Abstract
Nitric oxide (NO) plays an important role in the regulation of cardiovascular function. S-nitrosylation, the covalent attachment of an NO moiety to sulfhydryl residues of proteins, resulting in the formation of S-nitrosothiols (SNOs), is a prevalent posttranslational protein modification involved in redox-based cellular signaling. Under physiologic conditions, protein S>-nitrosylation and SNOs provide protection preventing further cellular oxidative and nitrosative stress. However, oxidative stress and the resultant dysfunction of NO signaling have been implicated in the pathogenesis of cardiovascular diseases.
INTRODUCTION
Oxidative stress in vivo can result from a reduction in endogenous antioxidant, burst formation of reactive oxygen species (ROS), or other imbalances between antioxidants and ROS. Increasing data suggest that physiologic levels of ROS may play an important role in normal cell signaling. In contrast, under pathophysiologic conditions, such as myocardial ischemia–reperfusion injury and cardiomyopathy, ROS production increases and exceeds the antioxidant defense of the cell. Thus, a large transient increase or a moderate sustained increase in ROS is suggested to be detrimental and to contribute to heart dysfunction and myocyte death (47, 77).
Nitric oxide (NO) plays an important role in the regulation of cardiac function (7, 22, 47). In addition to activating cyclic guanosine monophosphate (cGMP)-dependent signaling pathways, NO can directly modify sulfhydryl residues of proteins through S-nitrosylation, which has emerged as an important posttranslational protein modification based on prototypic redox mechanisms in signal transduction (12, 48, 68, 82). Under physiologic oxidative stress, NO might provide protection to cells by S-nitrosylation of some critical protein thiols, preventing them from further oxidative modification by ROS (Fig. 1). Conversely, increased oxidative stress and the resultant dysregulation of NO are implicated in the pathogenesis of cardiovascular diseases. Nitrosative stress occurs with an increase in reactive nitrogen species (RNS) and ROS formed from oxidative stress. For example, the peroxynitrite (OONO−), generated from NO and superoxide, is a very strong cytotoxic oxidant, which can irreversibly damage cells by oxidation of free thiols, nitration of tyrosine residues, and lipid peroxidation (100, 112). In cardiac myocytes, ROS and RNS induce stress-signaling pathways involved in mitochondrial dysfunction, intracellular Ca2+ overload, hypertrophy and heart failure, and apoptosis and necrosis (122).
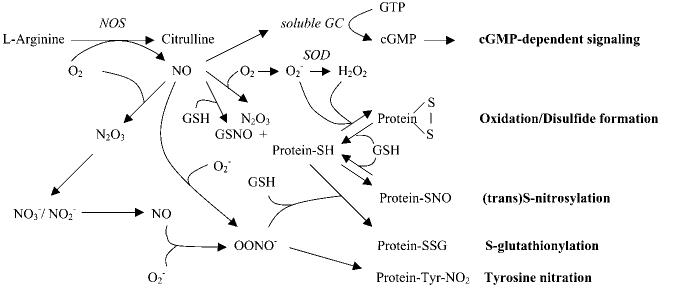
FIG. 1.
Redox-based NO-related signaling. Under physiologic condition, NO is produced from NOS and mediates cGMP-dependent and/or cGMP-independent signaling, which is dependent on the (sub)cellular redox status. NO can also be produced by nitrate/nitrite in nonenzymatic (such as low pH) and enzymatic ways (such as by XOR) in ischemic or anoxia conditions. The formation of N2O3 by autooxidation of NO or the formation of LMW-SNOs, such as GSNO, leads to protein S-nitrosylation by direct SNO formation or transnitrosylation. The ROS (O2−, H2O2, etc.) causes the oxidation of protein such as disulfide formation. Low physiologic levels of ONOO− via the reaction of NO and O2− can interact with GSH, resulting in reversible S-glutathiolation of proteins; however, high concentrations or sustained formation of NO and O2− increase ONOO− formation, leading to irreversible tyrosine nitration of proteins.
Redox and NO
Physiologic levels of ROS and NO can interact and modulate the signaling of one another. The redox status of the cell influences NO signaling in two major ways. First, the balance between NO, molecular oxygen (O2), superoxide anion (O2−) radical, and antioxidants determines what products are made. As shown in Fig. 1, depending on the localization and level of enzymes that produce or consume O2−, NO can (a) activate guanylyl cyclase and mediate cGMP-dependent signaling; (b) form N2O3 by autooxidation via the reaction with O2 and lead to protein S-nitrosylation; (c) generate GSNO in the presence of GSH, which can mediate transnitrosylation reactions; (d) produce ONOO− by reacting with O2−, which was originally suggested to be a toxic end product of high levels of NO and O2−, leading to irreversible nitration of proteins; however, recent data suggest that low physiologic levels of ONOO− can interact with reduced glutathione (GSH), resulting in reversible S-glutathiolation of proteins, such as the cardiac sarcoplasmic reticulum (SR) Ca2+-ATPase (i.e., SERCA2a) (1). In some disease states, the thiols in SERCA2a that are the target of S-glutathiolation are irreversibly oxidized, thereby blocking the NO-dependent activation of SERCA2a (1). It is also interesting that neuronal NO synthase (nNOS) and xanthine oxidoreductase (XOR), an enzyme that produces O2−, have been reported to colocalize in the cardiac SR. Furthermore, inhibition or deletion of nNOS results in an increase in XOR-mediated O2− production, suggesting that NO produced by nNOS inhibits the activity of the colocalized XOR (57). Taken together, these studies suggest that the alterations in the regulation of NO- and ROS-generating enzymes, or the levels of antioxidants such as superoxide dismutase (SOD) and GSH, will alter NO and ROS signaling and the resulting protein modifications. Also consistent with this theme, endothelial NOS (eNOS) and extracellular SOD (ecSOD) have been reported to localize in the sarcolemma of ventricular myocytes (11). The efficiency of NO synthase (NOS) can also be a factor, because it has been shown that NOS can produce O2− if the substrate L-arginine or other cofactors such as tetrahydrobiopterin are limiting (19).
ROS and NO can both interact with thiol groups, and this is a second mechanism by which redox and NO signaling interact. It has been reported that S-nitrosylation of thiol groups in proteins can protect these proteins against irreversible oxidative stress (41, 128). In contrast, as mentioned earlier, irreversible oxidation of thiols can block the physiologic modification by S-nitrosylation or S-glutathiolation and thereby interfere with normal physiologic signaling (1). It has been suggested that NO can protect cells from oxidative stress, whereas loss or inhibition of NOS enhances oxidative stress. Hare and Stamler (47) also suggested that ROS can alter the balance between phosphorylation and S-nitrosylation of key signaling molecules.
Protein S-nitrosylation and its detection
The S-nitrosylation reaction can be mediated through NO carriers such as S-nitrosothiols (SNOs), NO complexed with transition metals, or a direct reaction between NO and thiols in the presence of electron acceptors. NO is unable to react with nucleophiles under oxygen-free conditions, suggesting that its higher oxides, possibly N2O3, are actually the nitrosylating agents. It has been found that oxidation of NO to N2O3 is facilitated by micellar catalysis, which is mediated within the hydrophobic pocket of proteins (73, 97). The protein S-nitrosylation is redox reversible with high spatial and temporal specificity.
In most cases, the specificity of S-nitrosylation is governed by consensus acid–base motifs controlling targeted thiol pKa and nucleophilicity, and physiologic concentrations of NO can lead to S-nitrosylation of only a single cysteine thiol. However, there is complexity in the acid–base motif (i.e., that the target cysteine may not have to be juxtaposed with acidic and/or basic residues with respect to primary sequence, but such a juxtaposition may emerge in three-dimensional protein structure) (48). In addition, the acid–base motif may be limited in its application to hydrophilic environments, whereas NO-related signals may originate in membranes and other hydrophobic environments that facilitate protein S-nitrosylation (73, 97). Thus, the relative hydrophobicity of the region surrounding the target thiol may provide a “hydrophobic motif” for protein S-nitrosylation (48). A further determinant that governs the specificity of posttranslational protein modification by NO is provided by the colocalization of NO sources and targets proteins, which is based at least in part on specific protein–protein interactions with NO synthases. Conversely, the S-nitrosylation also is a temporal signaling event, which depends on the formation of NO by NOS and other nitrosylating equivalents. A subsequent transnitrosylation reaction may occur once a protein within a signaling complex or low-molecular-weight thiols such as GSH becomes S-nitrosylated, which may serve to deliver NO sequentially to its neighboring proteins within the complex, potentially creating a cascade of spatial and temporal S-nitrosylation–based NO signaling (15, 48).
Thus, a number of models have been proposed to provide for targeting S-nitrosylation to specific proteins, including (a) the consensus S-nitrosylation acid–base motif controlling targeted thiol pKa and nucleophilicity; (b) hydrophobic compartmentalization facilitating the reaction of NO and O2; (c) spatial subcellular compartmentalization of NOS and proximity to potential targets; (d) allosteric regulation of thiol accessibility and reactivity by cellular redox, oxygen, metal ions, and nitrosonium (NO+) addition reaction; and (e) subsequent transnitrosylation reactions (15, 48).
The multiplicity of effects of protein S-nitrosylation has prompted the development of reliable techniques for detection of SNO, including immunoassay with antibody against S-nitrosocysteine (119), NO-based (after breakdown of SNO by mercury) chemical reactions such as Saville–Griess colori-metric method (49) or 2,3-diaminonaphthylene (DAN) fluorescence assay (38, 85), and a photolytic/ozone chemiluminescence technique (31). A newly developed biotin switch method (54, 55) has become a widespread technique in combination with proteomic approaches (44, 65, 76, 81). However, a recent study showing that ascorbic acid is not a selective reductant for S-nitrosothiols raises concerns about the specificity of the biotin switch method (67).
SNO storage and transportation
NO is a lipophilic and short-lived free radical. In the cardiovascular system, NO can produce remote or long-lasting effects by formation of various SNOs, serving as “NO carriers” to store and transport NO in vivo, which induces slower but much more persistent effects than does pure NO (96). SNOs derived from proteins, peptides, and amino acids supply cellular compartments and extracellular fluids with reservoirs of NO bioactivity, playing key roles in human health and disease (34).
Low-molecular-weight SNO
Intracellular NO may be buffered by low-molecular-weight (LMW)-SNOs, such as S-nitrosoglutathione (GSNO), after the reaction of NO with LMW thiols like GSH. These LMW-SNOs have been proposed to function as major physiologic mediators of the actions of NO (96). GSNO is able to modify protein thiols via S-nitrosylation or glutathionylation, which is dependent on the surrounding redox equilibrium. Regulation of the cardiovascular system by GSNO appears to be of particular physiologic interest, because GSNO is the most abundant endogenous SNO and has been suggested to be a potential NO-storage and -transport species. GSNO decomposes slowly to generate NO, a reaction catalyzed by LMW thiols and trace metal ions (130). In addition, denitrosylation can also be achieved by GSNO reductase. An elevated level of SNOs in the plasma of GSNO reductase–deficient mice suggests that SNO proteins and GSNO are in a dynamic equilibrium and that transnitrosylation via GSNO is an integral mechanism for SNO-protein formation in vivo (72).
