Abstract
Apolipoprotein E (apoE) plays important roles in lipid homeostasis, anti-inflammation, and host defense. Since tissue apoE mRNA levels have been reported to decrease during inflammatory responses, we were surprised to find that plasma apoE levels were significantly elevated during septic infections in both humans and mice. This apparent paradox was also observed during lipopolysaccharide-induced acute inflammation in mice: plasma levels of apoE increased up to 4-fold despite sharply decreased apoE gene expression in the liver, macrophages, and extrahepatic tissues. We hypothesized that apoE levels were augmented by decreased plasma clearance. Our analysis revealed that apoE associated principally with HDL in mice and that apoE was cleared from the circulation principally via LDL receptors. The acute inflammatory response decreased LDL receptor expression in the liver and significantly reduced the rate of apoE clearance. In contrast, the same inflammatory stimuli increased LDL receptor expression in macrophages. Our results define a novel acute phase mechanism that increases circulating apoE levels as apoE production decreases. Diminished hepatic LDL receptor expression may thus cooperate with elevated LDL receptor expression in macrophages to facilitate the forward transport of apoE and its associated lipids to these key defense cells.
Keywords: sepsis, acute inflammation, lipopolysaccharide, high density lipoprotein, low density lipoprotein
Apolipoprotein E (apoE) is a 34 kDa secreted protein that associates with plasma lipoproteins. The importance of apoE in atherosclerosis has been attributed in part to its best-defined role as the key determinant for receptor-mediated uptake and catabolism of triglyceride-rich lipoproteins (VLDL and chylomicrons) and their remnants (1). ApoE also has functions that impact diverse disease processes, including Alzheimer's disease and other neuropathologies (2), renal disease (3), cancer (4), and infection. Numerous studies have shown that apoE is involved in immunoregulation and host defense (reviewed in Ref. 1). For example, apoE improves resistance to Klebsiella pneumoniae (5, 6) and Listeria monocytogenes (7) infection. ApoE can also inhibit malaria sporozoite invasion of hepatocytes (8). The mechanisms of immune modulation by apoE are varied and complex. ApoE protects against lipopolysaccharide (LPS) toxicity by neutralizing LPS and by redirecting LPS traffic to hepatocytes (5, 9, 10). It also modulates T-cell activation (1, 11), suppresses the type I inflammatory response (12), and promotes clearance of apoptotic bodies (13). ApoE also plays a role in antigen presentation to natural killer T-cells by transporting bacterial lipids to CD1 via dendritic cell LDL receptors (14). Although the impact of apoE on human immunity is poorly understood, the finding that an apoE polymorphism is associated with the risk of severe sepsis in surgical patients (15) supports a role for apoE in human host defense.
ApoE is most highly expressed by hepatocytes and macrophages. Its expression is controlled by liver-specific (16) and macrophage-specific (17) downstream enhancers that coordinately control the expression of other apolipoprotein genes in the apoE/C-I/C-IV/C-II gene cluster. ApoE and apoC-I collectively constitute the most abundant population of mRNAs in mature macrophages (18), and they are also highly expressed by the liver (19, 20). Liver transplantation studies have shown that essentially all of the plasma apoE is derived from the liver (21), and bone marrow transplantation studies have shown that macrophages do not contribute significantly to the circulating pool of apoE (22, 23). ApoE is produced at much lower levels by the brain, kidney, and possibly by epithelial cells in other tissues (19).
Although the majority of circulating apoE associates with HDL [50–75% in humans (24)], most apoE clearance studies have focused on VLDL remnant lipoproteins. ApoE-VLDL remnants are cleared principally by the liver by three mechanisms: the LDL receptor, the LDL receptor-related protein (LRP), and heparan sulfate proteoglycan (HSPG) (1). The LDL receptor binds apoE with high affinity and provides a major mechanism for apoE-dependent clearance of remnant lipoproteins. Jones et al. (25) showed that the clearance of VLDL remnants is dramatically decreased in LDL receptor-deficient mice and that the LDL receptor-dependent mechanism does not require ARH, an intracellular adaptor protein that is required for apoB-dependent internalization of LDL. Whereas the ARH-independent mechanism also does not require LRP or HSPG, others have shown that HSPG can mediate significant clearance of remnant lipoproteins independently of LDL receptor family members (26). Relatively little is known about the clearance of apoE-HDL, which presumably also occurs by the above mechanisms. ApoE appears to play a role in the selective uptake of HDL-cholesteryl esters by a mechanism that is not entirely clear (27).
The acute phase response is a complex pathophysiologic phenomenon that replaces normal homeostatic mechanisms with new “set points” that are important for defense and adaptation (28). Whereas acute inflammation and infection are potent triggers of the acute phase response, the finding that apoE is a negative acute phase gene (29) raises questions about the role of apoE in inflammation and host defense. The inflammatory response sharply reduces apoE mRNA levels in the liver (29), macrophages (30), and extrahepatic tissues (31). Infection and LPS also decrease the synthesis of apoE protein in the liver (32) and macrophages (30). ApoE production thus decreases in response to conditions in which apoE would presumably contribute to anti-inflammation and host defense. Under normal conditions, high expression of apoE promotes the efflux of cholesterol and phospholipids from macrophages, targeting these lipids for LDL receptor-mediated hepatic uptake as part of a process known as reverse cholesterol transport. During acute inflammation and infection, in contrast, a decrease in reverse cholesterol transport is thought to benefit the host by conserving lipids in peripheral sites for tissue repair and for use by activated immune cells (33). The negative acute phase response is also thought to benefit the host by conserving biosynthetic resources (e.g., ATP and amino acyl tRNA) by reducing the synthesis of highly expressed proteins such as albumin and apoE, allowing the production of important positive acute phase products and functions (28, 29). Reducing the expression of apoE in macrophages would thus contribute to the conservation of both lipids and biosynthetic resources in these cells. Likewise, reduced apoE synthesis in the liver would conserve hepatocyte resources.
Given such strong evidence that apoE production decreases during acute phase responses, we were surprised to find that circulating apoE protein levels were maintained or even high in the plasma of septic humans. Moreover, we found low levels of apoC-I, which is derived from a gene whose expression is coordinately regulated with that of apoE. Exploring the basis for this apparent paradox in murine models of acute infection and LPS-induced acute inflammation, we found evidence that high levels of apoE could be attributed to decreased apoE clearance resulting from diminished expression of hepatic LDL receptors. Whereas plasma levels of other acute phase proteins are regulated almost universally by changes in production (via changes in gene transcription) (28, 29), posttranslational mechanisms that enhance the levels of certain positive acute phase proteins (34) have not been described in association with negative acute phase genes. To the best of our knowledge, this is the first instance in which the plasma concentration of an acute phase protein increases, despite reduced production, due to diminished clearance from the circulation.
MATERIALS AND METHODS
Experimental subjects
Wild-type (WT) C57BL/6J mice and mice with a targeted deletion of the LDL receptor (LDLR−/−; C57BL/6J background, stock number 002207) were from the Jackson Laboratory (Bar Harbor, ME). The animal protocol was approved by the Institutional Animal Care and Use Committee (University of Texas Southwestern Medical Center). Human serum and plasma were obtained after informed consent was given by healthy volunteers and patients with severe sepsis according to protocols approved by the Institutional Review Board. HDL (1.063 < d < 1.21 g/ml) was prepared from freshly drawn blood from three healthy volunteers by ultracentrifugal flotation (35).
