Anderson S, Rambaut A, Austin J, Ward R. Complete mitochondrial genome sequences of two extinct moas clarify ratite evolution. Nature. 2001;409:704–707. doi: 10.1038/35055536. doi:10.1038/35055536 [DOI] [PubMed] [Google Scholar]
- Coulson S.J, Hodkinson I.D, Webb N.R, Harrison J.A. Survival of terrestrial soil-dwelling arthropods on and in seawater: implications for trans-oceanic dispersal. Funct. Ecol. 2002;16:353–356. doi:10.1046/j.1365-2435.2002.00636.x [Google Scholar]
- Courtright E.M, Wall D.H, Virginia R.A, Frisse L.M, Vida J.T, Thomas W.K. Nuclear and mitochondrial DNA sequence diversity in the Antarctic nematode Scottnema lindsayae. J. Nematol. 2000;32:143–153. [PMC free article] [PubMed] [Google Scholar]
- Cowen R.K, Paris C.B, Srinivasan A. Scaling of connectivity in marine populations. Science. 2006;311:522–527. doi: 10.1126/science.1122039. doi:10.1126/science.1122039 [DOI] [PubMed] [Google Scholar]
- Craw R.C, Grehan J.R, Heads M.J. Oxford University Press; Oxford, UK: 1999. Panbiogeography: tracking the history of life. pp. 229. [Google Scholar]
- Crochet P.-D, Chen J.Z, Pons J.-M, Lebreton J.-D, Hebert P.D.N, Bonhomme F. Genetic differentiation at nuclear and mitochondrial loci among large white-headed gulls: sex biased interspecific gene flow? Evolution. 2003;57:2865–2878. doi: 10.1111/j.0014-3820.2003.tb01527.x. doi:10.1554/03-008 [DOI] [PubMed] [Google Scholar]
- Dale T.M, Skotnicki M.L, Adam K.D, Selkirk P.M. Genetic diversity in the moss Hennediella heimiii in Miers Valley, southern Victoria Land, Antarctica. Polar Biol. 1999;21:228–233. doi:10.1007/s003000050357 [Google Scholar]
- Darling K.F, Wade C.M, Stewart I.A, Kroon D, Dingle R, Brown A.J.L. Molecular evidence for genetic mixing of Arctic and Antarctic subpolar populations of planktonic foraminifers. Nature. 2000;405:43–47. doi: 10.1038/35011002. doi:10.1038/35011002 [DOI] [PubMed] [Google Scholar]
- Darling K.F, Kucera M, Pudsey C.J, Wade C.M. Molecular evidence links cryptic diversification in polar planktonic protists to Quaternary climate dynamics. Proc. Natl Acad. Sci. USA. 2004;101:7657–7662. doi: 10.1073/pnas.0402401101. doi:10.1073/pnas.0402401101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Broyer C, Nyssen F, Dauby P. The crustacean scavenger guild in Antarctic shelf, bathyal and abyssal communities. Deep-Sea Res. II. 2004;51:1733–1752. doi:10.1016/j.dsr2.2004.06.032 [Google Scholar]
- De Queiroz K. Different species problems and their resolution. BioEssays. 2005a;27:1263–1269. doi: 10.1002/bies.20325. doi:10.1002/bies.20325 [DOI] [PubMed] [Google Scholar]
- De Queiroz K. Ernst Mayr and the modern concept of species. Proc. Natl. Acad. Sci. USA. 2005b;102(Suppl.1):6600–6607. doi: 10.1073/pnas.0502030102. doi:10.1073/pnas.0502030102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dell R.K. Antarctic benthos. Adv. Mar. Biol. 1972;10:1–216. [Google Scholar]
- Detrich H.W, Parker S.K, Williams R.C, Nogales E, Downing K.H. Cold adaptation of microtubule assembly and dynamics. J. Biol. Chem. 2000;275:37 038–37 047. doi: 10.1074/jbc.M005699200. doi:10.1074/jbc.M005699200 [DOI] [PubMed] [Google Scholar]
- di Priscu G, Cocca E, Parker S.K, Detrich H.W. Tracking the evolutionary loss of hemoglobin expression by the white-blooded Antarctic icefishes. Gene. 2002;295:185–191. doi: 10.1016/s0378-1119(02)00691-1. doi:10.1016/S0378-1119(02)00691-1 [DOI] [PubMed] [Google Scholar]
- Eastman J.T. Antarctic notothenioid fishes as subjects for research in evolutionary biology. Antarct. Sci. 2000;12:276–287. [Google Scholar]
- Eastman J.T. The nature of diversity of Antarctic fishes. Polar Biol. 2005;28:93–107. doi:10.1007/s00300-004-0667-4 [Google Scholar]
- Edmands S, Burton R.S. Cytochrome c oxidase activity in interpopulation hybrids of a marine copepod; a test for nuclear–nuclear or nuclear–cytoplasmic coadaptation. Evolution. 1999;53:1972–1978. doi: 10.1111/j.1558-5646.1999.tb04578.x. doi:10.2307/2640456 [DOI] [PubMed] [Google Scholar]
- Fanciulli P.P, Summa D, Dallai R, Frati F. High levels of genetic variability and population differentiation in Gressittacantha terranova (Collembola, Hexapoda) from Victoria Land, Antarctica. Antarct. Sci. 2001;13:246–254. doi:10.1017/S0954102001000360 [Google Scholar]
- Fevolden S.E. Genetic variation of Euphausia superba Dana in the Antarctic Peninsula waters. Sarsia. 1986;71:169–175. [Google Scholar]
- Fevolden S.E. Biochemical genetics and population structure of Euphausia superba. Comp. Biochem. Physiol. B. 1988;90:507–513. doi:10.1016/0305-0491(88)90289-1 [Google Scholar]
- Fevolden S.E, Ayala F.J. Enzyme polymorphism in Antarctic krill (Euphausiacea): genetic variation between populations and species. Sarsia. 1981;66:167–181. [Google Scholar]
- Fevolden S.E, Schneppenheim R. Genetic population structure of Euphausia superba DANA in the Atlantic sector of the Southern Ocean as demonstrated by different electrophorestic techniques. Polar Biol. 1988;9:1–8. doi:10.1007/BF00441759 [Google Scholar]
- Fevolden S.E, Schneppenheim R. Genetic homogeneity of krill (Euphausia superba Dana) in the Southern Ocean. Polar Biol. 1989;9:533–539. doi:10.1007/BF00261038 [Google Scholar]