SNO-albumin
Albumin is the most abundant transport and storage protein in the mammalian vasculature. At physiologic NO concentrations, plasma albumin accelerates formation of LMW-SNOs in vitro and in vivo via a mechanism of micellar catalysis of NO oxidation in the albumin hydrophobic core and subsequent transfer of NO+ to LMW thiols. The albumin-mediated LMW-SNO production, which is directly dependent on the concentration of circulating LMW thiols, contributes to vasodilatory vascular control (106). Therapeutically, inhaled NO enhances SNO-albumin formation and has been found to decrease ischemia–reperfusion injury (98).
SNO-hemoglobin
In red blood cells, it has been found that the binding of O2 to heme iron in hemoglobin (Hb) promotes NO binding to a particular cysteine residue (β chain-Cys93), forming SNO-Hb. Deoxygenation leads to an allosteric transition in SNO-Hb that releases the NO group in the microcirculation and regulates vascular tone (23, 114). The exportation of NO bioactivity from red blood cells is through transnitrosylation from SNO-Hb to vicinal cysteine residues in the cytoplasmic domain of anion-exchange protein AE-1, also known as Band 3 (51). Thus, red blood cell–derived SNO-Hb regulates the vascular response to changes of tissue oxygen tension, thereby matching regional blood flow with local metabolic demands (113).
SNO-myoglobin
Myoglobin (Mb) is a key element influencing redox pathways in cardiac muscle to protect the heart functionally and metabolically from oxidative damage (32). Mb has been suggested to be a scavenger of cellular NO in myocardium (33), which can protect the heart from iNOS-mediated nitrosative stress (40). The observed impairment of cardiac function and exercise endurance in Mb−/− mice can be partly attenuated by NO inhibition (90). Although direct evidence is still lacking, S-nitrosylation of Mb to form SNO-Mb is thought to be one of the molecular mechanisms for its NO-scavenging function, given the similarities in the kinetic and thermodynamic properties of NO interactions with Hb and Mb (33).
Nitrite
Although SNO-proteins have been found to exist in the human circulation, their role in the regulation of basal vascular tone has been challenged because of the presence of other bioavailable NO sources, such as nitrite. Nitrite, generated from the reaction of NO and oxygen, can be converted to NO by protons or via enzymatic conversion by XOR (9, 56, 127, 139). Hemoglobin also has been shown to function as a nitrite reductase (52). It has been found that circulating nitrite is bioactive and provides a source of intravascular NO (15, 39, 123). In models of heart and liver ischemia–reper-fusion injury, nitrite has been shown to reduce infarct size dramatically and to exert NO-dependent protective effects on cellular apoptosis and necrosis. Thus, tissue nitrite can serve as a significant extravascular pool and biologic storage reserve of NO during a period of hypoxia, subserving a critical function in tissue protection from ischemic injury (28, 53, 121, 125).
Regulation of cardiac function by protein S-nitrosylation
NO plays an important role in modulating myocardial function in both health and disease (7, 47). Increasing evidence suggests that nitrosative and oxidative stress play important roles in the regulation of cardiac myocyte function and survival (47, 111). Under physiologic oxidative stress, NO might provide protection to cells by S-nitrosylation of some critical protein thiols, preventing them from further oxidative damage.
Intracellular Ca2+ handling
Ca2+-induced Ca2+ release (CICR) is a well-known molecular mechanism of excitation–contraction (e-c) coupling in cardiac myocytes. In brief, membrane depolarization leads to Ca2+ entry via the sarcolemmal L-type Ca2+ channel, which then stimulates a larger Ca2+ release from the SR through the cardiac isoform of the ryanodine receptor (RyR2), ultimately activating systolic myocyte contraction. Diastolic myocyte relaxation is mediated by Ca2+ uptake via SERCA2a and Ca2+ efflux via the sarcolemmal sodium–calcium exchanger. Oxidative stress could impair intracellular Ca2+ regulation, and dysregulation of intracellular Ca2+ homeostasis is thought to be an important mechanism in many acute and chronic cardiovascular diseases (27).
Both eNOS and nNOS are constitutively expressed in cardiomyocytes. A recent study suggested that NO regulates cardiac function by spatial confinement of NOS isoforms (i.e., eNOS is localized in caveolae where it regulates the L-type Ca2+ channel in the sarcolemma, and nNOS is located in the SR where it regulates Ca2+ release from the SR) (5, 131). However, the molecular mechanisms responsible for spatial and temporal specificity of NO-mediated regulation of intra-cellular Ca2+ and myocardial function are still not clear. Although NO-mediated regulation is dependent at least in part on the activation of guanylyl cyclase and the subsequent modification of the phosphorylation state of channels, NO could also participate in the regulation of e-c coupling through cGMP-independent redox mechanisms, because all of the major Ca2+-handling proteins possess multiple free cysteine residues and are subjected to redox regulation (46, 95, 124).
L-type Ca2+ channel
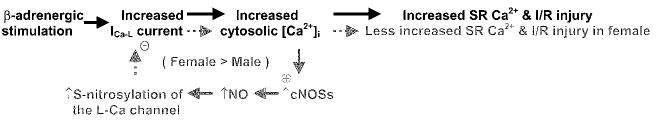
The L-type Ca2+ current (ICa-L) has been shown to be reversibly regulated by redox, with both activation (18) and inhibition (50, 91, 105) reported. Using the biotin switch method, Sun et al. (118) have found that the L-type Ca2+ channel α1 subunit is the predominant S-nitrosylated protein in the membrane fractions. Protein S-nitrosylation of the L-type Ca2+ channel in heart occurs endogenously in vivo, and the levels of the S-nitrosylation are significantly increased after β-adrenergic stimulation and ischemia–reperfusion, with the level of this increase being significantly higher in female than in male subjects. The higher level of SNO in females is due to a greater production of NO from constitutive isoforms of NOS. Compared with males, female mice have more caveolin-3–associated eNOS and a greater translocation of nNOS to the sarcolemma after -adrenergic stimulation and ischemia–reperfusion (118). This increase in SNO of the L-type Ca2+ channel in females is correlated with reduced ischemia–reperfusion injury under β-adrenergic stimulation. Isoproterenol treatment before ischemia and reperfusion results in higher levels of S-nitrosylation of the L-type Ca2+ channel, lower cardiomyocyte ICa-L, a smaller Ca2+ transient, less SR Ca2+ loading, less ischemic injury, and better functional recovery after reperfusion in females (Fig. 2), suggesting that the inhibition of ICa-L by S-nitrosylation of the L-type Ca2+ channel may play a cardioprotective role in hypercontractile hearts, such as with β-adrenergic stimulation (137) and ischemia–reperfusion (6, 75).
FIG. 2.
Cardioprotection role of S-nitrosylation of L-type Ca2+ channel in ischemic reperfused heart under adrenergic stimulation. An increase in Ca2+ before the ischemia, as occurs under β-adrenergic stimulation or other hypercontractile conditions, causes Ca2+ overload and an increased ischemia–reperfusion (I/R) injury (text in black). However, the increased cytosolic Ca2+ leads to a greater increase (+) in NO production and protein S-nitrosylation in females, because of increased constitutive NOS (cNOSs), eNOS, and nNOS association with caveolin-3 in females. The increase in S-nitrosylation of the L-type Ca2+ channel in females reduces (−) Ca2+ entry and SR Ca2+ loading at the start of ischemia, thereby reducing Ca2+ overload during ischemia and reperfusion and thus reducing ischemia–reperfusion injury (text in grey).
RyR2
It has been found that physiologic concentrations (submicromolar) of NO S-nitrosylate and activate the skeletal muscle isoform of RyR (RyR1) at a single cysteine (Cys3635, within the hydrophobic calmodulin-binding domain) from among ∼50 free thiols in each homotetrameric subunit, which is allosterically dependent on the presence of physiologic muscle O2 tension (31, 119, 120). A comparable study of cardiac RyR2 suggests that rather than direct NO-mediated specific S-nitrosylation, transnitrosylation via LMW-SNO may play a major role in regulation of RyR2 channel function (Sun and Meissner, unpublished study). It has been previously found that SNO compounds could poly-S-nitrosylate RyR2 and activate the channels in vitro (132). In addition, Petroff et al. (103) reported that stretching of cardiac muscle modulates Ca2+ release from RyR2, Ca2+ sparks, and the electrically stimulated Ca2+transient via activation of PtdIns-3-OH kinase (PI3K)/Akt/eNOS. The resultant production of NO exerts its action independent of cGMP, most probably through S-nitrosylation of the RyR2 or one of its regulatory components (103).
SERCA2a
Sequestration of Ca2+ by SERCA2a mediates cardiac muscle relaxation. NO has been reported to modulate the activity of SERCA2a (58, 131), but the molecular mechanism is not clear. Protein modification mediated by NO carriers could result from S-nitrosylation or from other secondary oxidative modifications (102). Recently, a dual role of NO/O2 in regulating SERCA2a has been elucidated, in which SERCA2a is activated by reversible S-glutathiolation at Cys674 via the formation of peroxynitrite in the presence of GSH (1). Chronically elevated levels of oxidative stress in some disease states, such as atherosclerosis, could irreversibly oxidize the responsible thiols and block NO-induced S-glutathiolation (1). Peroxynitrite has been found to be a mediator of cytotoxic damage during inflammation, by inducing irreversible tyrosine nitration and dysfunction of proteins (100, 112). However, the study from Adachi et al. (1) suggests that under certain redox conditions, peroxynitrite can readily react with reactive thiols to form S-glutathione adducts.
Antioxidant defense
S-nitrosylation is modulated by the cellular redox status; its formation is dependent on the state of redox equilibrium and is prevented by high levels of antioxidants (8, 17, 21). NO has been found to block cell death after GSH depletion by preserving the redox status of mitochondrial protein thiols, probably by a mechanism that involves S-nitrosylation of mitochondrial protein thiols (128). This may represent an endogenous protective mechanism for the mammalian cell against nitrosative/oxidative stress when intracellular thiols or other redox constituents have decreased below a critical concentration. In addition, NO generated by the coronary vasculature may serve as one of the antioxidant defenses in the heart, as blocking NO generation causes an increased oxidative stress in the heart (111).