Bacteria and LPS
K. pneumoniae (ATCC 43816) was obtained from the American Type Culture Collection. Colonies were selected from blood agar plates and expanded to mid-log phase in Luria-Bertani broth. The bacteria were washed in cold PBS, and colony-forming units were counted on agar plates. WT mice were inoculated by tail vein injection of 3,000 colony-forming units of freshly prepared bacteria in 0.15 ml of PBS. Bacteremia generally developed within 2 days after inoculation. The course and severity of infection varied among individuals; the bacteria were generally cleared within 4–5 days if the animal did not die. In general, the degree of bacteremia correlated with the degree of bacterial loads in the tissues (i.e., liver, spleen, lung, and peritoneal fluid). However, a few animals had detectable bacteria in tissues in the absence of detectable bacteremia (data not shown). LPS from Escherichia coli O111:B4 (Sigma-Aldrich, St. Louis, MO) was administered to mice intraperitoneally in PBS. The mice were anesthetized with avertin (2,2,2-tribromoethanol), blood was collected from the retro-orbital plexus, and tissues were snap-frozen in liquid nitrogen. Liver function analysis [alanine aminotransferase (ALT) and aspartate aminotransferase (AST) activity] of freshly isolated serum was performed by the Mouse Metabolic Phenotyping Core Lab (University of Texas Southwestern Medical Center). Thioglycollate-elicited peritoneal macrophages were isolated by peritoneal lavage, and adherent macrophages were cultured in RPMI 1640 (Cellgro) containing 10% heat-inactivated fetal bovine serum with or without penicillin/streptomycin.
mRNA analysis
Total RNA (RNeasy kit; Qiagen, Valencia, CA) was reverse-transcribed by SuperScript II (Invitrogen, Carlsbad, CA) using random hexamer primers. Relative levels of mRNA were quantitated using SYBR Green Master Mix in a model 7300 real-time PCR system (Applied Biosystems, Foster City, CA). Analysis of decreasing amounts of total RNA confirmed that real-time PCR measurements were within the dynamic range for each gene. Data were analyzed by the delta delta threshold cycle (ddCt) method after validation of each primer pair by the standard curve method; the results were normalized by levels of acidic ribosomal phosphoprotein P0 (36B4). 36B4 levels reflected those of 18S rRNA in tissues from both control and infected or LPS-treated mice, suggesting that 36B4 expression was not significantly altered by the inflammatory response. The primers (5′–3′) were as follows: 36B4, ACCTCCTTCTTCCAGGCTTTG and GCTGCACATCACTCAGAATTTCA; 18S rRNA, ACCGCAGCTAGGAATAATGGAA and CCGTCCCTCTTAATCATGGC; apoE, AGGTCCAGGAAGAGCTGCAG and CCTTTACTTCCGTCATAGTGTCCTC; apoC1, AGGCTCTTCATCGCTCTTCCT and GGGCTGGGCCTTCCAA; and LDL receptor, AGGCTGTGGGCTCCATAGG and TGCGGTCCAGGGTCATCT. Melting point analyses performed at the end of each PCR run revealed a single amplification product for each primer pair.
Western blotting
Livers or macrophages were homogenized in a protease inhibitor cocktail, and postnuclear supernatants (3,000 g for 5 min) were used to prepare the total membrane fractions by centrifugation for 30 min at 100,000 g as described previously (36). EGTA was omitted from the lysis buffer, and the protein content of membrane lysates were measured by the Pierce BCA Assay (Pierce Chemical Co., Rockford, IL). The lysate was heated to 95°C for 5 min in SDS sample buffer containing dithiothreitol, and 75 μg protein/lane was run on 7% or 10% SDS-PAGE electrophoresis and transferred to Immobilon-P membranes. Immunoblot detection antibodies were rabbit anti-mouse apoE (K23100R; BioDesign, Saco, ME; detects ∼34 kDa band), rabbit anti-LDL receptor (3143; a gift of Dr. Joachim Herz; detects ∼150 kDa band), rabbit anti-mouse ARH (4120; a gift of Dr. Helen Hobbs; detects ∼37 kDa band), and rabbit anti-mouse scavenger receptor class B type I (SR-BI; NB400-104; Novus, Littleton, CO; detects ∼80 kDa band). Detection was by donkey anti-rabbit IgG peroxidase followed by enhanced chemiluminescence on Hyperfilm ECL (Amersham Biosciences, Pittsburgh, PA). Semiquantitative analysis of the detected proteins was performed by histogram analysis using ImageJ software (National Institutes of Health, Bethesda, MD). Negative film images were scanned and inverted to obtain positive images. The “volume” of each protein band area was then measured as the sum of the pixel number multiplied by the brightness for each brightness level from 0 to 255. The volume was corrected for background by subtracting the volume of an equal adjacent area containing no specific signal. We found that band volumes were proportional to the amounts of target protein loaded onto the gel within a 2-fold range. More accurate measurements of larger differences required dilution of the more concentrated sample. The average volume of control samples was assigned the value 1.0.
ELISA
Human apoE was measured in the presence of mouse plasma by ELISA using a mouse monoclonal antibody to human apoE (H61529M) followed by biotin-goat anti-human apoE (K74180B) and streptavidin-peroxidase. ApoC-I was measured by a modification of a previous method (37) using goat anti-human apoC-I (31A-G1a) followed by goat anti-human apoC-I-biotin conjugate (31B-G1a; Academy Biomedical, Houston, TX), and streptavidin-peroxidase. For mouse plasma assays, samples were diluted 1:300, and normal mouse plasma was used as a standard. TMB substrate (BD Pharmingen, San Jose, CA) was used for detection. ApoE was measured in serum from human controls and septic patients by turbidimetric assay (Wako Chemicals USA, Richmond, VA).
Lipoprotein analysis
Mouse plasma was diluted with an equal volume of column buffer and fractionated on a Superose 6 HR 10/30 column as described previously (38). Total cholesterol (Wako Chemicals USA) and total triglycerides (Sigma) were measured colorimetrically in a microtiter format assay. ApoE was measured by Western blotting 10 μl of each fraction as described above. Non-HDL lipoproteins were removed from plasma by mixing with phosphotungstate reagent (HDL Cholesterol Reagent; Sigma) according to the manufacturer's protocol.
Statistics
The data were analyzed using Prism 5.0 software from GraphPad (San Diego, CA). Significant differences from controls were determined as two-tailed P values of the unpaired t-test to compare means or by the Mann-Whitney t-test to compare medians.
RESULTS
The acute inflammatory response selectively increases circulating apoE
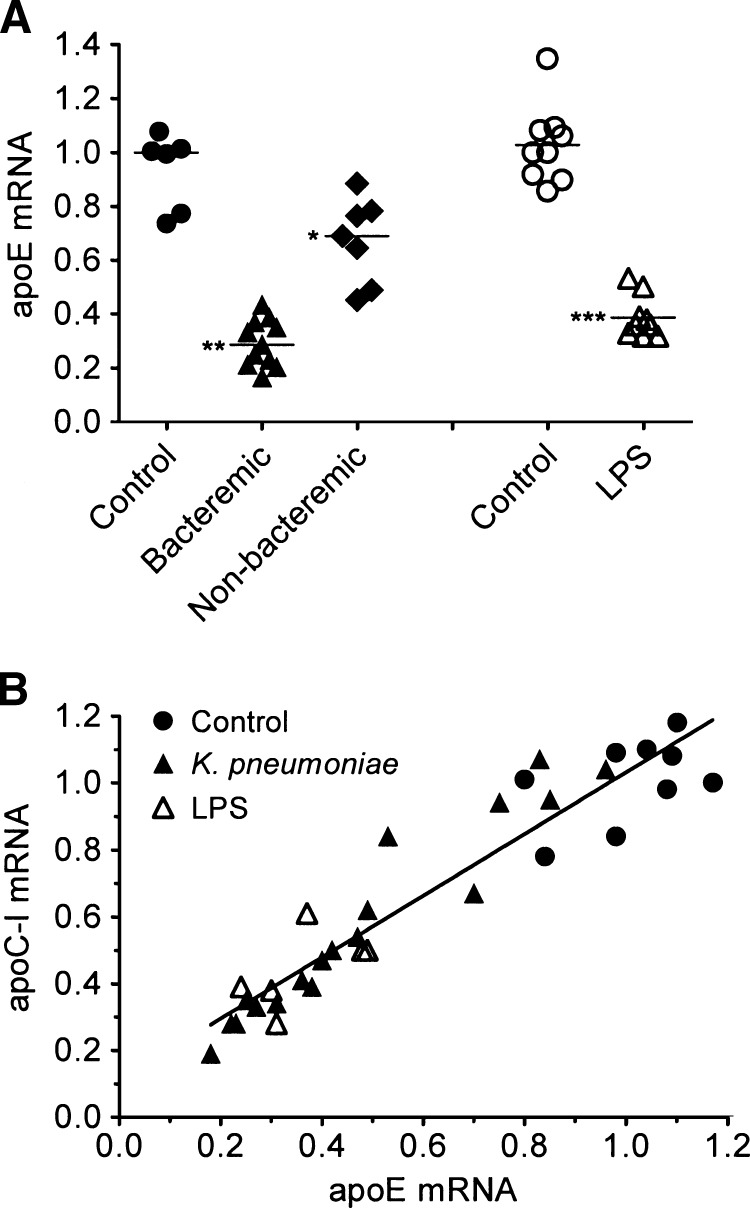
To determine whether apoE levels become elevated in infected humans, we measured apolipoprotein levels in 16 patients with severe sepsis and 9 healthy volunteers (see supplementary Table I for detailed information on subjects). As shown in Fig. 1A, serum apoE levels were significantly increased above those of the controls. In contrast, the same samples revealed a sharp decrease in levels of apoC-I, which is also produced from the coordinately controlled apoE/C-I/C-IV/C-II gene cluster. We observed a similar selective increase in plasma apoE in mouse models of infection and LPS-induced acute inflammation (Fig. 1B, C). Following intravenous inoculation with K. pneumoniae, plasma apoE levels were significantly higher in mice that had positive blood cultures 2–3 days later. Although the median plasma apoE level was not significantly elevated in nonbacteremic mice (Fig. 1B), individual mice with apoE levels that were above the normal range had significant numbers of bacteria in their tissues (data not shown). LPS administration also induced a significant increase in plasma apoE (Fig. 1B). In contrast, LPS administration significantly decreased apoC-I levels (Fig. 1C), and K. pneumoniae infection did not induce a significant change. In both humans and mice, severe infection was thus associated with higher plasma apoE concentrations, whereas apoC-I levels were either unchanged or decreased.