- Fields P.A, Somero G.N. Hot spots in cold adaptation: localised increases in conformational flexibility in lactate dehydrogenase A4 orthogs of Antarctic notothenioid fishes. Proc. Natl Acad. Sci. USA. 1998;95:11 476–11 481. doi: 10.1073/pnas.95.19.11476. doi:10.1073/pnas.95.19.11476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher G.L, Hew C.L, Davies P.L. Antifreeze proteins of teleost fish. Annu. Rev. Physiol. 2001;63:359–390. doi: 10.1146/annurev.physiol.63.1.359. doi:10.1146/annurev.physiol.63.1.359 [DOI] [PubMed] [Google Scholar]
- Fraser K.P.P, Clarke A, Peck L.S. Low-temperature metabolism: seasonal changes in protein synthesis and RNA dynamics in the Antarctic limpet Nacella concinna (Strebel, 1908) J. Exp. Biol. 2002;205:3077–3086. doi: 10.1242/jeb.205.19.3077. [DOI] [PubMed] [Google Scholar]
- Frati F, Spinsanti G, Dallai R. Genetic varation of mtCOII gene sequences in the collembolan Isotoma klovstadi from Victoria Land, Antarctica: evidence for population differentiation. Polar Biol. 2001;24:934–940. doi:10.1007/s003000100302 [Google Scholar]
- Freckman D.W, Virginia R.A. Low-diversity Antarctic soil nematode communities: distribution and response to disturbance. Ecology. 1997;78:363–369. doi:10.2307/2266013 [Google Scholar]
- Freckman D.W, Virginia R.A. Soil biodiversity and community structure in the McMurdo Dry Valleys, Antarctica. Antarct. Res. Ser. 1998;72:323–336. [Google Scholar]
- Gales N.J, Adams M, Burton H.R. Genetic relatedness of two populations of the southern elephant seal, Mirounga leonine. Mar. Mamm. Sci. 1989;5:57–67. doi:10.1111/j.1748-7692.1989.tb00213.x [Google Scholar]
- Garrick R.C, Sands C.J, Rowell D.M, Tait N.N, Greenslade P, Sunnucks P. Phylogeography recapitulates topography: very fine-scale local endemism of saproxylic ‘giant’ springtail at Tallaganda in the Great Dividing Range of south-east Australia. Mol. Ecol. 2004;13:3329–3344. doi: 10.1111/j.1365-294X.2004.02340.x. doi:10.1111/j.1365-294X.2004.02340.x [DOI] [PubMed] [Google Scholar]
- Giannini N.P, Bertelli S. Phylogeny of extant penguins based on integumentary and breeding characteristics. Auk. 2004;121:422–434. doi:10.1642/0004-8038(2004)121[0422:POEPBO]2.0.CO;2 [Google Scholar]
- Gill D.E, Chao L, Perkins S.L, Wolf J.B. Genetic mosaicism in plants and clonal animals. Annu. Rev. Ecol. Syst. 1995;26:423–444. doi:10.1146/annurev.es.26.110195.002231 [Google Scholar]
- Goldstein S.J, Gemmell N.J, Schiel D.R. Molecular phylogenetics and biogeography of the nacellid limpets of New Zealand (Mollusca: Patellogastropoda) Mol. Phylogenet. Evol. 2006;38:261–265. doi: 10.1016/j.ympev.2005.09.002. doi:10.1016/j.ympev.2005.09.002 [DOI] [PubMed] [Google Scholar]
- Gooday A.J, Holzmann M, Guiard J, Cornelius N, Pawlowski J. A new monothalamous foraminiferan from 1000 to 6300 m water depth in the Weddell Sea: morphological and molecular characterisation. Deep-Sea Res. II. 2004;51:1603–1616. doi:10.1016/j.dsr2.2004.06.025 [Google Scholar]
- Greenslade P. Collembola from the Scotia Arc and Antarctic Peninsula including descriptions of two new species and notes on biogeography. Polskie Pismo Entomologiczne. 1995;64:305–319. [Google Scholar]
- Gressitt J.L. Notes on arthropod populations in the Antarctic Peninsula—South Shetland Islands—South Orkney area. In: Gressitt J.L, editor. Entomology of Antarctica. Antarctica research series. vol. 10. American Geophysical Union; Washington, DC: 1967. pp. 373–391. [Google Scholar]
- Grosberg R.K, Levitan D.R, Cameron B.B. Characterization of genetic structure and genealogies using RAPD-PCR markers: a random primer for the novice and nervous. In: Ferraris J.D, Palumbi S.R, editors. Molecular zoology, advances, strategies and protocols. Wiley-Liss; New York, NY: 1996. pp. 67–100. [Google Scholar]
- Gutt J. On the distribution and ecology of holothurians on the Weddell Sea shelf (Antarctica) Polar Biol. 1991;24:145–155. [Google Scholar]
- Gutt J, Sirenko B.I, Smirnov I.S, Arntz W.E. How many macrozoobenthic species might inhabit the Antarctic shelf? Antarct. Sci. 2004;16:11–16. doi:10.1017/S0954102004001750 [Google Scholar]
- Hadrys H, Balick M, Schierwater B. Applications of random amplified polymorphic DNA (RAPD) in molecular ecology. Mol. Ecol. 1992;1:55–63. doi: 10.1111/j.1365-294x.1992.tb00155.x. [DOI] [PubMed] [Google Scholar]
- Hansen J, Ruedy R, Glascoe J, Sato M. GISS analysis of surface temperature change. J. Geophys. Res. 1999;104:30 997–31 022. doi:10.1029/1999JD900835 [Google Scholar]
- Hebert P.D.N, Cywinska A, Ball S.L, deWaard J.R. Biological identification through DNA barcodes. Proc. R. Soc. B. 2003;270:313–321. doi: 10.1098/rspb.2002.2218. doi:10.1098/rspb.2002.2218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebert P.D.N, Stoeckle M.Y, Zemlak T.S, Francis C.M. Identification of birds through DNA barcodes. PLoS Biol. 2004;2:1657–1663. doi: 10.1371/journal.pbio.0020312. doi:10.1371/journal.pbio.0020312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedgepeth, J. W. 1969 Introduction to Antarctic zoogeography. In Distribution of selected groups of marine invertebrates in waters south of 35° S latitude Antarctic map folio series, vol. 11 (eds V. C. Bushnell & J. W. Hedgepeth). Folio, pp. 1–29. New York, NY: American Geographical Society.