Many redox-related enzymes contain active cysteine(s), which are capable of undergoing NO-mediated S-nitrosylation. Among these are catalase (35), glutathione peroxidase (3), glutathione reductase (17), glutathione transferase P1–1 (74), and thioredoxin (41), with the S-nitrosylation of thioredoxin being best characterized (please see Haendeler's review in this issue). Thioredoxin and thioredoxin reductase are ubiquitously expressed antioxidant enzyme systems. Studies from Dimmeler's group (41, 49) suggest that thioredoxin is essential for maintaining the content of S-nitrosylated molecules in endothelial cells. Thioredoxin itself is S-nitrosylated at Cys69 under basal conditions, and this S-nitrosylation is required both for scavenging ROS and for preserving its own redox regulatory activity. S-nitrosylation of thioredoxin also contributes to its antiapoptotic function, possibly by transnitrosylation of proteins such as caspases, thereby inhibiting their activity (41). Shear stress increases the S-nitrosylation and the reductase activity of thioredoxin in endothelial cells (49). The antioxidant effect of statins is partially mediated via S-nitrosylation and activation of thioredoxin in endothelial cells (42). Conversely, it has been found that S-nitrosylation of thioredoxin at active-site Cys32/Cys35 leads to the dissociation and activation of apoptosis signal-regulating kinase 1 (ASK1), suggesting that S-nitrosylation of thioredoxin may also play a role in proapoptotic signaling under certain oxidative stresses (116, 134).
Cell death and survival
Apoptosis is characterized by an energy-dependent process of cell shrinkage, plasma membrane blebbing, chromatin condensation, and DNA fragmentation. Apoptosis can be initiated by binding of ligands such as tumor necrosis factor-α (TNF-α) and CD95/Fas to specific death receptors on the cell surface, leading to the formation of a death-inducing signaling complex (DISC). DISC then recruits and activates the protease zymogen procaspase-8, initiating a caspase cascade (26). Alternatively, apoptosis can be initiated by a mitochondrial pathway, which results in release of cytochrome c. Under some conditions, cytochrome c release is via the mitochondrial permeability transition pore (PTP), which undergoes a Ca2+-dependent transition that disrupts membrane potential and releases apoptogenic proteins. The pore is protected from opening by low pH, ADP, and a high electrochemical proton gradient (Δ ψ), while pore opening is enhanced by depleting ADP, by Pi, or by low Δ;ψ. PTP opening is promoted by specific oxidative stress targeted on a critical protein thiol. NO has been proposed to enhance the open probability of the PTP by S-nitrosylation on this specific cysteine residue, resulting in the release of cytochrome c and endonuclease G from mitochondria (104).
NO is implicated in both apoptotic and necrotic cell death, depending on the biologic milieu, such as the cellular redox state, NO concentration and exposure time, iron mobilization within the cell, and the combination with oxygen and other ROS (13, 61, 89). In addition, ATP depletion might modulate NO and affect cell death (69). On stimulation, NO can be either an antiapoptotic or a proapoptotic regulator, depending on the point in the pathway at which it interacts (20, 61). Protein S-nitrosylation may simultaneously inactivate several parts of the apoptotic machinery and serve to balance apoptosis and necrosis.
Akt/PKB
The serine/threonine kinase Akt/protein kinase B (PKB) is believed to play a crucial role in apoptosis and the insulin-signaling cascade in the cardiovascular system (99). The antiapoptotic effect of Akt/PKB has provided an intriguing therapeutic strategy for protecting against myocardial ischemia–reperfusion injury. In cardiovascular endothelial cells, it has been found that shear stress increases NO formation by Ca2+-independent activation of eNOS via Akt/PKB phosphorylation (25). However, a recent study suggests that NO might also inactivate Akt/PKB, providing negative feedback. In mouse C2C12 myoblasts, it has been found that S-nitrosylation of Cys296 of Akt/PKB blocks disulfide bond formation between Cys296 and Cys310 and suppresses the biologic effects of Akt/PKB (76). The involvement of S-nitrosylation of Akt/PKB in insulin signaling is addressed in the energy metabolism section.
Caspases
As cysteine aspartyl proteases, caspases are categorized into initiator (caspase-8, -9, -10) and executioner (caspase-3, -6, -7) subtypes. Most caspases contain a single cysteine at the catalytic site, which is subjected to redox modification and can be S-nitrosylated by NO (70). Inactive procaspases exist as a latent zymogen. Upon apoptotic stimulation, these procaspases are cleaved into active forms. S-nitrosylation of the redox-sensitive thiol in the catalytic site of caspases plays an essential role in the apoptotic signal cascade by inhibiting apoptotic cell death (61, 88). The activation of caspase-8 is known to involve sequential activation of other caspases and functions primarily upstream of Bcl-2 and cytochrome c (60). The activity of caspase-8 can be suppressed by NO-mediated S-nitrosylation, which inhibits the cleavage of Bid and Bcl-2 and blocks release of mitochondrial cytochrome c (62).
In lymphocytes and endothelial cells, S-nitrosylation of caspase-3 (Cys163 in p17 subunit) keeps the zymogen in an inactive state, which protects cells from unwanted apoptosis, whereas Fas activation results in denitrosylation of the catalytic cysteine as well as proteolytic cleavage of caspase-3 and induces apoptosis (43, 49, 110). S-nitrosylation of caspases is also dependent on subcellular localization. It has been reported that S-nitrosylation occurs frequently to mitochondrial, but not cytoplasmic caspase-3. Also, inhibition of endogenous NOS potentiates ischemia–reperfusion-induced myocardial apoptosis via a caspase-3–dependent pathway (126). A recent study using neonatal rat cardiomyocytes has demonstrated that the inhibition of apoptosis by S-nitrosylation of caspase-3 plays an important role in cardiomyocyte apoptosis (78). The ability of NO to inhibit downstream caspase-3 suggests that NO may be able to rescue cardiomyocytes from apoptosis even after the caspase cascade has been activated. In rat heart, 90% of caspase-9 zymogens are mitochondrial (64). In addition, the majority of mitochondrial caspase-9 is also S-nitrosylated (79). It would be interesting to know whether mitochondrial caspase-9 in heart undergoes S-nitrosylation as it does in other cells. Finding an appropriate dose of NO to affect caspase targets may provide a promising therapeutic strategy for preventing myocyte cell death.
Cyclooxygenase-2
Cyclooxygenase-2 (COX-2) is the rate-limiting enzyme in prostaglandin synthesis, which is induced in response to stress. It has been demonstrated that ischemic preconditioning upregulates the expression and activity of COX-2 in the heart, which mediates the protective effects of the late phase of ischemic preconditioning against both myocardial stunning and myocardial infarction (10). Recently, Atar et al. (4) found in rat heart that atorvastatin-induced cardioprotection is mediated by increasing inducible NOS (iNOS), which activates COX-2 in the heart by S-nitrosylation.
eNOS and Hsp90
Vascular endothelial cells express high levels of eNOS, and a recent study showed that eNOS is reversibly regulated by S-nitrosylation of its zinc-tetrathiolate cysteines (Cys96 and Cys101) and that the intracellular redox milieu mediates eNOS denitrosylation on enzyme activation (29, 30, 107). Thus, the dynamic receptor-mediated regulation of S-nitrosylation of eNOS provides a potentially important mechanism for the control of NO signaling pathways in the vascular wall (29, 30). In addition, eNOS specifically interacts with scaffolding proteins such as caveolin and heat shock protein 90 (Hsp90). It has been shown in endothelial cells that S-nitrosylation of Hsp90 not only abolishes the positive regulation of eNOS activity mediated by native Hsp90, but also inhibits its ability to hydrolyze ATP and disables its intrinsic properties as a chaperone (84).
Energy metabolism
The heart is capable of altering its metabolic rate during exercise or ischemia. The cytosolic and mitochondrial redox state is important in the regulation of oxidative phosphorylation and glycolysis under physiologic and pathophysiologic conditions. NO-mediated protein S-nitrosylation has been found to be involved in the regulation of energy metabolism.
Aldehyde dehydrogenase
Mitochondrial class 2 aldehyde dehydrogenase (ALDH2) is one of the key enzymes in the NAD+-dependent oxidation of various aldehydes produced during intermediary metabolism. Inactivation of ALDH2 is likely to cause marked accumulation of toxic aldehydes, leading to increased susceptibility to irreversible damage. It has been shown that S-nitrosylation of mitochondrial ALDH2 in intact cells leads to reversible inhibition of ALDH2 activity (94).
Creatine kinase
Creatine kinase (CK) plays a crucial role in energy metabolism and exists in both cytoplasmic and mitochondrial compartments. In myocytes, the CK system buffers ATP levels when oxygen supply is limited, such as during ischemia–reperfusion. It has been reported that GSNO dose-dependently inhibits CK, possibly via transnitrosylation (129). In adult rat ventricular myocytes, CK has been shown to be reversibly regulated by NO, possibly through S-nitrosylation of Cys283 (2). In addition, the concentration of GSH in myocytes seems to be an important determinant of the extent of S-nitrosylation of CK in situ. S-nitrosylation of CK with subsequent loss of enzyme activity may have important implications in heart during inotropic stimulation or under severe oxidative stress.
Glyceraldehyde-3-phosphate dehydrogenase
An essential glycolytic enzyme, glyceraldehyde-3-phosphate dehydrogenase (GAPDH) has been reported to bind to membranes of diverse organs including the heart. The binding of GAPDH to the membrane not only appears to be a direct regulatory mechanism for cell metabolism but also may prevent further oxidative modification of its active thiol. The most abundant S-nitrosylated protein in the resting endothelial cell is GAPDH, suggesting a regulatory function for NO/S-nitrosylation in glycolysis (133). It has been reported that NO or GSNO inhibits GAPDH activity by S-nitrosylation of Cys149; this S-nitrosylation is reversed by LMW thiols such as GSH (92, 93). S-nitrosylation of GAPDH is responsible for reversible enzyme inhibition, which initiates subsequent modification by the pyridinium cofactor NADH. The attachment of NADH causes enzyme inactivation. Thus, S-nitrosylation may serve to protect GAPDH from oxidant inactivation and to regulate glycolysis (92). In addition, S-nitrosylation of GAPDH has been found to decrease the binding affinity of GAPDH for the red blood cell membrane (36). Moreover, it has been shown that GSNO induces S-glutathionylation of GAPDH and inactivates the enzyme in ischemic myocardium (63). Using HEK293 cells, Hara et al. (45) demonstrated that S-nitrosylation of GADPH initiates apoptosis by nuclear translocation after Siah1 (an ubiqutin E3 ligase) binding.