Fig. 1.
Infection and lipopolysaccharide (LPS) selectively increase plasma apolipoprotein E (apoE) in humans and mice. A: ApoE and apoC-I levels were measured by ELISA in serum obtained from healthy volunteers (Controls) and infected patients with severe sepsis (Septic Patients). B: Wild-type (WT) mice were euthanized at 2–3 days after intravenous injection with PBS (Control) or K. pneumoniae. Infected animals were segregated according to the ability to detect bacteria in the blood (Bacteremic or Non-bacteremic). Fasted WT mice were euthanized at 18 h after intraperitoneal injection of PBS (Control) or LPS (100 μg/25 g body weight). Mouse plasma apoE levels were measured by quantitation of Western blots. C: Mouse plasma apoC-I levels were measured by ELISA. The data in B and C are shown in arbitrary units (median of control group = 1.0). Horizontal lines denote the median of each group. Significant differences from the controls are denoted as follows: * P = 0.006, ** P = 0.0006, *** P < 0.0001.
The acute inflammatory response decreases expression of apoE and apoC-I mRNA
Because plasma apoE and apoC-I are derived principally from the liver, we measured changes in hepatic apoE and apoC-I mRNA levels in our mouse models of acute inflammation. As shown in Fig. 2A, hepatic apoE mRNA was decreased 2- to 5-fold in the bacteremic mice, and a modest but significant decrease was found in the nonbacteremic mice compared with uninfected controls. LPS administration also decreased hepatic apoE mRNA by 2- to 3-fold. Both infection and LPS administration decreased hepatic apoC-I mRNA coordinately with apoE mRNA (Fig. 2B). Bacterial infection also decreased apoE mRNA in extrahepatic tissues: mRNA levels were 55 ± 9% of control in spleen (n = 7), 50 ± 13% of control in lung (n = 7), 81 ± 23% of control in kidney (n = 4), and 75 ± 42% of control in brain (n = 4). These results in extrahepatic tissues were in keeping with the previously reported effects of LPS in a wide variety of hamster tissues (31). To confirm that elevated plasma apoE levels were not derived from macrophages, we stimulated mouse peritoneal macrophages with K. pneumoniae or LPS and found that both stimuli sharply inhibited apoE mRNA levels (Fig. 3A) and that LPS suppressed apoE secretion into macrophage culture supernatants (Fig. 3B). Infection and LPS also decreased apoE mRNA in peritoneal lavage cell mixtures (see supplementary Fig. IA, B), which were enriched in macrophages. Our results confirm that apoE is a strongly negative acute phase gene in response to both infection and LPS. Therefore, we tested the hypothesis that increased levels of plasma apoE are due to decreased clearance of circulating apoE during acute inflammation.
Fig. 2.
Infection and LPS decrease hepatic apoE and apoC-I mRNA levels. A: WT mice were injected with K. pneumoniae or LPS as described for Figure 1. Liver apoE mRNA levels were measured by real-time PCR and normalized to acidic ribosomal phosphoprotein P0 (36B4) levels. mRNA levels are shown in arbitrary units; horizontal lines denote the median of each group. Significant differences from the controls are denoted as follows: * P = 0.02, ** P = 0.001, *** P = 0.0004. B: Liver mRNA levels of apoE and apoC-I were measured by real-time PCR. Data pairs from each mouse were plotted and analyzed by linear regression (r2 = 0.8934, r = 0.9452, P < 0.0001).
Fig. 3.
Bacteria and LPS decrease macrophage apoE expression. A: Adherent mouse peritoneal macrophages were cultured with K. pneumoniae (106 colony-forming units) or LPS (100 ng/ml) for the indicated times; a penicillin/streptomycin mixture was added 1 h after the addition of K. pneumoniae to prevent bacterial overgrowth. ApoE mRNA was measured by real-time PCR and normalized to 36B4. mRNA levels, shown in arbitrary units, were calculated as the change from mRNA levels of unstimulated control cultures measured at each time point expressed in hours. Mean and range (error bars) of duplicate determinations are denoted. B: Macrophages were cultured for 24 h with LPS (100 ng/ml) or PBS. The cells were then washed and challenged with LPS (100 ng/ml) or PBS for 16 h, and secreted apoE was measured in the culture supernatants by Western blotting. Both experiments (A and B) were repeated with similar results.
ApoE associates with HDL
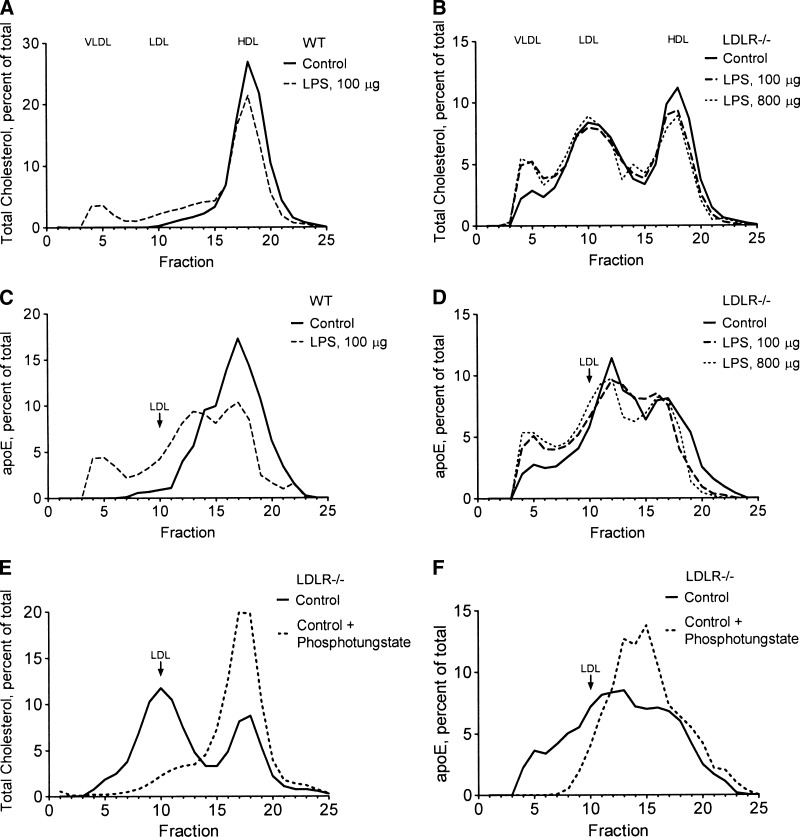
To study apoE clearance, we first determined the distribution of circulating apoE in the major lipoprotein fractions. As shown in Fig. 4, Superose 6 size exclusion chromatography of plasma revealed that essentially all of the circulating cholesterol was found in HDL in WT mice (Fig. 4A), whereas LDLR−/− mice carried a significant amount of cholesterol in LDL and a small amount in the VLDL fraction (Fig. 4B). In unstimulated WT mice, apoE overlapped with the HDL-cholesterol fraction and with a region of intermediate size between LDL and the HDL-cholesterol peak, whereas the LDL and VLDL fractions were essentially devoid of apoE (Fig. 4C). In LDLR−/− mice, apoE appeared mainly in particles that were intermediate in size between LDL and HDL-cholesterol; a minor fraction of apoE coeluted with VLDL, and some apoE trailed across, but did not peak in, the LDL fraction (Fig. 4D). To determine whether the intermediate-size apoE particles were in HDL, we precipitated apoB-containing lipoproteins (LDL and VLDL) from LDLR−/− plasma with phosphotungstate reagent. As shown in Fig. 4E, phosphotungstate removed essentially all of the cholesterol from the VLDL and LDL fractions, leaving only HDL-cholesterol in the supernatant. The phosphotungstate reagent also precipitated all of the apoE from the VLDL fraction and some apoE that overlapped with LDL, whereas the intermediate-size apoE particles remained in the supernatant (Fig. 4F). These results suggest that even when the LDL receptor is absent, the majority of apoE remains associated with HDL.