- Held C, Wägele J.-W. Cryptic speciation in the giant Antarctic isopod Glyptonotus antarcticus (Isopoda: Valvifera: Chaetiliidae) Sci. Mar. 2005;69(Suppl. 2):175–181. [Google Scholar]
- Hemmingsen E.A. Respiratory and cardiovascular adaptations in haemoglobin-free fish: resolved and unresolved problems. In: di Prisco G, Maresca B, Tota B, editors. Biology of the Antarctic fish. Springer; Berlin, Germany: 1991. pp. 191–203. [Google Scholar]
- Hewitt G. Ice ages, species distributions and evolution. In: Rothschild L.J, Lister A.M, editors. Evolution on planet earth. Academic Press; Oxford, UK: 2003. pp. 339–361. [Google Scholar]
- Hewitt G.M. Genetic consequences of climatic oscillations in the Quaternary. Phil. Trans. R. Soc. B. 2004;359:183–195. doi: 10.1098/rstb.2003.1388. doi:10.1098/rstb.2003.1388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoelzel A.R. Genetic structure of cetacean populations in sympatry, parapatry, and mixed assemblages: implications for conservation policy. J. Hered. 1998;89:451–458. doi:10.1093/jhered/89.5.451 [Google Scholar]
- Hoffman J.I, Amos W. Does kin selection influence fostering behaviour in Antarctic fur seals (Arctocephalus gazella)? Proc. R. Soc. B. 2005;272:2017–2022. doi: 10.1098/rspb.2005.3176. doi:10.1098/rspb.2005.3176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann G.E, Buckley B.A, Airaksinen S, Keen J.E, Somero G.N. Heat-shock protein expression is absent in the Antarctic fish Trematomus bernacchii (Family Nototheniidae) J. Exp. Biol. 2000;203:2331–2339. doi: 10.1242/jeb.203.15.2331. [DOI] [PubMed] [Google Scholar]
- Hoffman J.I, Boyd I.L, Amos W. Male reproductive strategy and the importance of maternal status in the Antarctic fur seal Arctocephalus gazella. Evolution. 2003;57:1917–1930. doi: 10.1111/j.0014-3820.2003.tb00598.x. doi:10.1554/02-530 [DOI] [PubMed] [Google Scholar]
- Hoffman J.I, Boyd I.L, Amos W. Exploring the relationship between parental relatedness and male reproductive success in the Antarctic fur seal Arctocephalus gazella. Evolution. 2004;58:2087–2099. doi: 10.1111/j.0014-3820.2004.tb00492.x. doi:10.1554/04-099 [DOI] [PubMed] [Google Scholar]
- Holdregger R, Stehlik I, Lewis-Smith R.I, Abbott R.J. Populations of Antarctic hairgrass (Deschampsia antarctica) show low genetic diversity. Arctic Antarct. Alpine Res. 2003;35:214–217. doi:10.1657/1523-0430(2003)035[0214:POAHDA]2.0.CO;2 [Google Scholar]
- Hsiao K, Cheng C.-H.C, Fernandes I.E, Detrich H.W. An antifreeze glycopeptide gene from the Antarctic cod Notothenia coriiceps neglecta encodes a polyprotein of high peptide copy number. Proc. Natl Acad. Sci. USA. 1990;87:9265–9269. doi: 10.1073/pnas.87.23.9265. doi:10.1073/pnas.87.23.9265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hugall A, Moritz C, Moussalli A, Stanisic J. Reconciling paleodistribution models and comparative phylogeography in the Wet Tropics rainforest land snail Gnarosophia bellendenkerensis (Brazier, 1875) Proc. Natl Acad. Sci. USA. 2002;99:6112–6117. doi: 10.1073/pnas.092538699. doi:10.1073/pnas.092538699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurr K.A, Lockhart P.J, Heenan P.B, Penny D. Evidence for recent dispersal of Sophora (Leguminosae) around the Southern Oceans: molecular data. J. Biogeogr. 1999;26:565–577. doi:10.1046/j.1365-2699.1999.00302.x [Google Scholar]
- Iwami I. Osteology and relationships of the family Channichthyidae. Mem. Natl. Inst. Polar Res., Tokyo E. 1985;36:1–69. [Google Scholar]
- Jackson G.D. Age, growth and maturation of the deepwater squid Moroteuthi ingens (Cephalopoda: Onychoteuthidae) in New Zealand waters. Polar Biol. 1997;17:268–274. doi:10.1007/s003000050131 [Google Scholar]
- Jarman S.N, Nicol S. Sources of variance in studies of krill population genetics. CCAMLR Sci. 2002;9:107–116. [Google Scholar]
- Jarman S.N, Elliot N.G, Nicol S, McMinn A. Genetic differentiation in the Antarctic coastal krill Euphausia crystallorophias. Heredity. 2002;88:280–287. doi: 10.1038/sj.hdy.6800041. doi:10.1038/sj.hdy.6800041 [DOI] [PubMed] [Google Scholar]
- Johns G.C, Avise J.C. Tests for ancient species flocks based on molecular phylogenetic appraisals of Sebastes rockfishes and other marine fishes. Evolution. 1998;52:3055–3074. doi: 10.1111/j.1558-5646.1998.tb01840.x. doi:10.2307/2411243 [DOI] [PubMed] [Google Scholar]
- Johnston I.A. Muscle metabolism and growth in Antarctic fishes (sub-order Notothenioidei): evolution in a cold environment. Comp. Biochem. Physiol. B. 2003;136:701–713. doi: 10.1016/s1096-4959(03)00258-6. doi:10.1016/S1096-4959(03)00258-6 [DOI] [PubMed] [Google Scholar]
- Knapp M, Stöckler K, Havell D, Delsuc F, Sebastiani F, Lockhart P.J. Relaxed molecular clock provides evidence for long-distance dispersal of Nothofagus (southern beech) PLoS Biol. 2005;3(e14):0038–0042. doi: 10.1371/journal.pbio.0030014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knowlton N. Sibling species in the sea. Annu. Rev. Ecol. Syst. 1993;24:189–216. doi:10.1146/annurev.es.24.110193.001201 [Google Scholar]
- Knowlton N. Molecular genetic analyses of species boundaries in the sea. Hydrobiologia. 2000;420:73–90. doi:10.1023/A:1003933603879 [Google Scholar]
- Komárková V, Poncet S, Quattro J.M. Two native vascular plants, Deschampsia antarctica and Colobanthus quitensis: a new southern-most locality and other localities in the Antarctic Peninsula area. Arctic Alpine Res. 1985;17:401–416. doi:10.2307/1550865 [Google Scholar]
- Korpelainen H, Pohjamo M, Laaka-Lindberg S. How efficiently does bryophyte dispersal lead to gene flow? J. Hattori Bot. Lab. 2005;97:195–205. [Google Scholar]