Mitochondrial respiratory chain components
It has been reported that NO inhibits mitochondrial ATP generation via inhibition of the mitochondrial respiratory chain, mainly at complex I and IV, leading to a switch from apoptosis to necrosis (69). In murine macrophage J774 cells, Clementi et al. (21) found that long-term exposure to NO leads to persistent inhibition of complex I, which appears to result from S-nitrosylation of complex I. A recent study using isolated rat heart mitochondria has shown that the 75-kDa subunit of complex I is S-nitrosylated by exogenously added GSNO, which results in significant inhibition of the complex. Furthermore, SNOs can be detected in mitochondria isolated from hearts subjected to ischemic preconditioning (16). Another study using endothelial cells has reported that mitochondrial complex IV/cytochrome c oxidase could also be persistently inhibited by S-nitrosylation at two active cysteine (Cys196 and Cys200) residues (138).
Insulin regulation
Oxidative and/or nitrosative stress has been implicated in many human diseases including insulin resistance. Insulin is an important metabolic regulator. A study using pancreatic cells suggests that S-nitrosylation of glucokinase may play an important role in glucose-stimulated insulin secretion (109). Yasukawa et al. (136) have shown that S-nitrosylation of Akt/PKB at Cys224 in skeletal muscle leads to its inactivation. In addition, an increase is found in the level of S-nitrosylation and inactivation of Akt in diabetic mice versus wild-type mice. These results suggest that S-nitrosylation–mediated inactivation of Akt/PKB may contribute to the pathogenesis of insulin resistance (136).
Transcription factors
Some redox-sensitive transcriptional factors (71) crucial for cell death and survival have been reported to be S-nitrosylated, including the following.
Estrogen receptor
Estrogen classically exerts its genomic effects by modifying gene expression through the activation of estrogen receptors (ERs). However, a rapid nongenomic action of estrogen in vascular cells appears to play a major cardioprotective role, mainly through NO production by activation of eNOS, which triggers downstream signaling cascades (59). NO-induced S-nitrosylation of the ER at cysteine residues in its zinc-finger domain, results in selective inhibition of DNA-binding at specific estrogen-responsive elements (EREs), which may favor activation of rapid nongenomic signaling pathways and subsequent modulation of downstream genomic activity (37).
Hypoxia-inducible factor-1
Hypoxia-inducible factor-1 (HIF-1) is a transcription regulator that responds to oxygen. HIF-1 is a heterodimer composed of subunits HIF-α1 and HIF-1α. Under normoxic condition, HIF-1α is hydroxylated at proline and arginine residues, which promotes ubiquitination and inhibits transactivation. Hypoxia impairs these hydroxylations and leads to HIF-1 accumulation and translocation to the nucleus, where it turns on hypoxia-responsive genes. During hypoxia, NO has been found to inhibit HIF-1 activation by the competitive inhibition of mitochondrial respiration, leading to increased oxygen availability (82). In normoxia, NO S-nitrosylates HIF-1α, which promotes HIF-1α stabilization, DNA binding, and activation of downstream target-gene expression (14, 101, 117, 135).
Nuclear factor-κB
Nuclear factor κB (NF-κB) is a transcription factor that plays a pivotal role in inflammation, cell survival, and cell proliferation. NF-κB, a heterodimer composed of p50/p65 subunits, is expressed constitutively in most mammalian cells. It has been shown that S-nitrosylation of NF-κB at Cys62 of the p50 subunit inhibits NF-κB–dependent DNA binding and gene transcription (80, 87). In addition, it has been established that NF-κB is complexed with and sequestered in the cytoplasm by NF-κB inhibitor (I-κB), which is phosphorylated by I-κB-kinase complex (IKK-a, -b, and -γ), initiating I-κB ubiquitination, releasing NF-κB which translocates to the nucleus. A recent study has shown that S-nitrosylation of Cys179 of the catalytic IKK-β subunit inhibits the IKK kinase complex and subsequent phosphorylation of I-κB, providing a mechanism for S-nitrosylation to be involved in the upstream regulation of NF-κB–mediated inflammatary responses (108).
SUMMARY
In summary, SNOs and protein S-nitrosylation can exert important effects and mediate redox signaling in the cardiovascular system (Table 1), and the accumulating evidence suggests that SNOs and S-nitrosylation play key roles in human health and disease (34, 83). Changes in the levels of SNOs depend on both enzymatic and nonenzymatic mechanisms of SNO formation, processing, and degradation. The S-nitrosylation of cysteine residues is redox reversible with high spatial and temporal specificity (48). The redox environment of targeted cysteine residue(s) in a protein influences the efficiency of S-nitrosylation and denitrosylation, and other allosteric effects can impose further control.
Table 1.
S-Nitrosylated Proteins in Cardiovascular System
| S-Nitrosylated proteins | Regulatory effects of S-nitrosylation | Cell/tissue type (species) | References |
|---|---|---|---|
| I. Inhibition by S-nitrosylation | |||
| Akt/PKB | Suppresses the biologic effects of Akt/PKB | C2C12 myoblasts (mouse) | 136 |
| Caspase-3 | Keeps the zymogen in an inactive state, which protects cells from unwanted apoptosis (antiapoptosis) |
Neonatal cardiomyocytes (rat) | 78 |
| Umbilical vein endothelial cells (human) | 49, 110 | ||
| Creatine kinase | Suppresses myocardial contractility | Ventricular myocytes (rat) | 2 |
| eNOS | eNOS is tonically S-nitrosylated in resting basal level, which is denitrosylated on activation |
Aortic endothelial cells (bovine) | 29, 30, 107 |
| GAPDH | Decreases GAPDH binding affinity of cell membrane and inhibiting the enzyme activity |
Aortic endothelial cells (human, bovine) | 133 |
| Red blood cells (human) | 36 | ||
| HSP90 | Inhibits ATP hydrolyzing ability, disables the chaperone property, and abolishes the positive regulation of eNOS |
Endothelial cells (EA.hy926 cell line) | 84 |
| L-type Ca2+ channel α1 subunit | Inhibits ICa-L in females under β-adrenergic stimulation | Ventricular myocytes (mouse) | 118 |
| Mitochondrial complex | Inhibits complex I | Isolated heart mitochondria (rat) | 16 |
| Persistently inhibits complex IV | Pulmonary artery endothelial cells (human) | 138 | |
| NF-κB | Inhibits gene transcription and antiinflammation | Alveolar type II epithelial cells (mouse) | 108 |
| N-ethylmaleimide-sensitive factor | Inhibits disassembly of SNARE and antiinflammation | Aortic endothelial cell (human) | 86 |
| Tissue-type plasminogen activator | Inhibits platelet aggregation | Aortic endothelial cell (bovine) | 115 |
| Attenuates necrosis after ischemia–reperfusion injury | Perfused heart in vivo (cat) | 24 | |
| Tissue transglutaminase | Inhibits enzyme activity and antiapoptosis | Aortic endothelial cell (bovine) | 66 |
| II. Activation by S-nitrosylation | |||
| COX-2 | Elicits preconditioning effect | Perfused hearts in vitro (rat) | 4 |
| HIF-1 | Provokes HIF-1β stabilization in normoxia | Pulmonary artery endothelial cells (bovine) | 101 |
| RyR2/SR Ca2+ release channel | Increases channel activity by poly-S-nitrosylation | Cardiac SR vesicles (dog) | 132 |
| Thioredoxin | Increases reductase activity and antiapoptosis | Vascular endothelial cells (human) | 41 |
The redox reversibility of S-nitrosylation provides two possible mechanisms of signal transduction [i.e., (a) S-nitrosylation of the specific active cysteine residue(s) not only leads to changes of protein structure and function, but also prevents these thiol(s) from further oxidative modification; and (b) release of NO can activate other signaling pathways]. Thus, SNOs and protein S-nitrosylation may serve multiple roles to mitigate oxidative stress. Further investigation of the biologic functions of endogenous SNOs and protein S-nitrosylation in vivo will help to understand better the molecular mechanisms of NO signaling and provide new therapeutic opportunities and targets for intervention in cardiovascular diseases.