Fig. 4.
ApoE associates primarily with HDL in normal and acute phase mouse plasma. A–D: PBS or LPS (100 or 800 μg/25 g body weight) was administered to WT mice (A, C) or LDL receptor knockout (LDLR−/−) mice (B, D) as described for Figure 1. E, F: Plasma from unstimulated LDLR−/− mice was treated with PBS or phosphotungstate reagent to remove non-HDL lipoproteins. Pooled plasma (0.25 ml) from groups of three mice was fractionated on a Superose 6 HR 10/30 column; 1 ml fractions were collected and assayed colorimetrically for total cholesterol (A, B, E) or by quantitation of apoE Western blots (C, D, F) and expressed as the percentage of the total material recovered from the column. Recovery of injected cholesterol from the column was 80–90%.
In LPS-treated WT mice, some cholesterol shifted to larger fractions that overlapped with the LDL and VLDL fractions (Fig. 4A). LPS caused some of the apoE to redistribute to a larger apoE-HDL fraction, and a small proportion of the apoE appeared in the VLDL fraction (Fig. 4C). LPS administration in LDLR−/− mice increased cholesterol only in the VLDL fraction (Fig. 4B). Although LPS administration to LDLR−/− mice produced very little increase in total plasma apoE (see Fig. 9 below), a small increase in apoE content of the VLDL fraction (Fig. 4D) reflected the increase of cholesterol in VLDL (Fig. 4B). These results suggest that whereas most apoE remains in HDL during the inflammatory response, a small proportion of the apoE increases in the VLDL fraction by an LDL receptor-independent mechanism.
Fig. 9.
LDL receptor deficiency increases plasma apoE without increasing liver apoE expression. Groups of three LDLR−/− mice were injected intraperitoneally with PBS (Control) or LPS (100 or 800 μg/25 g body weight) and fasted for 18 h. Plasma apoE (A) and liver LDL receptor (LDLR) and liver apoE (B) were measured by Western blotting as described for Figure 6. Both lines in A are from the same gel. The experiment was repeated with similar results.
The acute inflammatory response decreases the clearance of plasma apoE
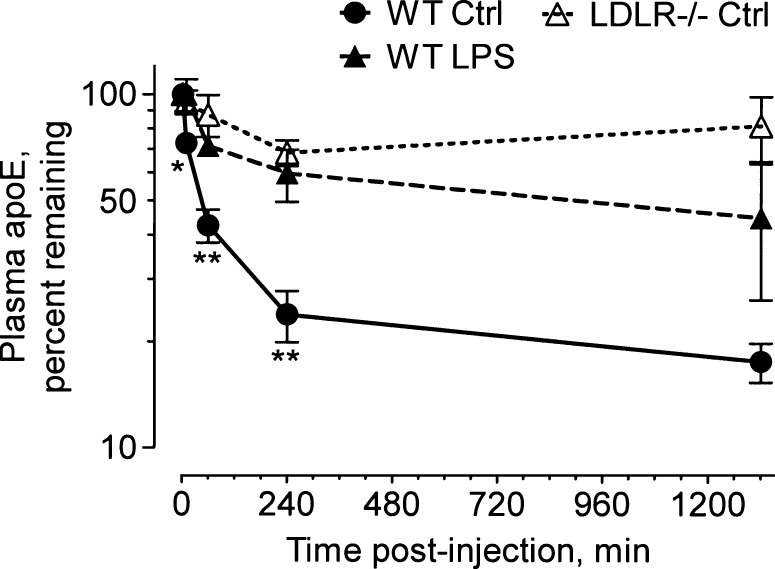
Because the overwhelming majority of apoE was found in HDL both before and during the inflammatory response, we measured the clearance of the apoE component of HDL by injecting human HDL intravenously into mice and following its clearance from plasma using a specific apoE ELISA that did not recognize mouse apoE. To minimize competition for receptor binding by endogenous apoE, we injected enough HDL to increase the total plasma cholesterol levels by 4- to 5-fold. As shown in Fig. 5, apoE was cleared rapidly in unstimulated control mice, whereas in LPS-treated mice, its clearance was slowed, revealing significantly increased apoE levels at 1 and 4 h after apoE administration. LPS treatment had little or no impact on the clearance of total HDL-cholesterol. In one experiment, the total cholesterol levels were slightly, but not significantly, increased in LPS-treated mice after 4 h [333 ± 48 mg/dl (n = 3) vs. 292 ± 50 mg/dl (n = 4) in control mice]. In LDLR−/− mice, 70–95% of the injected apoE persisted in the circulation for 24 h, suggesting that the LDL receptor is a critically important clearance mechanism for the apoE component of HDL. Clearance of apoE and apoB from the total human lipoprotein fraction was also decreased in mice that received LPS: 40–50% of the injected apoB was cleared in ∼4 h, whereas only 15% or less of the apoB was cleared during the same time period in LPS-treated mice (see supplementary Fig. II). We also found that endogenous non-HDL-cholesterol increased significantly in both LPS-treated and bacteremic mice (see supplementary Fig. IIIF). The ability to precipitate non-HDL lipoproteins with phosphotungstate suggests that they are associated with apoB. Taken together, our results support the hypothesis that LDL receptor activity is decreased during the acute inflammatory response, resulting in delayed clearance of apoE.
Fig. 5.
LPS decreases plasma clearance of apoE. WT mice were treated with PBS (Ctrl) or LPS (20 μg/25g body weight) and fasted for 18 h; unstimulated (PBS) LDLR−/− mice were used as a control. The mice were then injected intravenously with 0.15 ml of human HDL containing a total cholesterol concentration of 1,200–1,500 mg/dl. Blood (50–100 μl) was collected from the tail vein immediately after injection (2 min) and at timed intervals (10 min and 1, 4, and 24 h). Human apoE was measured by ELISA and expressed as the percentage remaining in the plasma. Error bars denote mean ± SD (n = 6–7) mice in two experiments using HDL isolated from three volunteers. Significant differences (WT control vs. LPS) at each time point are denoted as follows: * P = 0.03, ** P < 0.005.
The acute inflammatory response decreases hepatic LDL receptor expression
Because the liver is the primary site of apoE-mediated remnant lipoprotein clearance, we tested the effects of K. pneumoniae infection and LPS on hepatic LDL receptor protein levels. Western blots of liver membranes revealed dramatically reduced LDL receptor expression in some bacteremic mice (Figs. 6A, 7). LDL receptor protein levels were also decreased in LPS-treated mice (Figs. 6B, 7). The decline of LDL receptor expression was not accompanied by large elevations of serum ALT levels, indicating the absence of extensive liver damage as a contributing factor. ALT was elevated only 1.5-fold in animals treated with LPS [63 ± 3 U/l (n = 3) in mice treated with 100 μg of LPS and 60 ± 20 (n = 6) in mice treated with 20 μg of LPS vs. 39 ± 8 (n = 6) in control mice]. These LPS doses were far below those that caused lethality (LD50 ∼ 600 μg). A total of 100 μg/25 g body weight caused some diarrhea, ruffled fur, and decreased movement, and 20 μg of LPS had little or no overt effects. ALT levels were elevated by only 2.6-fold in mice infected with K. pneumoniae [128 ± 84 (n = 5) vs. 49 ± 6 (n = 3) in control mice]. AST/ALT ratios were approximately 2:1 in all experimental groups (data not shown).
Fig. 6.
Infection and LPS decrease LDL receptor protein in the liver. A: Nine WT mice (columns 1–9) were euthanized at 3 days after inoculation with K. pneumoniae or PBS as described for Figure 1. B: Fasted WT mice were euthanized at 18 h after intraperitoneal injection of PBS (Control) or LPS (20 or 100 μg/25 g body weight) using three mice per treatment group. Plasma apoE and the indicated liver proteins were measured by Western blotting of equal volumes of plasma or equal amounts of liver total membrane protein; semiquantitative analysis of the bands was performed as described in Materials and Methods. The experiments were repeated with similar results. SR-BI, scavenger receptor class B type I.
Fig. 7.