- Koufopanou V, Reid D.G, Ridgeway S.A, Thomas R.H. A molecular phylogeny of the patellid limpets (Gastropoda: Patellidae) and its implications for the origins of their antitropical distribution. Mol. Phylogenet. Evol. 1999;11:138–156. doi: 10.1006/mpev.1998.0557. doi:10.1006/mpev.1998.0557 [DOI] [PubMed] [Google Scholar]
- Kühl S, Schneppenheim R. Electrophoretic investigation of genetic variation in two krill species Euphausia superba and E. crystallorophias (Euphausiidae) Polar Biol. 1986;6:107–122. [Google Scholar]
- Kuhn K.L, Gaffney P.M. Preliminary assessment of population structure in the mackerel icefish (Champsocephalus gunnari) Polar Biol. 2006;29:927–935. doi:10.1007/s00300-006-0134-5 [Google Scholar]
- Lambert D.M, Ritchie P.A, Millar C.D, Holland B, Drummond A.J, Baroni C. Rates of evolution in ancient DNA from Adélie penguins. Science. 2002;295:2270–2273. doi: 10.1126/science.1068105. doi:10.1126/science.1068105 [DOI] [PubMed] [Google Scholar]
- Lawver L.A, Gahagen L.M. Evolution of Cenozoic seaways in the circum-Antarctic region. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2003;198:11–37. doi:10.1016/S0031-0182(03)00392-4 [Google Scholar]
- Lecointre G, Bonillo C, Ozouf-Costaz C, Hureau J.C. Molecular phylogeny of the Antarctic fishes: paraphyly of the Bovichtidae and no indication for the monophyly of the Notothenioidei (Teleostei) Polar Biol. 1997;18:193–208. doi:10.1007/s003000050176 [Google Scholar]
- Lee Y.-H, Song M, Lee S, Leon R, Godoy S.O, Canete I. Molecular phylogeny and divergence time of the Antarctic sea urchin (Sterechinus neumeyeri) in relation to the South American sea urchins. Antarct. Sci. 2004;16:29–36. doi:10.1017/S0954102004001786 [Google Scholar]
- Leiros H.-K.S, Willassen N.P, Smalås A.O. Structural comparisons of psychrophilic and mesophilic trypsins elucidating the molecular basis of cold-adaptation. Eur. J. Biochem. 1998;267:1039–1049. doi: 10.1046/j.1432-1327.2000.01098.x. doi:10.1046/j.1432-1327.2000.01098.x [DOI] [PubMed] [Google Scholar]
- Levin L.A. Recent progress in understanding larval dispersal: new directions and digressions. Integr. Comp. Biol. 2006;46:282–297. doi: 10.1093/icb/icj024. doi:10.1093/icb/icj024 [DOI] [PubMed] [Google Scholar]
- Lewis-Smith R.I. Terrestrial plant biology of the sub-Antarctic and Antarctic. In: Laws R.M, editor. Antarctic ecology. Academic Press; London, UK: 1984. pp. 61–162. [Google Scholar]
- Lewis-Smith R.I, Convey P. Enhanced sexual reproduction in bryophytes at high latitudes in the maritime Antarctic. J. Bryol. 2002;24:107–117. doi:10.1179/037366802125000962 [Google Scholar]
- Livermore R, Nankivell A, Eagles G, Morris P. Paleogene opening of Drake's Passage. Earth Planet. Sci. Lett. 2005;236:459–470. doi:10.1016/j.epsl.2005.03.027 [Google Scholar]
- Longton R.E. Cambridge University Press; Cambridge, UK: 1988. Biology of polar bryophytes and lichens. [Google Scholar]
- Lörz A.-N, Held C. A preliminary molecular and morphological phylogeny of Antarctic Epimeriidae and Iphimediidae (Crustacea, Amphipoda) Mol. Phylogenet. Evol. 2004;31:4–15. doi: 10.1016/j.ympev.2003.07.019. doi:10.1016/j.ympev.2003.07.019 [DOI] [PubMed] [Google Scholar]
- Lubimova, T. G., Makarov, R. R., Maslennikov, V. V., Shetsov, V. V. & Shust, K. V. 1985 The ecological peculiarities, stocks and role of E. superba in the trophic structure of the Antarctic ecosystem. Selected Scientific Papers, 1982–1984, Part II Hobart, Australia, CCAMLR, 391–505.
- Lud D, Moerdijk T.C.W, Van de Poll W.H, Buma A.G.J, Huiskies A.H.L. DNA damage and photosynthesis in Antarctic and Arctic Sanionia uncinata (Hedw.) Loeske under ambient and enhanced levels of UV-B radiation. Plant Cell Environ. 2002;25:1579–1589. doi:10.1046/j.1365-3040.2002.00914.x [Google Scholar]
- Lyrholm T, Leimar O, Johanneson B, Gyllensten U. Sex-biased dispersal in sperm whales: contrasting mitochondrial and nuclear genetic structure of global populations. Proc. R. Soc. B. 1999;266:347–354. doi: 10.1098/rspb.1999.0644. doi:10.1098/rspb.1999.0644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald C.M, Williams R, Adams M. Genetic variation and population structure of krill (Euphausia superba DANA) from the Prydz Bay region of Antarctic waters. Polar Biol. 1986;6:233–236. doi:10.1007/BF00443400 [Google Scholar]
- Mackintosh N.A. Life cycle of Antarctic krill in relation to ice and water conditions. Discov. Rep. 1972;36:1–94. [Google Scholar]
- Mackintosh N.A. Distribution of post-larval krill in the Antarctic. Discov. Rep. 1973;36:95–156. [Google Scholar]
- Marshall W.A. Biological particles over Antarctica. Nature. 1996;383:680. doi:10.1038/383680a0 [Google Scholar]
- Marshall D.J, Pugh P.J.A. Origin of the inland Acari of Continental Antarctica, with particular reference to Dronning Maud Land. Zool. J. Linn. Soc. 1996;118:101–118. doi:10.1006/zjls.1996.0051 [Google Scholar]
- Maslen N.R, Convey P. Nematode diversity and distribution in the southern maritime Antarctic—clues to history? Soil Biol.Soil Biol. Biochem. 2006;38:3141–3151. [Google Scholar]
- McDaniel S.F, Shaw A.J. Selective sweeps and intercontinental migration in the cosmopolitan moss Ceratodon purpureus (Hedw.) Brid. Mol. Ecol. 2005;14:1121–1132. doi: 10.1111/j.1365-294X.2005.02484.x. doi:10.1111/j.1365-294X.2005.02484.x [DOI] [PubMed] [Google Scholar]
- Medlin L.K, Lange M, Baumann M.E.M. Genetic differentiation among three colony-forming species of Phaeocystis: further evidence for the phylogeny of the Prymnesiophyta. Phycologia. 1994;33:199–212. [Google Scholar]