ABBREVIATIONS
- Akt/PKB
protein kinase B
- ADP
adenosine 5′-diphosphate
- ALDH2
mitochondrial class 2 aldehyde dehydrogenase
- ASK1
apoptosis signal-regulating kinase 1
- ATP
adenosine 5′-triphosphate
- cGMP
cyclic guanosine monophosphate
- CK
creatine kinase
- COX-2
cyclooxygenase-2
- Cys
cysteine residue
- eNOS
endothelial isoform of NOS
- ER
estrogen receptor
- GAPDH
glyceraldehydes-3-phosphate dehydrogenase
- GSH
reducing glutathione
- GSNO
S-nitrosoglutathione
- GTP
guanine 5′-triphosphate
- Hb
hemoglobin
- HIF-1
hypoxia-inducible factor-1
- HSP90
heat shock protein 90
- ICa-L
L-type Ca2+ current
- iNOS
inducible isoform of NOS
- LWM SNO
low-molecular-weight SNO
- Mb
myoglobin
- mitochondrial PTP
mitochondrial permeability transition pore
- NAD+/NADH
oxidized and reduced forms of nicotinamide adenine dinucleotide
- NF-κB
nuclear factor κB
- nNOS
neuronal isoform of NOS
- NO
nitric oxide
- NOS
nitric oxide synthase
- O2−
superoxide anion
- ONOO−
peroxynitrite
- RNS
reactive nitrogen species
- ROS
reactive oxygen species
- RyR2
cardiac isoform of ryanodine receptor/SR Ca2+ release channel
- SERCA2a
cardiac isoform of SR Ca2+-ATPase
- SNO
S-nitrosothiol
- SOD
superoxide dismutase
- SR
sarcoplasmic reticulum
- XOR
xanthine oxidoreductase
REFERENCES
- 1.Adachi T, Weibrod RM, Pimentel DR, Ying J, Sharov VS, Schoneich C, Cohen RA. S-glutathiolation by peroxynitrite activates SERCA during arterial relaxation by nitric oxide. Nat Med. 2004;10:1200–1207. doi: 10.1038/nm1119. [DOI] [PubMed] [Google Scholar]
- 2.Arstall MA, Bailey C, Gross WL, Bak M, Balligand JL, Kelly RA. Reversible S-nitrosation of creatine kinase by nitric oxide in adult rat ventricular myocytes. J Mol Cell Cardiol. 1998;30:979–988. doi: 10.1006/jmcc.1998.0662. [DOI] [PubMed] [Google Scholar]
- 3.Asahi M, Fujii J, Suzuki K, Seo HG, Kuzuya T, Hori M, Tada M, Fujii S, Taniguchi N. Inactivation of glutathione peroxidase by nitric oxide donor. J Biol Chem. 1995;270:21035–21039. doi: 10.1074/jbc.270.36.21035. [DOI] [PubMed] [Google Scholar]
- 4.Atar S, Ye Y, Lin Y, Freeberg SY, Nishi SP, Rosanio S, Huang MH, Uretsky BF, Perez-Polo JR, Birnbaum Y. Atorvastatin-induced cardioprotection is mediated by increasing inducible nitric oxide synthase and consequent S-nitrosylation of cycloxygenase-2. Am J Physiol. 2006;290:H1960–H1968. doi: 10.1152/ajpheart.01137.2005. [DOI] [PubMed] [Google Scholar]
- 5.Barouch LA, Harrison RW, Skaf MW, Rosas GO, Cappola TP, Kobeissi ZA, Hobai IA, Lemmon CA, Burnett AL, O'Rourke B, Rodriguez ER, Huang PL, Lima JA, Berkowitz DE, Hare JM. Nitric oxide regulates the heart by spatial confinement of nitric oxide synthase iso-forms. Nature. 2002;416:337–339. doi: 10.1038/416337a. [DOI] [PubMed] [Google Scholar]
- 6.Becker LB. New concepts in reactive oxygen species and cardiovascular reperfusion physiology. Cardiovasc Res. 2004;61:461–472. doi: 10.1016/j.cardiores.2003.10.025. [DOI] [PubMed] [Google Scholar]
- 7.Belge C, Massion PB, Pelat M, Balligand JL. Nitric oxide and the heart: update on new paradigms. Ann N Y Acad Sci. 2005;1047:173–182. doi: 10.1196/annals.1341.016. [DOI] [PubMed] [Google Scholar]
- 8.Beltran B, Orsi A, Clementi E, Moncada S. Oxidative stress and S-nitrosylation of proteins in cells. Br J Pharmacol. 2000;129:953–960. doi: 10.1038/sj.bjp.0703147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Berry CE, Hare JM. Xanthine oxidoreductase and cardiovascular disease: molecular mechanisms and pathophysiological implications. J Physiol. 2004;555:589–606. doi: 10.1113/jphysiol.2003.055913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bolli R, Shinmura K, Tang XL, Kodani E, Xuan YT, Guo Y, Dawn B. Discovery of a new function of cyclooxygenase (COX)-2: COX-2 is a cardioprotective protein that alleviates ischemia/reperfusion injury and mediates the late phase of preconditioning. Cardiovasc Res. 2002;55:506–519. doi: 10.1016/s0008-6363(02)00414-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brahmajothi MV, Campbell DL. Heterogeneous basal expression of nitric oxide synthase and superoxide dismutase isoforms in mammalian heart: implication for mechanisms governing indirect and direct nitric oxide-related effects. Circ Res. 1999;85:575–587. doi: 10.1161/01.res.85.7.575. [DOI] [PubMed] [Google Scholar]
- 12.Broillet MC. S-nitrosylation of proteins. Cell Mol Life Sci. 1999;55:1036–1042. doi: 10.1007/s000180050354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brune B. Nitric oxide: NO apoptosis or turning it ON? Cell Death Differ. 2003;10:864–869. doi: 10.1038/sj.cdd.4401261. [DOI] [PubMed] [Google Scholar]
- 14.Brune B, Zhou J. The role of nitric oxide (NO) in stability regulation of hypoxia inducible factor-1α (HIF-1α) Curr Med Chem. 2003;10:845–855. doi: 10.2174/0929867033457746. [DOI] [PubMed] [Google Scholar]
- 15.Bryan NS, Rassaf T, Maloney RE, Rodriguez CM, Saijo F, Rodriguez JR, Feelisch M. Cellular targets and mechanism of nitros(yl)ation: an insight into their nature and kinetics in vivo. Proc Natl Acad Sci U S A. 2004;101:4308–4313. doi: 10.1073/pnas.0306706101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Burwell LS, Nadtochiy SM, Tompkins AJ, Young SM, Brookes PS. Direct evidence for S-nitrosation of mitochondrial complex I. Biochem J. 2006;394:627–634. doi: 10.1042/BJ20051435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Butzer U, Weidenbach H, Gansauge S, Gansauge F, Beger HG, Nussler AK. Increased oxidative stress in the RAW 264.7 macrophage cell line is partially mediated via the S-nitrosothiol-induced inhibition of glutathione reductase. FEBS Lett. 1999;445:274–278. doi: 10.1016/s0014-5793(99)00139-8. [DOI] [PubMed] [Google Scholar]
- 18.Campbell DL, Stamler JS, Strauss HC. Redox modulation of L-type calcium channels in ferret ventricular myocytes: dual mechanism regulation by nitric oxide and Snitrosothiols. J Gen Physiol. 1996;108:277–293. doi: 10.1085/jgp.108.4.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cardounel AJ, Xia Y, Zweier JL. Endogenous methylarginines modulate superoxide as well as nitric oxide generation from neuronal nitric oxide synthase: differences in the effects of monomethyl- and dimethylarginines in the presence and absence of tetrahydrobiopterin. J Biol Chem. 2005;280:7540–7549. doi: 10.1074/jbc.M410241200. [DOI] [PubMed] [Google Scholar]
- 20.Choi BM, Pae HO, Jang SI, Kim YM, Chung HT. Nitric oxide as a pro-apoptotic as well as anti-apoptotic modulator. J Biochem Mol Biol. 2002;35:116–126. doi: 10.5483/bmbrep.2002.35.1.116. [DOI] [PubMed] [Google Scholar]
- 21.Clementi E, Brown GC, Feelisch M, Moncada S. Persistent inhibition of cell respiration by nitric oxide: crucial role of S-nitrosylation of mitochondrial complex I and protective action of glutathione. Proc Natl Acad Sci U S A. 1998;95:7631–7636. doi: 10.1073/pnas.95.13.7631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Danson EJ, Choate JK, Paterson DJ. Cardiac nitric oxide: emerging role of nNOS in regulating physiological function. Pharmacol Ther. 2005;106:57–74. doi: 10.1016/j.pharmthera.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 23.Datta B, Tufnell-Barrett T, Bleasdale RA, Jones CJH, Beeton I, Paul V, Frenneaux M, James P. Red blood cell nitric oxide as an endocrine vasoregulator: a potential role in congestive heart failure. Circulation. 2004;109:1339–1342. doi: 10.1161/01.CIR.0000124450.07016.1D. [DOI] [PubMed] [Google Scholar]
- 24.Delyani JA, Nossuli TO, Scalia R, Thomas G, Garvey DS, Lefer AM. S-nitrosylated tissue-type plasminogen activator protects against myocardial ischemia/reperfusion injury in cats: role of the endothelium. J Pharmacol Exp Ther. 1996;279:1174–1180. [PubMed] [Google Scholar]
- 25.Dimmeler S, Fleming I, Fisslthaler B, Hermann C, Busse R, Zeiher AM. Activation of nitric oxide synthase in endothelial cells by Akt-dependent phosphorylation. Nature. 1999;399:601–605. doi: 10.1038/21224. [DOI] [PubMed] [Google Scholar]
- 26.Dimmeler S, Haendeler J, Sause A, Zeiher AM. Nitric oxide inhibits APO-1/Fas-mediated cell death. Cell Growth Differ. 1998;9:415–422. [PubMed] [Google Scholar]
- 27.Dong Z, Saikumar P, Weinberg JM, Venkatachalam MA. Calcium in cell injury and death. Annu Rev Pathol Mech Dis. 2006;1:405–434. doi: 10.1146/annurev.pathol.1.110304.100218. [DOI] [PubMed] [Google Scholar]
- 28.Duranski MR, Greer JJ, Dejam A, Jaganmohan S, Hogg N, Langston W, Patel RP, Yet SF, Wang X, Kevil CG, Gladwin MT, Lefer DJ. Cytoprotective effects of nitrite during in vivo ischemia-reperfusion of the heart and liver. J Clin Invest. 2005;115:1232–1240. doi: 10.1172/JCI22493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Erwin PA, Lin AJ, Golan DE, Michel T. Receptor-regulated dynamic S-nitrosylation of endothelial nitric-oxide synthase in vascular endothelial cells. J Biol Chem. 2005;280:19888–19894. doi: 10.1074/jbc.M413058200. [DOI] [PubMed] [Google Scholar]
- 30.Erwin PA, Mitchell DA, Sartoretto J, Marletta MA, Michel T. Subcellular targeting and differential S-nitrosylation of endothelial nitric oxide synthase. J Biol Chem. 2006;281:151–157. doi: 10.1074/jbc.M510421200. [DOI] [PubMed] [Google Scholar]