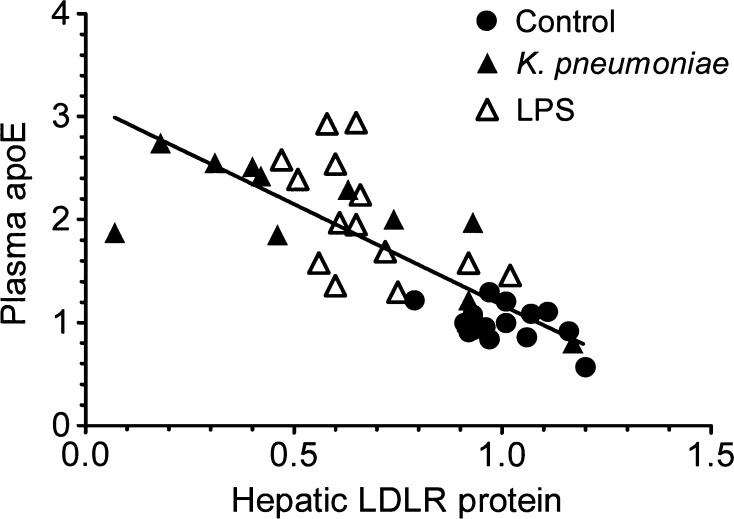
Plasma apoE correlates inversely with hepatic LDL receptor protein. Mice were infected with K. pneumoniae or treated with LPS (100 μg/25g body weight) as described for Figure 1. Plasma apoE and liver LDL receptor protein were quantitated from Western blots as described for Figure 6 and other experiments (data not shown). Data pairs from each mouse were plotted and analyzed by linear regression (r2 = 0.6319, r = 0.7949, P < 0.0001).
Plasma apoE increased in mice with decreased LDL receptor expression during the inflammatory response, whereas low levels of apoE protein in the liver (Fig. 6) reflected low apoE mRNA levels (Fig. 2). When LDL receptor protein levels were plotted against the plasma apoE levels from all experiments, we found a highly significant inverse correlation (Fig. 7), supporting the hypothesis that modulation of the LDL receptor contributes importantly to the regulation of plasma apoE levels. Hepatic LDL receptor levels also correlated inversely with non-HDL cholesterol levels (see supplementary Fig. IIID, F). We also measured the expression of ARH, an essential intracellular component of the LDL receptor (25), and found that ARH protein levels were not affected (Fig. 6). As an additional control, we performed Western blotting for SR-BI protein in one experiment and found it to be unchanged in all but one infected mouse (Fig. 6A). Contrary to a previous report (39), LPS did not significantly reduce LDL receptor protein levels in Tlr4-deficient mice (C57BL/10ScN strain; data from two experiments not shown).
To determine whether the decreased LDL receptor protein levels reflected a decrease in mRNA levels, we measured LDL receptor mRNA levels and found that they were also decreased in both infected and LPS-treated mice (Fig. 8). Decreased food intake can contribute to the suppression of LDL receptor mRNA without greatly affecting hepatic LDL receptor protein expression. To minimize dietary effects, we used fasted mice to assess the effects of LPS administration and found that LDL receptor mRNA levels were markedly decreased even when both groups were fasted (Fig. 8). We also measured the effect of LPS on apoE levels in LDLR−/− mice. In fasted LDLR−/− mice, plasma apoE levels were increased 5- to 6-fold above those of WT mice (Fig. 9A), whereas hepatic apoE mRNA [1.01 ± 0.15 vs. 1.00 ± 0.10 in WT controls (n = 3)] and protein levels (Fig. 9B) were normal. We also found that the lack of LDL receptor expression gave rise to a disproportionately higher fold increase in plasma apoE [5.7 ± 1-fold (n = 6)] than total cholesterol (2.6-fold) in fasted control animals (see supplementary Fig. IIIA). Thus, the apoE/cholesterol ratio in fasting LDLR−/− mice was increased >2-fold above that of WT mice. To determine whether LPS increased plasma apoE levels in LDLR−/− mice, we administered LPS intraperitoneally at doses of 100 and 800 μg/25 g body weight. The high amount of LPS was used to compensate for any decreased LPS response in LDLR−/− mice, which require 8-fold higher amounts of LPS than WT mice to induce lethality (40). As shown in Fig. 9, the administration 100 and 800 μg of LPS resulted in a modest increase in plasma apoE (31% and 4%, respectively) in LDLR−/− mice (Fig. 9). In a similar experiment (data not shown), administration of 100 and 800 μg of LPS increased plasma apoE by 26% and 36%, respectively. Although the LDL receptor is a key mediator of the ability of LPS to augment plasma apoE levels, other factors may also contribute to maintaining or increasing apoE levels.
Fig. 8.
Infection and LPS decrease hepatic LDL receptor (LDLR) mRNA levels. WT mice were infected with K. pneumoniae, and fasting mice were treated with LPS as described for Figure 1. LDLR mRNA was measured by real-time PCR as described for Figure 2. Significant differences from the controls are denoted as follows: * P = 0.003, ** P < 0.0003.
The inflammatory response increases LDL receptor expression in macrophages
In contrast to the liver, macrophages increased their expression of the LDL receptor in response to K. pneumoniae and LPS. As shown in Fig. 10A, LDL receptor mRNA levels increased up to 3-fold in cultured peritoneal macrophages at 6 h after exposure to LPS or K. pneumoniae. In another experiment, macrophage LDL receptor mRNA levels were increased 3-fold after 16 h of exposure to either LPS or K. pneumoniae (data not shown). Western blots of peritoneal macrophage membranes revealed that LDL receptor protein levels increased at 6 h after exposure to LPS and decreased to normal or subnormal levels by 24 h (Fig. 10B). Whereas LDL receptor mRNA increased transiently in cultured macrophages, it did not increase at early time points in the liver: liver LDL receptor mRNA decreased sharply 3 h after LPS administration [0.39 ± 0.09 vs. 1.01 ± 0.21 in controls (n = 3)]. In the spleen, a macrophage-rich organ, LPS maintained or increased LDL receptor mRNA levels, whereas apoE mRNA declined in the same samples (see supplementary Fig. ID). In peritoneal lavage cells, which are also enriched in macrophages, elevated LDL receptor mRNA levels persisted for 2–3 days after inoculation with K. pneumoniae, and preliminary data also suggest that LDL receptor mRNA levels were maintained or increased at 18 h after LPS administration (see supplementary Fig. IA, B). Our data thus reveal marked cell-specific differences in the regulation of LDL receptor expression during the acute inflammatory response.
Fig. 10.
Bacteria and LPS increase macrophage LDL receptor expression. A: LDL receptor (LDLR) mRNA levels were measured in cultured peritoneal macrophages at the indicated times after exposure to K. pneumoniae or LPS as described for Figure 3. The mean and range (error bars) of duplicate determinations are denoted. The experiment was repeated with similar results. B: LDL receptor protein levels were measured in peritoneal macrophages by Western blotting at the indicated times after exposure to LPS. Equal amounts of membrane protein were loaded in each gel lane. The results of two experiments are shown. In experiment 1, ARH was measured as a control. In experiment 2, proteins were stained on the membrane by India ink to confirm equal loading.
DISCUSSION
Our results show that infection and acute inflammation trigger mechanisms that conserve circulating apoE during a strongly negative acute phase apoE mRNA response.
Inasmuch as an acute phase protein is empirically defined as one whose plasma concentration changes by 25% or more following an inflammatory stimulus (34), our results indicate that apoE is a positive acute phase protein in response to bacterial stimuli. Our analysis of human serum also suggests that circulating apoE can reach positive acute phase levels during septic infection; in contrast, circulating apoC-I levels declined in the same patients (Fig. 1A). Since apoE and apoC-I are coordinately regulated by factors that influence the expression of genes in the apoE/C-I/C-IV/C-II gene cluster, the sharp increase in apoE/apoC-I ratios in septic patients and in LPS-treated mice supports the hypothesis that apoE levels are selectively maintained or increased because apoE clearance decreases. These results are consistent with those of a previous study that found increased apoE and decreased apoC-I content of HDL isolated from infected humans (41); however, those authors did not report total plasma apoE levels. Others have also reported that circulating apoE levels increase transiently in mice after LPS administration (9).