- Medlin L.K, Lange M, Nöthig E.-M. Genetic diversity in the marine phytoplankton: a review and a consideration of Antarctic phytoplankton. Antarct. Sci. 2000;12:325–333. [Google Scholar]
- Montresor M, Lovejoy C, Orsini L, Procaccini G, Roy S. Biploar distribution of the cyst-forming dinoflagellate Polarella glacialis. Polar Biol. 2003;26:186–194. [Google Scholar]
- Moore D.M. Anthony Nelson; Oswestry, UK: 1983. Flora of Tierra del Fuego. [Google Scholar]
- Moylan T.J, Sidell B.D. Concentrations of myoglobin and myoglobin mRNA in heart ventricles from Antarctic fishes. J. Exp. Biol. 2000;203:1277–1286. doi: 10.1242/jeb.203.8.1277. [DOI] [PubMed] [Google Scholar]
- Muñoz J, Felicísimo M, Cabezas F, Burgaz A.R, Martínez I. Wind as a long-distance dispersal vehicle in the southern hemisphere. Science. 2004;304:1144–1147. doi: 10.1126/science.1095210. doi:10.1126/science.1095210 [DOI] [PubMed] [Google Scholar]
- Murphy E.J, et al. Spatial and temporal operation of the Scotia Sea ecosystem: a review of large-scale links in a krill-centred food web. Phil. Trans. R. Soc. B. 2007;362:113–148. doi: 10.1098/rstb.2006.1957. doi:10.1098/rstb.2006.1957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Near T.J. Estimating divergence times of notothenioid fishes using a fossil-calibrated molecular clock. Antarct. Sci. 2004;16:37–44. doi:10.1017/S0954102004001798 [Google Scholar]
- Near T.J, Pesavento J.J, Cheng C.-H.C. Mitochondrial DNA, morphology and the phylogenetic relationships of Antarctic icefishes (Notothenioidei: Channichthyidae) Mol. Phylogenet. Evol. 2003;28:87–98. doi: 10.1016/s1055-7903(03)00029-0. doi:10.1016/S1055-7903(03)00029-0 [DOI] [PubMed] [Google Scholar]
- Near T.J, Pesavento J.J, Cheng C.-H.C. Phylogenetic investigations of Antarctic notothenioid fishes (Perciformes: Notothenioidei) using complete gene sequences of the mitochondrial encoded 16S rRNA. Mol. Phylogenet. Evol. 2004;32:881–891. doi: 10.1016/j.ympev.2004.01.002. doi:10.1016/j.ympev.2004.01.002 [DOI] [PubMed] [Google Scholar]
- Nevo E. Evolution of genome–phenome diversity under environmental stress. Proc. Natl Acad. Sci. USA. 2001;98:6233–6240. doi: 10.1073/pnas.101109298. doi:10.1073/pnas.101109298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newsham K.K, Hodgson D.A, Murray A.W.A, Peat H.J, Lewis-Smith R.I. Response of two Antarctic bryophytes to stratospheric ozone depletion. Global Change Biol. 2002;8:972–983. doi:10.1046/j.1365-2486.2002.00509.x [Google Scholar]
- Nolan L, Hogg I.D, Stevens M.I, Haase M. Fine scale distribution of mtDNA haplotypes for the springtail Gomphiocephalus hodgsoni (Collembola) corresponds to an ancient shoreline in Taylor Valley, continental Antarctica. Polar Biol. 2006;29:813–819. doi:10.1007/s00300-006-0119-4 [Google Scholar]
- Nunn G.B, Cooper J, Jouventin P, Robertson C.J.R, Robertson G.G. Evolutionary relationships among extant albatrosses (Procellariiformes: Diomedeidae) established from complete cytochrome-b gene sequences. Auk. 1996;113:784–801. [Google Scholar]
- Page T.J, Linse K. More evidence of speciation and dispersal across the Antarctic Polar Front through molecular systematics of Southern Ocean Limatula (Bivalvia: Limidae) Polar Biol. 2002;25:818–826. [Google Scholar]
- Page D.M, Cruickshank R.H, Dickens M, Furness R.W, Kennedy M, Palma R.L, Smith V.S. Phylogeny of “Philoceanus complex” seabird lice (Phthiraptera: Ischnocera) inferred from mitochondrial DNA sequences. Mol. Phylogenet. Evol. 2004;30:633–652. doi: 10.1016/S1055-7903(03)00227-6. doi:10.1016/S1055-7903(03)00227-6 [DOI] [PubMed] [Google Scholar]
- Palsbøll P.J, Clapham P.J, Mattila D.K, Larsen F, Sears R, Siegismund H.R, Sigurjónsson J, Vasquez O, Arctander P. Distribution of mtDNA haplotypes in North Atlantic humpback whales: the influence of behaviour on population structure. Mar. Ecol. Prog. Ser. 1995;116:1–10. [Google Scholar]
- Palumbi S.R, Baker C.S. Contrasting population structure from nuclear intron sequences and mtDNA of humpback whales. Mol. Biol. Evol. 1994;11:426–435. doi: 10.1093/oxfordjournals.molbev.a040115. [DOI] [PubMed] [Google Scholar]
- Parker S.K, Detrich H.W. Evolution, organization, and expression of α-tubulin genes in the Antarctic fish Notothenia coriiceps. J. Biol. Chem. 1998;273:34 358–34 369. doi: 10.1074/jbc.273.51.34358. doi:10.1074/jbc.273.51.34358 [DOI] [PubMed] [Google Scholar]
- Pastene, L. A., Goto, M., Kanda, N. & Nishiwaki, S. 2005a Genetic analyses on stock identification in the Antarctic humpback and fin whales based on samples collected under the JARPA. Paper submitted to the Review Meeting of the Japanese Whale Research Program Under Special Permit in the Antarctic (JARPA), Government of Japan, Tokyo, 18th–20th January, 2005. See http://www.icrwhale.org/eng/JA-J05-JR16.pdf
- Pastene, L. A., Goto, M., Kanda, N., Bando, T., Zenitani, R., Hakamada, T., Otani, S. & Fujise, Y. 2005b A new interpretation of the stock identity in the Antarctic minke whale based on analyses of genetics and non-genetics markers. Paper submitted to the Review Meeting of the Japanese Whale Research Program Under Special Permit in the Antarctic (JARPA), Government of Japan, Tokyo, 18th–20th January, 2005. See http://www.icrwhale.org/eng/JA-J05-JR03.pdf
- Patarnello T, Bargelloni L, Varotto V, Battaglia B. Krill evolution and the Antarctic ocean currents: evidence of vicariant speciation as inferred by molecular data. Mar. Biol. 1996;126:603–608. doi:10.1007/BF00351327 [Google Scholar]