- 31.Eu JP, Sun J, Xu L, Stamler JS, Meissner G. The skeletal muscle calcium release channel: coupled O2 sensor and NO signaling functions. Cell. 2000;102:499–509. doi: 10.1016/s0092-8674(00)00054-4. [DOI] [PubMed] [Google Scholar]
- 32.Flogel U, Godecke A, Klotz LO, Schrader J. Role of myoglobin in the antioxidant defense of the heart. FASEB J. 2004;18:1156–1158. doi: 10.1096/fj.03-1382fje. [DOI] [PubMed] [Google Scholar]
- 33.Flogel U, Merx MW, Godecke A, Decking UK, Schrader J. Myoglobin: a scavenger of bioactive NO. Proc Natl Acad Sci U S A. 2001;98:735–740. doi: 10.1073/pnas.011460298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Foster MW, McMahon TJ, Stamler JS. S-nitrosylation in health and disease. Trends Mol Med. 2003;9:160–168. doi: 10.1016/s1471-4914(03)00028-5. [DOI] [PubMed] [Google Scholar]
- 35.Foster MW, Stamler JS. New insights into protein S-nitrosylation: mitochondria as a model system. J Biol Chem. 2004;279:25891–25897. doi: 10.1074/jbc.M313853200. [DOI] [PubMed] [Google Scholar]
- 36.Galli F, Rovidati S, Ghibelli L, Canestrari F. S-nitrosylation of glyceraldehyde-3-phosphate dehydrogenase decreases the enzyme affinity to the erythrocyte membrane. Nitric Oxide. 1998;2:17–27. doi: 10.1006/niox.1997.0148. [DOI] [PubMed] [Google Scholar]
- 37.Garban HJ, Marquez-Garban DC, Pietras RJ, Ignarro LJ. Rapid nitric oxide-mediated S-nitrosylation of estrogen receptor: regulation of estrogen-dependent gene transcription. Proc Natl Acad Sci U S A. 2005;102:2632–2636. doi: 10.1073/pnas.0409854102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ghelardoni S, Frascarelli S, Ronca-Testoni S, Zucchi R. S-nitrosothiol detection in isolated perfused rat heart. Mol Cell Biochem. 2003;252:347–357. doi: 10.1023/a:1025504611433. [DOI] [PubMed] [Google Scholar]
- 39.Gladwin MT, Shelhamer JH, Schechter AN, Pease-Fye ME, Waclawiw MA, Panza JA, Ognibene FP, Cannon RO., III Role of circulating nitrite and S-nitrosohemoglobin in the regulation of regional blood flow in humans. Proc Natl Acad Sci U S A. 2000;97:11482–11487. doi: 10.1073/pnas.97.21.11482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Godecke A, Molojavyi A, Heger J, Flogel U, Ding Z, Jacoby C, Schrader J. Myoglobin protects the heart from inducible nitric-oxide synthase (iNOS)-mediated nitrosative stress. J Biol Chem. 2003;278:21761–21766. doi: 10.1074/jbc.M302573200. [DOI] [PubMed] [Google Scholar]
- 41.Haendeler J, Hoffmann J, Tischler V, Berk BC, Zeiher AM, Dimmeler S. Redox regulatory and anti-apoptotic functions of thioredoxin depend on S-nitrosylation at cysteine 69. Nat Cell Biol. 2002;4:743–749. doi: 10.1038/ncb851. [DOI] [PubMed] [Google Scholar]
- 42.Haendeler J, Hoffmann J, Zeiher AM, Dimmeler S. Antioxidant effects of statins via S-nitrosylation and activation of thioredoxin in endothelial cells: a novel vasculo-protective function of statins. Circulation. 2004;110:856–861. doi: 10.1161/01.CIR.0000138743.09012.93. [DOI] [PubMed] [Google Scholar]
- 43.Haendeler J, Weiland U, Zeiher AM, Dimmeler S. Effects of redox-related congeners of NO on apoptosis and caspase-3 activity. Nitric Oxide. 1997;1:282–293. doi: 10.1006/niox.1997.0134. [DOI] [PubMed] [Google Scholar]
- 44.Hao G, Derakhshan B, Shi l, Campagne F, Gross SS. SNOSID, a proteomic method for identification of cysteine S-nitrosylation sites in complex protein mixtures. Proc Natl Acad Sci U S A. 2006;103:1012–1017. doi: 10.1073/pnas.0508412103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hara MR, Agrawal N, Kim SF, Cascio MB, Fujimuro M, Ozeki Y, Takahashi M, Cheah JH, Tankou SK, Hester LD, Ferris CD, Hayward SD, Snyder SH, Sawa A. S-nitrosylated GAPDH initiates apoptotic cell death by nuclear translocation following Siah1 binding. Nat Cell Biol. 2005;7:665–674. doi: 10.1038/ncb1268. [DOI] [PubMed] [Google Scholar]
- 46.Hare JM. Nitric oxide and excitation–contraction coupling. J Mol Cell Cardiol. 2003;35:719–729. doi: 10.1016/s0022-2828(03)00143-3. [DOI] [PubMed] [Google Scholar]
- 47.Hare JM, Stamler JS. NO/redox disequilibrium in the failing heart and cardiovascular system. J Clin Invest. 2005;115:509–517. doi: 10.1172/JCI200524459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hess DT, Matsumoto A, Kim SO, Marshall HE, Stamler JS. Protein S-nitrosylation: purview and parameters. Nat Rev Mol Cell Biol. 2005;6:150–166. doi: 10.1038/nrm1569. [DOI] [PubMed] [Google Scholar]
- 49.Hoffmann J, Dimmeler S, Haendeler J. Shear stress increases the amount of S-nitrosylated molecules in endothelial cells: important role for signal transduction. FEBS Lett. 2003;551:153–158. doi: 10.1016/s0014-5793(03)00917-7. [DOI] [PubMed] [Google Scholar]
- 50.Hu H, Chiamvimonvat N, Yamagishi T, Marban E. Direct inhibition of expressed cardiac L-type Ca2+ channels by S-nitrosothiol nitric oxide donors. Circ Res. 1997;81:742–752. doi: 10.1161/01.res.81.5.742. [DOI] [PubMed] [Google Scholar]
- 51.Huang KT, Han TH, Hyduke DR, Vaughn MW, Van Herle H, Hein TW, Zhang C, Kuo L, Liao JC. Modulation of nitric oxide bioavailability by erythrocytes. Proc Natl Acad Sci U S A. 2001;98:11771–11776. doi: 10.1073/pnas.201276698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Huang Z, Shiva S, Kim-Shapiro DB, Patel RP, Ringwood LA, Irby CE, Huang KT, Ho C, Hogg N, Schechter AN, Gladwin MT. Enzymatic function of hemoglobin as a nitrite reductase that produces NO under allosteric control. J Clin Invest. 2005;115:2099–2107. doi: 10.1172/JCI24650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hunter CJ, Dejam A, Blood AB, Shields H, Kim-Shapiro DB, Machado RF, Tarekegn S, Mulla N, Hopper AO, Schechter AN, Power GG, Gladwin MT. Inhaled nebulized nitrite is a hypoxia-sensitive NO-dependent selective pulmonary vasodilator. Nat Med. 2004;10:1122–1127. doi: 10.1038/nm1109. [DOI] [PubMed] [Google Scholar]
- 54.Jaffrey SR, Erdjument-Bromage H, Ferris CD, Tempst P, Snyder SH. Protein S-nitrosylation: a physiological signal for neuronal nitric oxide. Nat Cell Biol. 2001;3:193–197. doi: 10.1038/35055104. [DOI] [PubMed] [Google Scholar]
- 55.Jaffrey SR, Snyder SH. The biotin switch method for the detection of S-nitrosylated proteins. Sci STKE. 2001;86:PL1. doi: 10.1126/stke.2001.86.pl1. [DOI] [PubMed] [Google Scholar]
- 56.Kelm M. Nitric oxide metabolism and breakdown. Biochim Biophys Acta. 1999;1411:273–289. doi: 10.1016/s0005-2728(99)00020-1. [DOI] [PubMed] [Google Scholar]
- 57.Khan SA, Lee K, Minhas KM, Gonzalez DR, Raju SVY, Tejani AD, Li D, Berkowitz DE, Hare JM. Neuronal nitric oxide synthase negatively regulates xanthine oxidoreductase inhibition of cardiac excitation-contraction coupling. Proc Natl Acad Sci U S A. 2004;101:15944–15948. doi: 10.1073/pnas.0404136101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Khan SA, Skaf MW, Harrison RW, Lee K, Minhas KM, Kumar A, Fridley M, Shoukas AA, Berkowitz DE, Hare JM. Nitric oxide regulation of myocardial contractility and calcium cycling: Independent impact of neuronal and endothelial nitric oxide synthases. Circ Res. 2003;92:1322–1329. doi: 10.1161/01.RES.0000078171.52542.9E. [DOI] [PubMed] [Google Scholar]
- 59.Kim KH, Nebder JR. Rapid, estrogen receptor-mediated signaling: why is the endothelium so special? Sci STKE. 2005;288:PE28. doi: 10.1126/stke.2882005pe28. [DOI] [PubMed] [Google Scholar]
- 60.Kim KM, Kim PK, Kwon YG, Bai SK, Nam WD, Kim YM. Regulation of apoptosis by nitrosative stress. J Biochem Mol Biol. 2002;35:127–133. doi: 10.5483/bmbrep.2002.35.1.127. [DOI] [PubMed] [Google Scholar]
- 61.Kim PK, Kwon YG, Chung HT, Kim YM. Regulation of caspases by nitric oxide. Ann N Y Acad Sci. 2002;962:42–52. doi: 10.1111/j.1749-6632.2002.tb04054.x. [DOI] [PubMed] [Google Scholar]
- 62.Kim YM, Kim TH, Seol DW, Talanian RV, Billiar TR. Nitric oxide suppression of apoptosis occurs in association with an inhibition of Bcl-2 cleavage and cytochrome c release. J Biol Chem. 1998;273:31437–31441. doi: 10.1074/jbc.273.47.31437. [DOI] [PubMed] [Google Scholar]
- 63.Knight RJ, Kofoed KF, Schelbert HR, Buxton DB. Inhibition of glyceraldehyde-3-phosphate dehydrogenase in post-ischaemic myocardium. Cardiovasc Res. 1996;32:1016–1023. doi: 10.1016/s0008-6363(96)00137-x. [DOI] [PubMed] [Google Scholar]
- 64.Krajewski S, Krajewska M, Ellerby LM, Welsh K, Xie Z, Deveraux QL, Salvesen GS, Bredesen DE, Rosenthal RE, Fiskum G, Reed JC. Release of caspase-9 from mitochondria during neuronal apoptosis and cerebral ischemia. Proc Natl Acad Sci U S A. 1999;96:5752–5757. doi: 10.1073/pnas.96.10.5752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kuncewicz T, Sheta EA, Goldknopf IL, Kone BC. Proteomic analysis of S-nitrosylated proteins in mesangial cells. Mol Cell Proteomics. 2003;2:156–163. doi: 10.1074/mcp.M300003-MCP200. [DOI] [PubMed] [Google Scholar]
- 66.Lai TS, Hausladen A, Slaughter TF, Eu JP, Stamler JS, Greenberg CS. Calcium regulates S-nitrosylation, denitrosylation, and activity of tissue transglutaminase. Biochemistry. 2001;40:4904–4910. doi: 10.1021/bi002321t. [DOI] [PubMed] [Google Scholar]
- 67.Landino LM, Koumas MT, Mason CE, Alston JA. Ascorbic acid reduction of microtubule protein disulfides and its relevance to protein S-nitrosylation assays. Biochem Biophys Res Commun. 2006;340:347–352. doi: 10.1016/j.bbrc.2005.12.013. [DOI] [PubMed] [Google Scholar]