We found that the LDL receptor provides the major clearance mechanism for the apoE component of HDL (Fig. 5), in keeping with the receptor's known role as the major mechanism of apoE-mediated remnant lipoprotein clearance (25). Whereas remnant lipoprotein clearance occurs mainly in the liver, our findings that LPS decreased hepatic LDL receptor protein levels in mice and inhibited apoE clearance supports the hypothesis that reduced hepatic LDL receptor expression contributes importantly to the conservation of circulating apoE. We elected to use human HDL to measure apoE clearance, because apoE associates almost entirely with HDL in WT mice, and most of the apoE remains in HDL after the inflammatory stimulus (Fig. 4). Maeda and coworkers (42) showed that both human and mouse apoE bind to mouse LDL receptors with similar affinity and binding maximum, whereas human apoE is cleared poorly by LDL receptor-independent mechanisms in the mouse. In contrast, human apoB (LDL) binds to mouse LDL receptors with lower affinity than that of mouse apoB (43), and the hepatic clearance rate of human LDL in the mouse is 2.4-fold slower than that of mouse LDL (44). Thus, although our clearance data for the apoB component of the total lipoprotein fraction (see supplementary Fig. IIB) may be more compromised by competition from endogenous ligands than those of apoE, our results support the hypothesis that LPS administration decreased LDL receptor activity. The importance of the LDL receptor in apoE clearance is supported by our findings that apoE clearance was strongly inhibited in LDL receptor-deficient mice (Fig. 5), that LPS administration significantly slowed apoE clearance in WT mice (Fig. 5), and that endogenous apoE levels were increased by the lack of the LDL receptor (Fig. 9). In contrast, apoC-I does not bind to the LDL receptor; thus changes in LDL receptor expression are expected to have relatively little impact on apoC-I clearance. Thus, decreased plasma apoC-I levels in LPS-treated mice (Fig. 1C) probably result from decreased hepatic apoC-I expression (Fig. 2B).
The effects of acute phase changes in hepatic LDL receptor expression on plasma total cholesterol and triglyceride levels in mice are unclear. Mice have very little LDL and carry essentially all of their cholesterol in HDL. HDL particles are heterogeneous in composition, and apoE is carried by a small subset of the total HDL particles (45). Although the absence of LDL receptor expression causes hypercholesterolemia and hypertriglyceridemia, LPS administration in WT mice did not increase total cholesterol levels, whereas triglycerides were increased (see supplementary Fig. IIIA, B). Whereas LPS treatment slowed the clearance of the apoE component of HDL, it appeared to have little or no ability to slow the clearance of HDL-cholesterol. In contrast, total cholesterol levels increased by ∼50% in bacteremic mice, whereas total triglyceride levels were not elevated significantly (see supplementary Fig. IIIA, B), for reasons that are unclear. However, elevated plasma non-HDL cholesterol levels were a common finding in both LPS-treated and infected mice; these levels correlated inversely with hepatic LDL receptor levels (see supplementary Fig. IIID, F), which suggests that hepatic LDL receptor activity was decreased in both mouse models.
The regulation of cholesterol levels during acute inflammation in humans differs markedly from that of mice. Our septic patients were hypocholesterolemic, whereas their total triglyceride levels were maintained or increased (see supplementary Fig. IVA); we previously found that the hypocholesterolemia in our septic patients is due to a selective loss of esterified cholesterol in both HDL and LDL (38), which may be attributed to low LCAT activity. Also unlike those in mice, human HDL levels decline sharply during infection, as measured by their content of cholesterol, phospholipids, and major HDL apolipoproteins (38, 41), whereas apoE-HDL levels are maintained or elevated in most patients (see supplementary Fig. IV). Although apoE was also maintained or increased in non-HDL fractions, our analysis did not reveal a significant correlation between total circulating apoE and total triglycerides in the patient population.
Our data do not rule out the possibility that LPS and other bacterial products may induce a signal that inhibits the activity of the LDL receptor system or the intracellular fate of its apoE ligand. However, changes in plasma apoE and hepatic LDL receptor protein expression did not occur rapidly: at 3 h after LPS administration, we found normal levels of both plasma apoE (1.00 ± 0.13 vs. 1.00 ± 0.11 in controls) and hepatic LDL receptor protein [1.10 ± 0.05 vs. 1.00 ± 0.12 in controls (n = 3)]. An LDL receptor-independent mechanism may also play a significant role in regulating plasma apoE levels, as evidenced by our finding that apoE levels increased somewhat (4–36% above control) after LPS administration to LDLR−/− mice. The increase in apoE in the LPS-treated LDLR−/− mice occurred mainly in fractions containing VLDL and chylomicrons (Fig. 4D). This may result from a decline in LPL, which plays an important role in the clearance of triglyceride-rich lipoproteins. Feingold et al. (46) showed that decreased clearance of triglyceride- and cholesterol-labeled chylomicrons accompanied decreased plasma LPL activity in LPS-treated rats. However, the LPS-induced decline of LPL activity does not appear to play a key role in increasing plasma triglycerides (33, 47). Although LPS increased plasma triglycerides in our mice, septic infection did not, and we did not find a significant correlation between total plasma apoE and triglyceride levels during the acute inflammatory response to LPS (see supplementary Fig. III). It is also possible that LPS could contribute to the increase of plasma apoE by altering apoE uptake by other receptors [e.g., LRP (1), HSPG (26), or SR-BI (27)]. We also cannot rule out potential effects of LPS on other apoE posttranscriptional or posttranslational processes. For example, apoE is relatively resistant to intracellular degradation, and a large amount of apoE is recycled and released from cells by retroendocytosis (48). A decline in LDL receptor activity might also promote increased secretion of cellular apoE (49). Thus, it is possible that low hepatic LDL receptor activity may be part of a more complex acute phase program to conserve plasma apoE.
Although the polysaccharide portion of LPS has been reported to play a role in the inhibition of LDL uptake by a hepatocyte cell line (50), we found that “rough”-form Ra-LPS that lacked the O polysaccharide chain stimulated elevated levels of apoE in vivo (data not shown). Contrary to a previous report that LPS suppressed hepatic LDL receptor protein expression in C3H/HeJ mice, which lack functional Tlr4 (39), we found that Tlr4-deficient mice were unresponsive to the effects of LPS. The cited study showed a Tlr4-dependent shift of apoE and triglycerides to the VLDL fraction, but the authors did not report total plasma apoE levels.
The mechanism by which LPS and bacterial infection induce a decline in hepatic LDL receptor protein levels may be attributed to reduced mRNA levels. We found that LPS sharply reduced hepatic LDL receptor mRNA levels by 3 h. However, LDL receptor protein levels do not always decrease along with declining mRNA. For example, fasting decreases LDL receptor mRNA without greatly affecting hepatic protein levels of the receptor. Fasting suppresses sterol-regulatory element binding protein-2 activity, resulting in decreased transcription of both the LDL receptor and proprotein convertase subtilisin-like kexin type 9 (PCSK9), a protease that regulates the LDL receptor by promoting its degradation (51). However, our results show that LPS decreases LDL receptor mRNA levels below the baseline levels of fasting mice (Fig. 8B). We have not determined how PCSK9 activity is affected by LPS or whether it contributes significantly to LDL receptor degradation under these conditions. Although LPS and bacteria increase LDL receptor mRNA and protein transiently in cultured peritoneal macrophages, we found that after 24 h the LDL receptor protein declines below the levels found in unstimulated macrophages. The presence of a somewhat smaller immunoreactive band on the LDL receptor Western blots (Fig. 10B) suggests proteolytic degradation. However, we failed to detect any PCSK9 mRNA in the macrophages, suggesting that another protease may target the LDL receptor.
Our results suggest that hepatic and macrophage LDL receptor expression is differentially regulated at the mRNA level. Whereas acute inflammatory stimuli rapidly downregulated hepatic LDL receptor mRNA, macrophage LDL receptor mRNA was rapidly upregulated by the same stimuli (Fig. 10). It is unclear whether elevated macrophage LDL receptor levels persist in vivo during bacterial infection. Although our in vitro results suggest that LDL receptor mRNA and protein levels increase only transiently during the first 24 h, these results may not reflect the changing macrophage populations and traffic patterns that may occur in vivo. Our preliminary findings suggest that LDL receptor mRNA levels remain elevated in peritoneal lavage cells for up to 3 days after K. pneumoniae inoculation (see supplementary Fig. IA). Although we have not measured the effects of inflammatory stimuli on apoE uptake by macrophages, others have found an association between increased LDL receptor expression during dendritic cell maturation and increased apoE-mediated internalization of a variety of bacterial lipids (14). Those authors found that bacterial lipids internalized by the LDL receptor are targeted to CD1, which then presents the lipids to natural killer T-cells. Thus, macrophage/dendritic cell LDL receptors may be involved in both antigen presentation and uptake of host lipids.
These experiments have revealed a novel acute phase mechanism that enhances the circulating levels of a protein while decreasing its production. We hypothesize that this acute phase program benefits the host in several ways. First, decreasing the production of apoE in the liver and macrophages should conserve biosynthetic resources to serve other functions during the acute response to infection. Second, decreased expression of hepatic LDL receptors should decrease the hepatic uptake of important lipids, making them available for use within peripheral sites of infection and injury. Third, increased levels of plasma apoE would be expected to increase apoE concentrations in lymph and interstitial fluids, where apoE may exert many of its immunomodulatory effects. Finally, increased expression of the LDL receptor by macrophages may contribute to the uptake of lipids by these important defense cells. Thus, differential expression of LDL receptors by the liver and macrophages underlies an acute phase program that impacts apoE-mediated lipid traffic during infection.