- Patarnello T, Marcato S, Zane L, Varotto V, Bargelloni L. Phylogeography of the Chionodraco genus (Perciformes, Channichthyidae) in the Southern Ocean. Mol. Phylogenet. Evol. 2003;28:420–429. doi: 10.1016/s1055-7903(03)00124-6. doi:10.1016/S1055-7903(03)00124-6 [DOI] [PubMed] [Google Scholar]
- Paulay G, Meyer C. Dispersal and divergence across the greatest ocean region: do larvae matter? Integr. Comp. Biol. 2006;46:269–281. doi: 10.1093/icb/icj027. doi:10.1093/icb/icj027 [DOI] [PubMed] [Google Scholar]
- Peat H.J, Clarke A, Convey P. Diversity and biogeography of the Antarctic flora. J. Biogeogr. 2007;34:132–146. doi:10.1111/j.1365-2699.2006.01565.x [Google Scholar]
- Peck L.S, Conway L.Z. The myth of metabolic cold adaptation: oxygen consumption in stenothermal Antarctic bivalves. In: Harper E.M, Taylor J.D, Crame J.A, editors. The evolutionary biology of the bivalvia. Special publications. vol. 177. Geological Society; London, UK: 2000. pp. 441–445. [Google Scholar]
- Peck L.S, et al. Genomics: applications to Antarctic ecosystems. Polar Biol. 2005;28:351–365. doi:10.1007/s00300-004-0671-8 [Google Scholar]
- Peterson M.A, Denno R.F. The influence of dispersal and diet breadth on patterns of genetic isolation by distance in phytophagous insects. Am. Nat. 1998;152:428–446. doi: 10.1086/286180. doi:10.1086/286180 [DOI] [PubMed] [Google Scholar]
- Picard D, Plantard O, Scurrah M. Mugniéry Inbreeding and population structure of the potato cyst nematode (Globodera pallida) in its native area (Peru) Mol. Ecol. 2004;13:2899–2908. doi: 10.1111/j.1365-294X.2004.02275.x. doi:10.1111/j.1365-294X.2004.02275.x [DOI] [PubMed] [Google Scholar]
- Plantard O, Porte C. Population genetic structure of the sugar beet cyst nematode Heterodera schachtii: a gonochoristic and amphimictic species with highly inbred but weakly differentiated populations. Mol. Ecol. 2004;13:33–41. doi: 10.1046/j.1365-294x.2003.02023.x. doi:10.1046/j.1365-294X.2003.02023.x [DOI] [PubMed] [Google Scholar]
- Pörtner H.O, Peck L, Somero G. Thermal limits and adaptation in marine ectotherms: an integrative view. Phil. Trans. R. Soc. B. 2007;362:2233–2258. doi: 10.1098/rstb.2006.1947. doi:10.1098/rstb.2006.1947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pugh P.J.A, Convey P. Scotia Arc Acari: antiquity and origin. Zool. J. Linn. Soc. 2000;130:309–328. doi:10.1006/zjls.1999.0219 [Google Scholar]
- Quayle W.C, Peck L.S, Peat H, Ellis-Evans C.J, Harrigan P.R. Extreme responses to climate change in Antarctic lakes. Science. 2002;295:645. doi: 10.1126/science.1064074. doi:10.1126/science.1064074 [DOI] [PubMed] [Google Scholar]
- Raguá-Gil J.M, Gutt J, Clarke A, Arntz W.E. Antarctic shallow-water mega-epibenthos: shaped by circumpolar dispersion or local conditions. Mar. Biol. 2004;144:829–839. doi:10.1007/s00227-003-1269-3 [Google Scholar]
- Raven P.H, Axelrod D.I. Angiosperm biogeography and past continental movements. Ann. Miss. Bot. Gard. 1974;61:539–673. doi:10.2307/2395021 [Google Scholar]
- Ribera I, Vogler A.P. Speciation of Iberian diving beetles in Pleistocene refugia (Coleoptera, Dytiscidae) Mol. Ecol. 2004;13:179–193. doi: 10.1046/j.1365-294x.2003.02035.x. doi:10.1046/j.1365-294X.2003.02035.x [DOI] [PubMed] [Google Scholar]
- Ritchie P.A, Lavoue S, Lecointre G. Molecular phylogenetics and the evolution of Antarctic notothenioid fishes. Comp. Biochem. Physiol. A. 1997;118:1009–1025. doi: 10.1016/s0300-9629(97)86790-4. doi:10.1016/S0300-9629(97)86790-4 [DOI] [PubMed] [Google Scholar]
- Ritchie P.A, Millar C.D, Gibb G.C, Baroni C, Lambert D.M. Ancient DNA enables timing of the Pleistocene origin and Holocene expansion of two Adélie penguin lineages in Antarctica. Mol. Biol. Evol. 2004;21:240–248. doi: 10.1093/molbev/msh012. doi:10.1093/molbev/msh012 [DOI] [PubMed] [Google Scholar]
- Roeder A.D, et al. Gene flow on the ice: genetic differentiation among Adélie penguin colonies around Antarctica. Mol. Ecol. 2001;10:1645–1656. doi: 10.1046/j.0962-1083.2001.01312.x. doi:10.1046/j.0962-1083.2001.01312.x [DOI] [PubMed] [Google Scholar]
- Rogers A.D. The role of oceanic oxygen minima in generating biodiversity in the deep sea. Deep-Sea Res. II. 2000;47:119–148. doi:10.1016/S0967-0645(99)00107-1 [Google Scholar]
- Rogers A.D, Clarke A, Peck L.S. Population genetics of the Antarctic heteronemertean Parborlasia corrugatus from the South Orkney Islands. Mar. Biol. 1998;131:1–13. doi:10.1007/s002270050290 [Google Scholar]
- Rogers A.D, Morley S, Fitzcharles E, Jarvis K, Belchier M. Genetic structure of Patagonian toothfish (Dissostichus eleginoides) populations on the Patagonian Shelf and Atlantic and western Indian Ocean Sectors of the Southern Ocean. Mar. Biol. 2006;149:915–924. doi:10.1007/s00227-006-0256-x [Google Scholar]
- Römisch K, Collie N, Soto N, Logue J, Lindsay M, Scheper W, Cheng C.-H.C. Protein translocation across the endoplasmic reticulum membrane in cold-adapted organisms. J. Cell Sci. 2003;116:2875–2883. doi: 10.1242/jcs.00597. doi:10.1242/jcs.00597 [DOI] [PubMed] [Google Scholar]
- Sands C.J, Jarman S.N, Jackson G.D. Genetic differentiation in the squid Moroteuthis ingens inferred from RAPD analysis. Polar Biol. 2003;26:166–170. [Google Scholar]
- Sanmartín I, Ronquist F. Southern hemisphere biogeography inferred by event-based models: plant versus animal patterns. Syst. Biol. 2004;53:216–243. doi: 10.1080/10635150490423430. doi:10.1080/10635150490423430 [DOI] [PubMed] [Google Scholar]