- 68.Lane P, Hao G, Gross SS. S-nitrosylation is emerging as a specific and fundamental posttranslational protein modification: head-to-head comparison with O-phosphorylation. Sci STKE. 2001;2001:RE1. doi: 10.1126/stke.2001.86.re1. [DOI] [PubMed] [Google Scholar]
- 69.Leist M, Single B, Naumann H, Fava E, Simon B, Kuhnle S, Nicotera P. Inhibition of mitochondrial ATP generation by nitric oxide switches apoptosis to necrosis. Exp Cell Res. 1999;249:396–403. doi: 10.1006/excr.1999.4514. [DOI] [PubMed] [Google Scholar]
- 70.Li J, Billiar TR, Talanian RV, Kim YM. Nitric oxide reversibly inhibits seven members of the caspase family via S-nitrosylation. Biochem Biophys Res Commun. 1997;240:419–424. doi: 10.1006/bbrc.1997.7672. [DOI] [PubMed] [Google Scholar]
- 71.Liu H, Colavitti R, Rovira II, Finkel T. Redox-dependent transcriptional regulation. Circ Res. 2005;97:967–974. doi: 10.1161/01.RES.0000188210.72062.10. [DOI] [PubMed] [Google Scholar]
- 72.Liu L, Yan Y, Zeng M, Zhang J, Hanes MA, Ahearn G, McMahon TJ, Dickfeld T, Marshall HE, Que LG, Stamler JS. Essential roles of S-nitrosothiols in vascular homeostasis and endotoxic shock. Cell. 2004;116:617–628. doi: 10.1016/s0092-8674(04)00131-x. [DOI] [PubMed] [Google Scholar]
- 73.Liu X, Miller MJS, Joshi MS, Thomas DD, Lancaster JR., Jr Accelerated reaction of nitric oxide with O2 within the hydrophobic interior of biological membranes. Proc Natl Acad Sci U S A. 1998;95:2175–2179. doi: 10.1073/pnas.95.5.2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lo Bello M, Nuccetelli M, Caccuri AM, Stella L, Parker MW, Rossjohn J, McKinstry WJ, Mozzi AF, Federici G, Polizio F, Pedersen JZ, Ricci G. Human glutathione transferase P1–1 and nitric oxide carriers: a new role for an old enzyme. J Biol Chem. 2001;276:42138–42145. doi: 10.1074/jbc.M102344200. [DOI] [PubMed] [Google Scholar]
- 75.Logue SE, Gustafsson AB, Samali A, Gottlieb RA. Ischemia/reperfusion injury at the intersection with cell death. J Mol Cell Cardiol. 2005;38:21–33. doi: 10.1016/j.yjmcc.2004.11.009. [DOI] [PubMed] [Google Scholar]
- 76.Lu XM, Lu M, Tompkins RG, Fischman AJ. Site-specific detection of S-nitrosylated PKB alpha/Akt1 from rat soleus muscle using CapLC-Q-TOF(micro) mass spec-trometry. J Mass Spectrom. 2005;40:1140–1148. doi: 10.1002/jms.885. [DOI] [PubMed] [Google Scholar]
- 77.Madamanchi NR, Vendrov A, Runge MS. Oxidative stress and vascular disease. Arterioscler Thromb Vasc Biol. 2005;25:29–38. doi: 10.1161/01.ATV.0000150649.39934.13. [DOI] [PubMed] [Google Scholar]
- 78.Maejima Y, Adachi S, Morikawa K, Ito H, Isobe M. Nitric oxide inhibits myocardial apoptosis by preventing caspase-3 activity via S-nitrosylation. J Mol Cell Cardiol. 2005;38:163–174. doi: 10.1016/j.yjmcc.2004.10.012. [DOI] [PubMed] [Google Scholar]
- 79.Mannick JB, Schonhoff C, Papeta N, Ghafourifar P, Szibor M, Fang K, Gaston B. S-Nitrosylation of mitochondrial caspases. J Cell Biol. 2001;154:1111–1116. doi: 10.1083/jcb.200104008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Marshall HE, Stamler JS. Nitrosative stress-induced apoptosis through inhibition of NF-kappa B. J Biol Chem. 2002;277:34223–34228. doi: 10.1074/jbc.M201638200. [DOI] [PubMed] [Google Scholar]
- 81.Martinez-Ruiz A, Lamas S. Detection and proteomic identification of S-nitrosylated proteins in endothelial cells. Arch Biochem Biophys. 2004;423:192–199. doi: 10.1016/j.abb.2003.12.006. [DOI] [PubMed] [Google Scholar]
- 82.Martinez-Ruiz A, Lamas S. S-nitrosylation: a potential new paradigm in signal transduction. Cardiovasc Res. 2004;62:43–52. doi: 10.1016/j.cardiores.2004.01.013. [DOI] [PubMed] [Google Scholar]
- 83.Martinez-Ruiz A, Lamas S. Nitrosylation of thiols in vascular homeostasis and diseases. Curr Ather Rep. 2005;7:213–218. doi: 10.1007/s11883-005-0009-1. [DOI] [PubMed] [Google Scholar]
- 84.Martinez-Ruiz A, Villanueva L, Gonzalez de Orduna C, Lopez-Ferrer D, Higueras MA, Tarin C, Rodriguez-Crespo I, Vazquez J, Lamas S. S-nitrosylation of Hsp90 promotes the inhibition of its ATPase and endothelial nitric oxide synthase regulatory activities. Proc Natl Acad Sci U S A. 2005;102:8525–8530. doi: 10.1073/pnas.0407294102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Marzinzig M, Nussler AK, Stadler J, Marzinzig E, Barthlen W, Nussler NC, Beger HG, Morris SM, Jr, Bruckner UB. Improved methods to measure end products of nitric oxide in biological fluids: nitrite, nitrate, and S-nitrosothiols. Nitric Oxide. 1997;1:177–189. doi: 10.1006/niox.1997.0116. [DOI] [PubMed] [Google Scholar]
- 86.Matsushita K, Morrell CN, Cambien B, Yang S, Yamakuchi M, Bao C, Hara MR, Quick RA, Cao W, O'Rourke B, Lowenstein JM, Pevsner J, Wagner DD, Lowenstein CJ. Nitric oxide regulates exocytosis by S-nitrosylation of N-ethylmaleimide-sensitive factor. Cell. 2003;115:139–150. doi: 10.1016/s0092-8674(03)00803-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Matthews JR, Botting CH, Panico M, Morris HR, Hay RT. Inhibition of NF-kappaB DNA binding by nitric oxide. Nucleic Acids Res. 1996;24:2236–2242. doi: 10.1093/nar/24.12.2236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Melino G, Bernassola F, Knight RA, Corasaniti MT, Nistico G, Finazzi-Agro A. S-nitrosylation regulates apoptosis. Nature. 1997;388:432–433. doi: 10.1038/41237. [DOI] [PubMed] [Google Scholar]
- 89.Melino G, Catani MV, Corazzari M, Guerrieri P, Bernassola F. Nitric oxide can inhibit apoptosis or switch it into necrosis. Cell Mol Life Sci. 2000;57:612–622. doi: 10.1007/PL00000723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Merx MW, Godecke A, Flogel U, Schrader J. Oxygen supply and nitric oxide scavenging by myoglobin contribute to exercise endurance and cardiac function. FASEB J. 2005;19:1015–1017. doi: 10.1096/fj.04-2886fje. [DOI] [PubMed] [Google Scholar]
- 91.Mery PF, Pavoine C, Belhassen L, Pecker F, Fischmeister R. Nitric oxide regulates cardiac Ca2+ current: involvement of cGMP-inhibited and cGMP-stimulated phosphodiesterases through guanylyl cyclase activation. J Biol Chem. 1993;268:26286–26295. [PubMed] [Google Scholar]
- 92.Mohr S, Stamler JS, Brüne B. Posttranslational modification of glyceraldehyde-3-phosphate dehydrogenase by S-nitrosylation and subsequent NADH attachment. J Biol Chem. 1996;271:4209–4214. doi: 10.1074/jbc.271.8.4209. [DOI] [PubMed] [Google Scholar]
- 93.Molina y Vedia L, McDonald B, Reep B, Brune B, Di Silvio M, Billiar TR, Lapetina EG. Nitric oxide-induced S-nitrosylation of glyceraldehyde-3-phosphate dehydrogenase inhibits enzymatic activity and increases endogenous ADP-ribosylation. J Biol Chem. 1992;267:24929–24932. [PubMed] [Google Scholar]
- 94.Moon KH, Kim BJ, Song BJ. Inhibition of mitochondrial aldehyde dehydrogenase by nitric oxide-mediated Snitrosylation. FEBS Lett. 2005;579:6115–6120. doi: 10.1016/j.febslet.2005.09.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Morad M, Suzuki YJ. Redox regulation of cardiac muscle calcium signaling. Antioxid Redox Signal. 2000;2:65–71. doi: 10.1089/ars.2000.2.1-65. [DOI] [PubMed] [Google Scholar]
- 96.Muller B, Kleschyov AL, Alencar JL, Vanin A, Stoclet J-C. Nitric oxide transport and storage in the cardiovascular system. Ann N Y Acad Sci. 2002;962:131–139. doi: 10.1111/j.1749-6632.2002.tb04063.x. [DOI] [PubMed] [Google Scholar]
- 97.Nedospasov A, Rafikov R, Beda N, Nudler E. An autocatalytic mechanism of protein nitrosylation. Proc Natl Acad Sci U S A. 2000;97:13543–13548. doi: 10.1073/pnas.250398197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ng ES, Jourd'heuil D, McCord JM, Hernandez D, Yasui M, Knight D, Kubes P. Enhanced S-nitroso-albumin formation from inhaled NO during ischemia reperfusion. Circ Res. 2004;94:559–565. doi: 10.1161/01.RES.0000117771.63140.D6. [DOI] [PubMed] [Google Scholar]
- 99.O'Neil BT, Abel ED. Akt1 in the cardiovascular system: friend or foe? J Clin Invest. 2005;115:2059–2064. doi: 10.1172/JCI25900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Pacher P, Schulz R, Liaudet L, Szabo C. Nitosative stress and pharmacological modulation of heart failure. Trends Pharmacol Sci. 2005;26:302–310. doi: 10.1016/j.tips.2005.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Palmer LA, Gaston B, Johns RA. Normoxic stabilization of hypoxia-inducible factor-1 expression and activity: redox-dependent effect of nitrogen oxides. Mol Pharmacol. 2000;58:1197–1203. doi: 10.1124/mol.58.6.1197. [DOI] [PubMed] [Google Scholar]
- 102.Paolocci N, Ekelund UE, Isoda T, Ozaki M, Vandegaer K, Georgakopoulos D, Harrison RW, Kass DA, Hare JM. cGMP-independent inotropic effects of nitric oxide and peroxynitrite donors: Potential role for nitrosylation. Am J Physiol. 2000;279:H1982–H1988. doi: 10.1152/ajpheart.2000.279.4.H1982. [DOI] [PubMed] [Google Scholar]
- 103.Petroff MG, Kim SH, Pepe S, Dessy C, Marban E, Balligand JL, Sollott SJ. Endogenous nitric oxide mechanisms mediate the stretch dependence of Ca2+ release in cardiomyocytes. Nat Cell Biol. 2001;3:867–873. doi: 10.1038/ncb1001-867. [DOI] [PubMed] [Google Scholar]
- 104.Piantadosi CA, Tatro LG, Whorton AR. Nitric oxide and differential effects of ATP on mitochondrial permeability transition. Nitric Oxide. 2002;6:45–60. doi: 10.1006/niox.2001.0368. [DOI] [PubMed] [Google Scholar]