Supplementary Material
Acknowledgments
The authors thank Dr. Robert Munford for critical reading of the manuscript. The authors also thank Drs. John Dietschy, Helen Hobbs, Jonathan Cohen, Jay Horton, and Guosheng Liang for helpful advice and discussions.
Abbreviations
ALT, alanine aminotransferase
apoE, apolipoprotein E
AST, aspartate aminotransferase
HSPG, heparan sulfate proteoglycan
LDLR−/−, LDL receptor knockout
LRP, LDL receptor-related protein
LPS, lipopolysaccharide
PCSK9, proprotein convertase subtilisin-like kexin type 9
SR-BI, scavenger receptor class B type I
WT, wild-type
Published, JLR Papers in Press, May 24, 2008.
Footnotes
This work was supported by National Institutes of Health Grant AI-45896 from the National Institute of Allergy and Infectious Diseases.
The online version of this article (available at http://www.jlr.org) contains supplementary data in the form of one table and four figures.
References
- 1.Mahley R. W., and S. C. Rall, Jr. 2000. Apolipoprotein E: far more than a lipid transport protein. Annu. Rev. Genomics Hum. Genet. 1 507–537. [DOI] [PubMed] [Google Scholar]
- 2.Mahley R. W., K. H. Weisgraber, and Y. Huang. 2006. Inaugural article. Apolipoprotein E4: a causative factor and therapeutic target in neuropathology, including Alzheimer's disease. Proc. Natl. Acad. Sci. USA. 103 5644–5651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liberopoulos E., K. Siamopoulos, and M. Elisaf. 2004. Apolipoprotein E and renal disease. Am. J. Kidney Dis. 43 223–233. [DOI] [PubMed] [Google Scholar]
- 4.Moore R. J., R. M. Chamberlain, and F. R. Khuri. 2004. Apolipoprotein E and the risk of breast cancer in African-American and non-Hispanic white women. Oncology. 66 79–93. [DOI] [PubMed] [Google Scholar]
- 5.De Bont N., M. G. Netea, P. N. Demacker, I. Verschueren, B. J. Kullberg, K. W. Van Dijk, J. W. van der Meer, and A. F. Stalenhoef. 1999. Apolipoprotein E knock-out mice are highly susceptible to endotoxemia and Klebsiella pneumoniae infection. J. Lipid Res. 40 680–685. [PubMed] [Google Scholar]
- 6.de Bont N., M. G. Netea, P. N. Demacker, B. J. Kullberg, J. W. van der Meer, and A. F. Stalenhoef. 2000. Apolipoprotein E-deficient mice have an impaired immune response to Klebsiella pneumoniae. Eur. J. Clin. Invest. 30 818–822. [DOI] [PubMed] [Google Scholar]
- 7.Roselaar S. E., and A. Daugherty. 1998. Apolipoprotein E-deficient mice have impaired innate immune responses to Listeria monocytogenes in vivo. J. Lipid Res. 39 1740–1743. [PubMed] [Google Scholar]
- 8.Sinnis P., T. E. Willnow, M. R. S. Briones, J. Herz, and V. Nussenzweig. 1996. Remnant lipoproteins inhibit malaria sporozoite invasion of hepatocytes. J. Exp. Med. 184 945–954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Van Oosten M. V., P. C. N. Rensen, E. S. Van Amersfoort, M. Van Eck, A. M. Van Dam, J. J. P. Brevé, T. Vogel, A. Panet, T. J. C. Van Berkel, and J. Kuiper. 2001. Apolipoprotein E protects against-bacterial lipopolysaccharide-induced lethality—a new therapeutic approach to treat Gram-negative sepsis. J. Biol. Chem. 276 8820–8824. [DOI] [PubMed] [Google Scholar]
- 10.Rensen P. C. N., M. Van Oosten, E. Van de Bilt, M. Van Eck, J. Kuiper, and T. J. C. Van Berkel. 1997. Human recombinant apolipoprotein E redirects lipopolysaccharide from Kupffer cells to liver parenchymal cells in rats in vivo. J. Clin. Invest. 99 2438–2445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Laskowitz D. T., D. M. Lee, D. Schmechel, and H. F. Staats. 2000. Altered immune responses in apolipoprotein E-deficient mice. J. Lipid Res. 41 613–620. [PubMed] [Google Scholar]
- 12.Ali K., M. Middleton, E. Pure, and D. J. Rader. 2005. Apolipoprotein E suppresses the type I inflammatory response in vivo. Circ. Res. 97 922–927. [DOI] [PubMed] [Google Scholar]
- 13.Grainger D. J., J. Reckless, and E. McKilligin. 2004. Apolipoprotein E modulates clearance of apoptotic bodies in vitro and in vivo, resulting in a systemic proinflammatory state in apolipoprotein E-deficient mice. J. Immunol. 173 6366–6375. [DOI] [PubMed] [Google Scholar]
- 14.van den Elzen P., S. Garg, L. Leon, M. Brigl, E. A. Leadbetter, J. E. Gumperz, C. C. Dascher, T. Y. Cheng, F. M. Sacks, P. A. Illarionov, et al. 2005. Apolipoprotein-mediated pathways of lipid antigen presentation. Nature. 437 906–910. [DOI] [PubMed] [Google Scholar]
- 15.Moretti E. W., R. W. Morris, M. Podgoreanu, D. A. Schwinn, M. F. Newman, E. Bennett, V. G. Moulin, U. U. Mba, and D. T. Laskowitz. 2005. APOE polymorphism is associated with risk of severe sepsis in surgical patients. Crit. Care Med. 33 2521–2526. [DOI] [PubMed] [Google Scholar]
- 16.Allan C. M., S. Taylor, and J. M. Taylor. 1997. Two hepatic enhancers, HCR.1 and HCR.2, coordinate the liver expression of the entire human apolipoprotein E/C-I/C-IV/C-II gene cluster. J. Biol. Chem. 272 29113–29119. [DOI] [PubMed] [Google Scholar]
- 17.Shih S. J., C. Allan, S. Grehan, E. Tse, C. Moran, and J. M. Taylor. 2000. Duplicated downstream enhancers control expression of the human apolipoprotein E gene in macrophages and adipose tissue. J. Biol. Chem. 275 31567–31572. [DOI] [PubMed] [Google Scholar]
- 18.Hashimoto S., T. Suzuki, H. Y. Dong, N. Yamazaki, and K. Matsushima. 1999. Serial analysis of gene expression in human monocytes and macrophages. Blood. 94 837–844. [PubMed] [Google Scholar]
- 19.Zannis V. I., S. Cole, C. L. Jackson, D. M. Kurnit, and S. K. Karathanasis. 1985. Distribution of apolipoprotein A-I, C-II, C-III, and E mRNA in fetal human tissues. Time-dependent induction of apolipoprotein E mRNA by cultures of human monocyte-macrophages. Biochemistry. 24 4450–4455. [DOI] [PubMed] [Google Scholar]
- 20.Jong M. C., M. H. Hofker, and L. M. Havekes. 1999. Role of apoCs in lipoprotein metabolism: functional differences between apoC1, apoC2, and apoC3. Arterioscler. Thromb. Vasc. Biol. 19 472–484. [DOI] [PubMed] [Google Scholar]
- 21.Kraft H. G., H. J. Menzel, F. Hoppichler, W. Vogel, and G. Utermann. 1989. Changes of genetic apolipoprotein phenotypes caused by liver transplantation: implications for apolipoprotein synthesis. J. Clin. Invest. 83 137–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Linton M. F., J. B. Atkinson, and S. Fazio. 1995. Prevention of atherosclerosis in apolipoprotein E-deficient mice by bone marrow transplantation. Science. 267 1034–1037. [DOI] [PubMed] [Google Scholar]
- 23.Fazio S., V. R. Babaev, A. B. Murray, A. H. Hasty, K. J. Carter, L. A. Gleaves, J. B. Atkinson, and M. F. Linton. 1997. Increased atherosclerosis in mice reconstituted with apolipoprotein E null macrophages. Proc. Natl. Acad. Sci. USA. 94 4647–4652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cheung M. C., and J. J. Albers. 1984. Characterization of lipoprotein particles isolated by immunoaffinity chromatography. Particles containing A-I and A-II and particles containing A-I but no A-II. J. Biol. Chem. 259 12201–12209. [PubMed] [Google Scholar]
- 25.Jones C., R. Garuti, P. Michaely, W. P. Li, N. Maeda, J. C. Cohen, J. Herz, and H. H. Hobbs. 2007. Disruption of LDL but not VLDL clearance in autosomal recessive hypercholesterolemia. J. Clin. Invest. 117 165–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.MacArthur J. M., J. R. Bishop, K. I. Stanford, L. Wang, A. Bensadoun, J. L. Witztum, and J. D. Esko. 2007. Liver heparan sulfate proteoglycans mediate clearance of triglyceride-rich lipoproteins independently of LDL receptor family members. J. Clin. Invest. 117 153–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Arai T., F. Rinninger, L. Varban, V. Fairchild-Huntress, C. P. Liang, W. Chen, T. Seo, R. Deckelbaum, D. Huszar, and A. R. Tall. 1999. Decreased selective uptake of high density lipoprotein cholesteryl esters in apolipoprotein E knock-out mice. Proc. Natl. Acad. Sci. USA. 96 12050–12055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gabay C., and I. Kushner. 1999. Acute-phase proteins and other systemic responses to inflammation. N. Engl. J. Med. 340 448–454. [DOI] [PubMed] [Google Scholar]
- 29.Aldred, A. R., and G. Schreiber. 1993. The negative acute phase proteins. In Acute Phase Proteins. A. Mackiewicz, I. Kushner, and H. Baumann, editors. CRC Press, Boca Raton, FL, 21–37.