- Scher H.D, Martin E.E. Timing and climatic consequences of the opening of Drake Passage. Science. 2006;312:428–430. doi: 10.1126/science.1120044. doi:10.1126/science.1120044 [DOI] [PubMed] [Google Scholar]
- Schneppenheim R, MacDonald C.M. Genetic variation and population structure of krill (Euphausia superba) in the Atlantic sector of Antarctic waters. Polar Biol. 1984;3:19–28. doi:10.1007/BF00265563 [Google Scholar]
- Selkirk P.M, Skotnicki M, Adam K.D, Connett M.B, Dale T, Joe T.W, Armstrong J. Genetic variation in Antarctic populations of the moss Sarconeurum glaciale. Polar Biol. 1997;18:344–350. doi:10.1007/s003000050198 [Google Scholar]
- Selkirk P.M, Skotnicki M.L, Ninham J, Connett M.B, Armstrong J. Genetic variation and dispersal of Bryum argenteum and Hennediella heimii populations in the Garwood Valley, southern Victoria Land, Antarctica. Antarct. Sci. 1998;10:423–430. [Google Scholar]
- Seppelt R.D, Green T.G.A, Skotnicki M. Notes on the flora, vertebrate fauna and biological significance of Beaufort Island, Ross Sea, Antarctica. Polarforschung. 1996 (1999);66:53–59. [Google Scholar]
- Shaw P.W, Arkhipkin A.I, Al-Khairulla H. Genetic structuring of Patagonian toothfish populations in the Southwest Atlantic Ocean: the effect of the Antarctic Polar Front and deep-water troughs as barriers to genetic exchange. Mol. Ecol. 2004;13:3293–3304. doi: 10.1111/j.1365-294X.2004.02327.x. doi:10.1111/j.1365-294X.2004.02327.x [DOI] [PubMed] [Google Scholar]
- Shepherd L.D, Millar C.D, Ballard G, Ainley D.G, Wilson P.R, Haynes G.D, Baroni C, Lambert D.M. Microevolution and mega-icebergs in the Antarctic. Proc. Natl Acad. Sci. USA. 2005;102:16 717–16 722. doi: 10.1073/pnas.0502281102. doi:10.1073/pnas.0502281102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skotnicki M.L, Selkirk P.M, Dale T.M. RAPD profiling of genetic variation in Antarctic mosses. In: Lyons W.B, Howard-Williams C, Hawes I, editors. Ecosystem processes in Antarctic ice-free landscapes. A. A. Balkema; Rotterdam, UK: 1997. pp. 129–135. [Google Scholar]
- Skotnicki M.L, Selkirk P.M, Beard C. RAPD profiling of genetic diversity in two populations of the moss Ceratodon purpureus in Victoria Land, Antarctica. Polar Biol. 1998a;19:172–176. doi:10.1007/s003000050231 [Google Scholar]
- Skotnicki M.L, Selkirk P.M, Ninham J.A. RAPD analysis of genetic variation and dispersal of the moss Bryum pseudotriquetrum from Southern Victoria Land, Antarctica. Polar Biol. 1998b;20:121–126. doi:10.1007/s003000050285 [Google Scholar]
- Skotnicki M.L, Selkirk P.M, Ninham J.A. RAPD analysis of genetic variation and dispersal of the moss Bryum argenteum in Ross Island and Victoria Land, Antarctica. Polar Biol. 1999;21:417–422. doi:10.1007/s003000050382 [Google Scholar]
- Skotnicki M.L, Ninham J.A, Selkirk P.M. Genetic diversity, mutagenesis and dispersal of Antarctic mosses—a review of progress with molecular studies. Antarct. Sci. 2000;12:363–373. [Google Scholar]
- Skotnicki M.L, Selkirk P.M, Broady P, Adam K.D, Ninham J.A. Dispersal of the moss Campylopus pyriformis on geothermal ground near the summits of Mount Erebus and Mount Melbourne, Victoria Land, Antarctica. Antarct. Sci. 2001;13:280–285. doi:10.1017/S0954102001000396 [Google Scholar]
- Skotnicki M.L, Bargagli R, Ninham J.A. Genetic diversity in the moss Pohlia nutans on geothermal ground of Mount Rittmann, Victoria Land, Antarctica. Polar Biol. 2002;25:771–777. [Google Scholar]
- Skotnicki M.L, Mackenzie A.M, Ninham J.A, Selkirk P.M. High levels of genetic variability in the moss Ceratodon purpureus from continental Antarctica, subantarctic Heard and Macquarie Islands and Australasia. Polar Biol. 2004;27:687–698. doi:10.1007/s00300-004-0640-2 [Google Scholar]
- Skotnicki M.L, Mackenzie A.M, Clements M.A, Selkirk P.M. DNA sequencing and genetic diversity of the 18S–26S nuclear ribosomal internal transcribed spacers (ITS) in nine Antarctic moss species. Antarct. Sci. 2005;17:377–384. doi:10.1017/S0954102005002816 [Google Scholar]
- Slade R.W, Moritz C, Hoelzel A.R, Burton H.R. Molecular population genetics of the southern elephant seal Mirounga leonine. Genetics. 1998;149:1945–1957. doi: 10.1093/genetics/149.4.1945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith P, McVeagh M. Allozyme and microsatellite DNA markers of toothfish population structure in the Southern Ocean. J. Fish Biol. 2000;57:72–83. [Google Scholar]
- Stevens M.I, Hogg I.D. Long-term isolation and recent range expansion from glacial refugia revealed for the endemic springtail Gomphiocephalus hodgsoni from Victoria Land, Antarctica. Mol. Ecol. 2003;12:2357–2369. doi: 10.1046/j.1365-294x.2003.01907.x. doi:10.1046/j.1365-294X.2003.01907.x [DOI] [PubMed] [Google Scholar]
- Stevens M.I, Hogg I.D. Contrasting levels of mitochondrial DNA variability between mites (Penthalodidae) and springtails (Hypogastruridae) from the Trans-Antarctic Mountains suggest long-term effects of glaciation and life history on substitution rates, and speciation processes. Soil Biol. Biochem. 2006;38:3171–3180. doi:10.1016/j.soilbio.2006.01.009 [Google Scholar]
- Stevens M.I, Greenslade P, Hogg I.D, Sunnucks P. Southern hemisphere springtails: could any have survived glaciation of Antarctica? Mol. Biol. Evol. 2006;23:874–882. doi: 10.1093/molbev/msj073. doi:10.1093/molbev/msj073 [DOI] [PubMed] [Google Scholar]
- Tambussi C.P, Reguero M.A, Marenssi S.A, Santillana S.N. Crossvallia unienwillia, a new Spheniscidae (Spenisciformes, Aves) from the Late Paleocene of Antarctica. Geobios. 2005;38:667–675. doi:10.1016/j.geobios.2004.02.003 [Google Scholar]