- 105.Poteser M, Romanin C, Schreibmayer W, Mayer B, Groschner K. S-nitrosation controls gating and conductance of the 1 subunit of class C L-type Ca2+ channels. J Biol Chem. 2001;276:14797–14803. doi: 10.1074/jbc.M008244200. [DOI] [PubMed] [Google Scholar]
- 106.Rafikova O, Rafikov R, Nudler E. Catalysis of S-nitrosothiols formation by serum albumin: the mechanism and implication in vascular control. Proc Natl Acad Sci U S A. 2002;99:5913–5918. doi: 10.1073/pnas.092048999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ravi K, Brennan LA, Levic S, Ross PA, Black SM. S-nitrosylation of endothelial nitric oxide synthase is associated with monomerization and decreased enzyme activity. Proc Natl Acad Sci U S A. 2004;101:2619–2624. doi: 10.1073/pnas.0300464101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Reynaert NL, Ckless K, Korn SH, Vos N, Guala AS, Wouters EF, van der Vliet A, Janssen-Heininger YM. Nitric oxide represses inhibitory kappaB kinase through S-nitrosylation. Proc Natl Acad Sci U S A. 2004;101:8945–8950. doi: 10.1073/pnas.0400588101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Rizzo MA, Piston DW. Regulation of cell glucokinase by S-nitrosylation and association with nitric oxide synthase. J Cell Biol. 2001;161:243–248. doi: 10.1083/jcb.200301063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Rossig L, Fichtlscherer B, Breitschopf K, Haendeler J, Zeiher AM, Mulsch A, Dimmeler S. Nitric oxide inhibits caspase-3 by S-nitrosation in vivo. J Biol Chem. 1999;274:6823–6826. doi: 10.1074/jbc.274.11.6823. [DOI] [PubMed] [Google Scholar]
- 111.Sabri AK, Hughie HH, Lucchesi PAA. Regulation of hypertrophic and apoptotic signaling pathways by reactive oxygen species in cardiac myocytes. Antioxid Redox Signal. 2003;5:731–740. doi: 10.1089/152308603770380034. [DOI] [PubMed] [Google Scholar]
- 112.Schopfer FJ, Baker PRS, Freeman BA. NO-dependent protein nitration: a cell signaling event or an oxidative inflammatory response? Trends Biochem Sci. 2003;28:646–654. doi: 10.1016/j.tibs.2003.10.006. [DOI] [PubMed] [Google Scholar]
- 113.Singel DJ, Stamler JS. Chemical physiology of blood flow regulation by red blood cells: the role of nitric oxide and S-nitrosohemoglobin. Annu Rev Physiol. 2005;67:99–145. doi: 10.1146/annurev.physiol.67.060603.090918. [DOI] [PubMed] [Google Scholar]
- 114.Sonveaux P, Kaz AM, Snyder SA, Richardson RA, Cardenas-Navia LI, Braun RD, Pawloski JR, Tozer GM, Bonaventura J, McMahon TJ, Stamler JS, Dewhirst MW. Oxygen regulation of tumor perfusion by S-nitroso-hemoglobin reveals a pressor activity of nitric oxide. Circ Res. 2005;96:1119–1126. doi: 10.1161/01.RES.0000168740.04986.a7. [DOI] [PubMed] [Google Scholar]
- 115.Stamler JS, Simon DI, Jaraki O, Osborne JA, Francis S, Mullins M, Singel DJ, Loscalzo J. S-nitrosylation of tissue-type plasminogen activator confers vasodilatory and antiplatelet properties on the enzyme. Proc Natl Acad Sci U S A. 1992;89:8087–8091. doi: 10.1073/pnas.89.17.8087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Sumbayev VV. S-nitrosylation of thioredoxin mediates activation of apoptosis signal-regulating kinase 1. Arch Biochem Biophys. 2003;415:133–136. doi: 10.1016/s0003-9861(03)00199-1. [DOI] [PubMed] [Google Scholar]
- 117.Sumbayev VV, Budde A, Zhou J, Brune B. HIF-1 alpha protein as a target for S-nitrosation. FEBS Lett. 2003;535:106–112. doi: 10.1016/s0014-5793(02)03887-5. [DOI] [PubMed] [Google Scholar]
- 118.Sun J, Picht E, Ginsburg KS, Bers DM, Steenbergen C, Murphy E. Hypercontractile female hearts exhibit increased S-nitrosylation of the L-type Ca2+ channel α1 subunit and reduced ischemia-reperfusion injury. Circ Res. 2006;98:403–411. doi: 10.1161/01.RES.0000202707.79018.0a. [DOI] [PubMed] [Google Scholar]
- 119.Sun J, Xin C, Eu JP, Stamler JS, Meissner G. Cysteine 3635 is responsible for skeletal muscle ryanodine receptor modulation by NO. Proc Natl Acad Sci U S A. 2001;98:11158–11162. doi: 10.1073/pnas.201289098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Sun J, Xu L, Eu JP, Stamler JS, Meissner G. Nitric oxide, NOC-12, and S-nitrosoglutathione modulate the skeletal muscle calcium release channel/ryanodine receptor by different mechanisms: an allosteric function for O2 in S-nitrosylation of the channel. J Biol Chem. 2003;278:8184–8189. doi: 10.1074/jbc.M211940200. [DOI] [PubMed] [Google Scholar]
- 121.Tsuchiya K, Kanematsu Y, Yoshizumi M, Ohnishi H, Kirima K, Izawa Y, Shikishima M, Ishida T, Kondo S, Kagami S, Takiguchi Y, Tamaki T. Nitrite is an alternative source of NO in vivo. Am J Physiol. 2005;288:H2163–H2170. doi: 10.1152/ajpheart.00525.2004. [DOI] [PubMed] [Google Scholar]
- 122.Ungvari Z, Gupte SA, Recchia FA, Batkai S, Pacher P. Role of oxidative-nitrosative stress and downstream pathways in various forms of cardiomyopathy and heart failure. Curr Vasc Pharmacol. 2005;3:221–229. doi: 10.2174/1570161054368607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Wang X, Tanus-Santos JE, Reiter CD, Dejam A, Shiva S, Smith RD, Hogg N, Gladwin MT. Biological activity of nitric oxide in the plasmatic compartment. Proc Natl Acad Sci U S A. 2004;101:11477–11482. doi: 10.1073/pnas.0402201101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Waring P. Redox active calcium ion channels and cell death. Arch Biochem Biophys. 2005;434:33–42. doi: 10.1016/j.abb.2004.08.001. [DOI] [PubMed] [Google Scholar]
- 125.Webb A, Bond R, McLean P, Uppal R, Benjamin N, Ahluwalia A. Reduction of nitrite to nitric oxide during ischemia protects against myocardial ischemia-reperfusion damage. Proc Natl Acad Sci U S A. 2004;101:13683–13688. doi: 10.1073/pnas.0402927101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Weiland U, Haendeler J, Ihling C, Albus U, Scholz W, Ruetten H, Zeiher AM, Dimmeler S. Inhibition of endogenous nitric oxide synthase potentiates ischemia-reperfusion-induced myocardial apoptosis via a caspase-3 dependent pathway. Cardiovasc Res. 2000;45:671–678. doi: 10.1016/s0008-6363(99)00347-8. [DOI] [PubMed] [Google Scholar]
- 127.Weitzberg E, Lundberg JON. Nonenzymatic nitric oxide production in humans. Nitric Oxide. 1998;2:17–27. doi: 10.1006/niox.1997.0162. [DOI] [PubMed] [Google Scholar]
- 128.Whiteman M, Chua YL, Zhang D, Duan W, Liou YC, Armstrong JS. Nitric oxide protects against mitochondrial permeabilization induced by glutathione depletion: role of S-nitrosylation? Biochem Biophys Res Commun. 2006;339:255–262. doi: 10.1016/j.bbrc.2005.10.200. [DOI] [PubMed] [Google Scholar]
- 129.Wolosker H, Panizzutti R, Engelender S. Inhibition of creatine kinase by S-nitrosoglutathione. FEBS Lett. 1996;392:274–276. doi: 10.1016/0014-5793(96)00829-0. [DOI] [PubMed] [Google Scholar]
- 130.Xu A, Vita JA, Heaney JF., Jr Ascorbic acid and glutathione modulate the biological activity of S-nitrosoglutathione. Hypertension. 2000;36:291–295. doi: 10.1161/01.hyp.36.2.291. [DOI] [PubMed] [Google Scholar]
- 131.Xu KY, Huso DL, Dawson TM, Bredt DS, Becker LC. Nitric oxide synthase in cardiac sarcoplasmic reticulum. Proc Natl Acad Sci U S A. 1999;96:657–662. doi: 10.1073/pnas.96.2.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Xu L, Eu JP, Meissner G, Stamler JS. Activation of the cardiac calcium release channel (ryanodine receptor) by poly-S-nitrosylation. Science. 1998;279:234–237. doi: 10.1126/science.279.5348.234. [DOI] [PubMed] [Google Scholar]
- 133.Yang Y, Loscalzo J. S-nitrosoprotein formation and localization in endothelial cells. Proc Natl Acad Sci U S A. 2005;102:117–122. doi: 10.1073/pnas.0405989102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Yasinska IM, Kozhukhar AV, Sumbayev VV. S-nitrosation of thioredoxin in the nitrogen monoxide/superoxide system activates apoptosis signal-regulating kinase 1. Arch Biochem Biophys. 2004;428:198–203. doi: 10.1016/j.abb.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 135.Yasinska IM, Sumbayev VV. S-nitrosation of Cys-800 of HIF-1alpha protein activates its interaction with p300 and stimulates its transcriptional activity. FEBS Lett. 2003;549:105–109. doi: 10.1016/s0014-5793(03)00807-x. [DOI] [PubMed] [Google Scholar]
- 136.Yasukawa T, Tokunaga E, Ota H, Sugita H, Martyn JA, Kaneki M. S-nitrosylation-dependent inactivation of Akt/protein kinase B in insulin resistance. J Biol Chem. 2005;280:7511–7518. doi: 10.1074/jbc.M411871200. [DOI] [PubMed] [Google Scholar]
- 137.Zhang GX, Kimura S, Nishiyama A, Shokoji T, Rahman M, Yao L, Nagai Y, Fujisawa Y, Miyatake A, Abe Y. Cardiac oxidative stress in acute and chronic isoproterenol-infused rats. Cardiovasc Res. 2005;65:230–238. doi: 10.1016/j.cardiores.2004.08.013. [DOI] [PubMed] [Google Scholar]
- 138.Zhang J, Jin B, Li L, Block ER, Patel JM. Nitric oxide-induced persistent inhibition and nitrosylation of active site cysteine residues of mitochondrial cytochrome-c oxidase in lung endothelial cells. Am J Physiol. 2005;288:C840–C849. doi: 10.1152/ajpcell.00325.2004. [DOI] [PubMed] [Google Scholar]
- 139.Zweier JL, Samouilov A, Kuppusamy P. Non-enzymatic nitric oxide synthesis in biological systems. Biochim Biophys Acta. 1999;1411:250–262. doi: 10.1016/s0005-2728(99)00018-3. [DOI] [PubMed] [Google Scholar]