- 30.Werb Z., and J. R. Chin. 1983. Endotoxin suppresses expression of apoprotein E by mouse macrophages in vivo and in culture. J. Biol. Chem. 258 10642–10648. [PubMed] [Google Scholar]
- 31.Hardardottir I., J. Sipe, A. H. Moser, C. J. Fielding, K. R. Feingold, and C. Grunfeld. 1997. LPS and cytokines regulate extra hepatic mRNA levels of apolipoproteins during the acute phase response in Syrian hamsters. Biochim. Biophys. Acta. 1344 210–220. [DOI] [PubMed] [Google Scholar]
- 32.Tripp R. J., A. Tabares, H. Wang, and S. Lanza-Jacoby. 1993. Altered hepatic production of apolipoproteins B and E in the fasted septic rat: factors in the development of hypertriglyceridemia. J. Surg. Res. 55 465–472. [DOI] [PubMed] [Google Scholar]
- 33.Khovidhunkit W., M. S. Kim, R. A. Memon, J. K. Shigenaga, A. H. Moser, K. R. Feingold, and C. Grunfeld. 2004. Effects of infection and inflammation on lipid and lipoprotein metabolism mechanisms and consequences to the host. J. Lipid Res. 45 1169–1196. [DOI] [PubMed] [Google Scholar]
- 34.Kushner, I., and A. Mackiewicz. 1993. The acute phase response: an overview. In Acute Phase Proteins. A. Mackiewicz, I. Kushner, and H. Baumann, editors. CRC Press, Boca Raton, FL, 4–19.
- 35.Kitchens R. L., G. Wolfbauer, J. J. Albers, and R. S. Munford. 1999. Plasma lipoproteins promote the release of bacterial lipopolysaccharide from the monocyte cell surface. J. Biol. Chem. 274 34116–34122. [DOI] [PubMed] [Google Scholar]
- 36.Engelking L. J., H. Kuriyama, R. E. Hammer, J. D. Horton, M. S. Brown, J. L. Goldstein, and G. Liang. 2004. Overexpression of Insig-1 in the livers of transgenic mice inhibits SREBP processing and reduces insulin-stimulated lipogenesis. J. Clin. Invest. 113 1168–1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Westerterp M., W. de Haan, J. F. P. Berbee, L. M. Havekes, and P. C. N. Rensen. 2006. Endogenous apoC-I increases hyperlipidemia in apoE-knockout mice by stimulating VLDL production and inhibiting LPL. J. Lipid Res. 47 1203–1211. [DOI] [PubMed] [Google Scholar]
- 38.Kitchens R. L., P. A. Thompson, R. S. Munford, and G. E. O'Keefe. 2003. Acute inflammation and infection maintain circulating phospholipid levels and enhance lipopolysaccharide binding to plasma lipoproteins. J. Lipid Res. 44 2339–2348. [DOI] [PubMed] [Google Scholar]
- 39.Liao W., M. Rudling, and B. Angelin. 1999. Endotoxin suppresses mouse hepatic low-density lipoprotein-receptor expression via a pathway independent of the Toll-like receptor 4. Hepatology. 30 1252–1256. [DOI] [PubMed] [Google Scholar]
- 40.Netea M. G., P. N. M. Demacker, B. J. Kullberg, O. C. Boerman, I. Verschueren, A. F. H. Stalenhoef, and J. W. M. van der Meer. 1996. Low-density lipoprotein receptor-deficient mice are protected against lethal endotoxemia and severe Gram-negative infections. J. Clin. Invest. 97 1366–1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Barlage S., D. Fröhlich, A. Böttcher, M. Jauhiainen, H. P. Müller, F. Noetzel, G. Rothe, C. Schütt, R. P. Linke, K. J. Lackner, et al. 2001. ApoE-containing high density lipoproteins and phospholipid transfer protein activity increase in patients with a systemic inflammatory response. J. Lipid Res. 42 281–290. [PubMed] [Google Scholar]
- 42.Knouff C., O. Briand, S. Lestavel, V. Clavey, M. Altenburg, and N. Maeda. 2004. Defective VLDL metabolism and severe atherosclerosis in mice expressing human apolipoprotein E isoforms but lacking the LDL receptor. Biochim. Biophys. Acta. 1684 8–17. [DOI] [PubMed] [Google Scholar]
- 43.Corsini A., M. Mazzotti, A. Villa, F. M. Maggi, F. Bernini, L. Romano, C. Romano, R. Fumagalli, and A. L. Catapano. 1992. Ability of the LDL receptor from several animal species to recognize the human apoB binding domain: studies with LDL from familial defective apoB-100. Atherosclerosis. 93 95–103. [DOI] [PubMed] [Google Scholar]
- 44.Osono Y., L. A. Woollett, J. Herz, and J. M. Dietschy. 1995. Role of the low density lipoprotein receptor in the flux of cholesterol through the plasma and across the tissues of the mouse. J. Clin. Invest. 95 1124–1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Böttcher A., J. Schlosser, F. Kronenberg, H. Dieplinger, G. Knipping, K. J. Lackner, and G. Schmitz. 2000. Preparative free-solution isotachophoresis for separation of human plasma lipoproteins: apolipoprotein and lipid composition of HDL subfractions. J. Lipid Res. 41 905–915. [PubMed] [Google Scholar]
- 46.Feingold K. R., I. Staprans, R. A. Memon, A. H. Moser, J. K. Shigenaga, W. T. Doerrler, C. A. Dinarello, and C. Grunfeld. 1992. Endotoxin rapidly induces changes in lipid metabolism that produce hypertriglyceridemia: low doses stimulate hepatic triglyceride production while high doses inhibit clearance. J. Lipid Res. 33 1765–1776. [PubMed] [Google Scholar]
- 47.Feingold K. R., M. Marshall, R. Gulli, A. H. Moser, and C. Grunfeld. 1994. Effect of endotoxin and cytokines on lipoprotein lipase activity in mice. Arterioscler. Thromb. 14 1866–1872. [DOI] [PubMed] [Google Scholar]
- 48.Rensen P. C. N., M. C. Jong, L. C. van Vark, H. van der Boom, W. L. Hendriks, T. J. C. Van Berkel, E. A. L. Biessen, and L. M. Havekes. 2000. Apolipoprotein E is resistant to intracellular degradation in vitro and in vivo: evidence for retroendocytosis. J. Biol. Chem. 275 8564–8571. [DOI] [PubMed] [Google Scholar]
- 49.Lucic D., Z. H. Huang, D. S. Gu, M. K. Altenburg, N. Maeda, and T. Mazzone. 2007. Regulation of macrophage apoE secretion and sterol efflux by the LDL receptor. J. Lipid Res. 48 366–372. [DOI] [PubMed] [Google Scholar]
- 50.Liao W., and C. H. Florén. 1992. Both the polysaccharide and lipid A parts of endotoxins are needed for the inhibitory effects of endotoxins on cellular LDL uptake. Scand. J. Clin. Lab. Invest. 52 183–188. [DOI] [PubMed] [Google Scholar]
- 51.Horton J. D., J. C. Cohen, and H. H. Hobbs. 2007. Molecular biology of PCSK9: its role in LDL metabolism. Trends Biochem. Sci. 32 71–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.