- Thingsgaard K. Population structure and genetic diversity of the amphiatlantic haploid peatmoss Sphagnum affine (Sphagnopsida) Heredity. 2001;87:485–496. doi: 10.1046/j.1365-2540.2001.00939.x. doi:10.1046/j.1365-2540.2001.00939.x [DOI] [PubMed] [Google Scholar]
- Tripati A, Backman J, Elderfield H, Ferretti P. Eocene bipolar glaciation associated with global carbon cycle changes. Nature. 2005;436:341–346. doi: 10.1038/nature03874. doi:10.1038/nature03874 [DOI] [PubMed] [Google Scholar]
- van der Velde M, Bijlsma R. Phylogeography of five Polytrichum species within Europe. Biol. J. Linn Soc. 2003;78:203–213. doi:10.1046/j.1095-8312.2003.00151.x [Google Scholar]
- Vaughan D.G, Marshall G.J, Connolley W.M, King J.C, Mulvaney R. Devil in the detail. Science. 2001;293:1777–1779. doi: 10.1126/science.1065116. doi:10.1126/science.1065116 [DOI] [PubMed] [Google Scholar]
- Wada S, Numachi K.I. Allozyme analyses of genetic differentiation among the populations and species of Balaenoptera. Rep. Int. Whaling Commun. 1991;13:125–154. [Google Scholar]
- Wagstaff S.J, Garnock-Jones P.J. Patterns of diversification in Chionohebe and Parahebe (Scrophulariaceae) inferred from ITS sequences. N. Zeal. J. Bot. 2000;38:389–407. [Google Scholar]
- Wagstaff S.J, Martinsson K, Swenson U. Divergence estimates of Tetrachondra hamiltonii and T. patagonica (Tetrachondraceae) and their implications for austral biogeography. N. Zeal. J. Bot. 2000;38:587–596. [Google Scholar]
- Wallwork J.A. Zoogeography of some terrestrial micro-arthropoda in Antarctica. Biol. Rev. 1973;48:233–259. [Google Scholar]
- Wanntorp L, Wanntorp H.-E. The biogeography of Gunnera L.:vicariance and dispersal. J. Biogeogr. 2003;30:979–987. [Google Scholar]
- Waters J.M, Dijkstra L.H, Wallis G.P. Biogeography of a southern hemisphere freshwater fish: how important is marine dispersal? Mol. Ecol. 2000;9:1815–1821. doi: 10.1046/j.1365-294x.2000.01082.x. doi:10.1046/j.1365-294x.2000.01082.x [DOI] [PubMed] [Google Scholar]
- Watling L, Thurston M.H. Antarctica as an evolutionary incubator; evidence from the cladistic biogeography of the amphipod family Iphimediidae. In: Crame J.A, editor. Origins and evolution of the Antarctic biota. Geological society special publication. vol. 47. Geological society; London, UK: 1989. pp. 297–313. [Google Scholar]
- White A.G. Marine benthos. In: Laws R.M, editor. Antarctic ecology. vol. 2. Academic Press; London, UK: 1984. pp. 421–461. [Google Scholar]
- Wiens J.J. What is speciation and how should we study it? Am. Nat. 2004;163:914–923. doi: 10.1086/386552. doi:10.1086/386552 [DOI] [PubMed] [Google Scholar]
- Willett C.S, Burton R.S. Viability of cytochrome c genotypes depends on cytoplasmic backgrounds. Evolution. 2001;55:1592–1599. doi: 10.1111/j.0014-3820.2001.tb00678.x. doi:10.1554/0014-3820(2001)055[1592:VOCCGD]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Williams S.T, Reid D.G, Littlewood D.T.J. A molecular phylogeny of the Littorininae (Gastropoda: Littorinidae): unequal evolutionary rates, morphological parallelism, and biogeography of the Southern Ocean. Mol. Phylogenet. Evol. 2003;28:60–86. doi: 10.1016/s1055-7903(03)00038-1. doi:10.1016/S1055-7903(03)00038-1 [DOI] [PubMed] [Google Scholar]
- Winckworth R.C, Grau J, Robertson A.W, Lockhart P.J. The origins and evolution of the genus Myosotis L. (Boraginaceae) Mol. Phylogenet. Evol. 2002a;24:180–193. doi: 10.1016/s1055-7903(02)00210-5. doi:10.1016/S1055-7903(02)00210-5 [DOI] [PubMed] [Google Scholar]
- Winckworth R.C, Wagstaff S.J, Glenny D, Lockhart P.J. Plant dispersal N.E.W.S. from New Zealand. Trends Ecol. Evol. 2002b;17:514–520. doi:10.1016/S0169-5347(02)02590-9 [Google Scholar]
- Wise K.A.J. Collembola (Springtails) In: Gressitt J.L, editor. Entomology of Antarctica. Antarctica research series. vol. 10. American Geophysical Union; Washington, DC: 1967. pp. 123–148. [Google Scholar]
- Wright S.D, Yong C.G, Dawson J.W, Whittaker D.J, Gardner R.C. Riding the ice age El Niño? Pacific biogeography and evolution of Metrosideros subg. Metrosideros (Myrtaceae) inferred from nuclear ribosomal DNA. Proc. Natl Acad. Sci. USA. 2000;97:4118–4123. doi: 10.1073/pnas.050351197. doi:10.1073/pnas.050351197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wynen L.P, Goldsworthy S.D, Guinet C, Bester M.N, Boyd I.L, Gjertz I, Hofmeyr G.J.G, White R.W.G, Slade R. Postsealing genetic variation and population structure of two species of fur seal (Arctocephalus gazella and A. tropicalis) Mol. Ecol. 2000;9:299–314. doi: 10.1046/j.1365-294x.2000.00856.x. doi:10.1046/j.1365-294x.2000.00856.x [DOI] [PubMed] [Google Scholar]
- Zachos J.C, Shackleton N.J, Revenaugh J.S, Pälike H, Flower B.P. Climatic responses to orbital forcing across the Oligocene–Miocene boundary. Science. 2001;292:274–278. doi: 10.1126/science.1058288. doi:10.1126/science.1058288 [DOI] [PubMed] [Google Scholar]
- Zane L, Patarnello T. Krill: a possible model for investigating the effects of ocean currents on the genetic structure of a pelagic invertebrate. Can. J. Fish. Aquat. Sci. 2000;57(Suppl. 2):16–23. doi:10.1139/cjfas-57-S3-16 [Google Scholar]
- Zane L, Ostellari L, Maccatrozzo L, Bargelloni L, Battaglia B, Patarnello T. Molecular evidence for genetic sub-division of Antarctic krill (Euphausia superba Dana) populations. Proc. R. Soc. B. 1998;265:2387–2393. doi: 10.1098/rspb.1998.0588. doi:10.1098/rspb.1998.0588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinsmeister W.J. Late Cretaceous—Early Tertiary molluscan biogeography of the southern circum-Pacific. J. Paleontol. 1982;54:1–14. [Google Scholar]