Abstract
We use mathematical models to study the relationship between HIV and the immune system during the natural course of infection and in the context of different antiviral treatment regimes. The models suggest that an efficient cytotoxic T lymphocyte (CTL) memory response is required to control the virus. We define CTL memory as long-term persistence of CTL precursors in the absence of antigen. Infection and depletion of CD4+ T helper cells interfere with CTL memory generation, resulting in persistent viral replication and disease progression. We find that antiviral drug therapy during primary infection can enable the development of CTL memory. In chronically infected patients, specific treatment schedules, either including deliberate drug holidays or antigenic boosts of the immune system, can lead to a re-establishment of CTL memory. Whether such treatment regimes would lead to long-term immunologic control deserves investigation under carefully controlled conditions.
Antiviral therapy for HIV-infected patients has greatly improved the recent years. Administration of drug cocktails consisting of three or more different drugs can reduce and maintain virus load below detection limit in many patients. Nevertheless considerable problems remain such as viral resistance, side effects, and lack of compliance during prolonged therapy (1–4). Furthermore it is unlikely that combination therapy alone can eradicate HIV from infected patients because of long-lived infected cells and sites within the body where drugs may not achieve effective levels (5–8). Hence there is considerable interest in searching for therapy regimes that may reduce virus load and restimulate immune responses, thereby turning the balance between HIV and the immune system in favor of the immune system.
There is convincing evidence that antiviral immune responses play an important role in determining virus load and the rate of disease progression in infected patients (9–12). Long-term nonprogressors often have good CD4- and CD8-mediated immune responses against HIV and low virus load, whereas rapid progressors tend to have weak antiviral immunity and high virus load (13–21). Experimental depletion of CD8 cells in simian immunodeficiency virus-infected macaques leads to a dramatic increase in virus load (22).
Here we design a mathematical model to study the interaction between HIV and the immune system and to analyze how specific antiviral treatment regimes can lead to the establishment of effective immune responses and long-term control of HIV.
Results and Discussion
We analyze a model containing four variables: uninfected CD4+ T cells (x), infected CD4+ T cells (y), cytotoxic T lymphocyte (CTL) precursors (CTLp) (w), and CTL effectors (z). The model is given as follows.
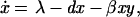 |
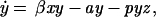 |
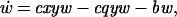 |
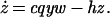 |
Uninfected CD4+ T cells are produced at a rate λ, die at a rate dx, and become infected by free virus at a rate βxy. Infected cells decay at a rate ay and are killed by CTL effectors at a rate pyz. In accordance with experimental findings (23–26) we assume that establishment of a lasting CTL response depends on CD4+ T cell help, and that HIV impairs T helper cell function. Thus, proliferation of the CTLp population is given by cxyw and is proportional to both virus load (y) and the number of uninfected T helper cells (x). CTLp die at a rate bw and differentiate into effectors at a rate cqyw. CTL effectors die at a rate hz.
The model has the interesting property that after viral infection the system may go to one of two equilibria: (i) either an effective, sustained CTL response becomes established and virus load is contained at low levels or (ii) an effective, sustained CTL response is not established and viral load is at high levels. In the model, the development of a lasting CTL response depends on host and viral parameters as well as initial conditions. More specifically, the dynamics between virus and CTL depend on the balance between the rate of viral replication (β) and the quality of the CTL response (CTL activation rate, c and CTL death rate, b), reflected in the virus load attained during primary infection. If the rate of viral replication is below a threshold and is low compared with the immune responsiveness of the host, an effective CTL response is always established. On the other hand, if the rate of viral replication is above a threshold and is fast relative to the immune responsiveness of the host, the virus may replicate to high levels and an effective, sustained CTL response fails to become established because of high degrees of immune impairment. For intermediate rates of viral replication, the outcome of the dynamics depends on the initial conditions: a sustained CTL response is unlikely to become established in naïve hosts, because a low initial number of CTLp allows the virus to replicate to high levels and to impair the T helper cell response before the CTL had time to act. In addition, a high initial virus load and a low initial CD4+ T cell count interfere with CTLp expansion.
If a sustained CTL response is successfully established, it is an important determinant of virus load. Virus load is suppressed to low levels by long-term persistence of CTLp in the absence of antigen (low b) and by a high activation rate of CTLp (high c). The development of such T helper cell-dependent, persistent, readily activated CTLp has been used as a criterion for defining CTL memory associated with protection from reinfection with a pathogen (27–31). However, our model suggests that these properties, traditionally associated with CTL memory, also may be required for clearance or effective containment of HIV.
Infection of CD4 cells by HIV may impair T helper function, resulting in the absence of CTLp that are long-lived in the absence of antigen. According to our models, this impairment may be the reason for persistent replication of HIV in the host, eventually leading to the development of AIDS. This notion is supported by the finding that specific CTLp in chronically HIV-infected patients tend to decay rapidly after antiretroviral therapy, thus having a relatively short life-span at low levels of antigen (32, 33). Our analysis of the dynamics of HIV infection concentrates on memory CTL responses that depend on the presence of CD4 T cell help, because the model suggests that these have the potential to control the virus in the long term. Note, however, that we do not take into account CTLs that are maintained mainly by active viral replication and that may become established without the presence of significant CD4 cell help (25, 26), because they are predicted to lose control of the infection. Therefore, when our model suggests the absence of an efficient CTL memory response, this result does not exclude the presence of less efficient CTL maintained mainly by persisting antigen.
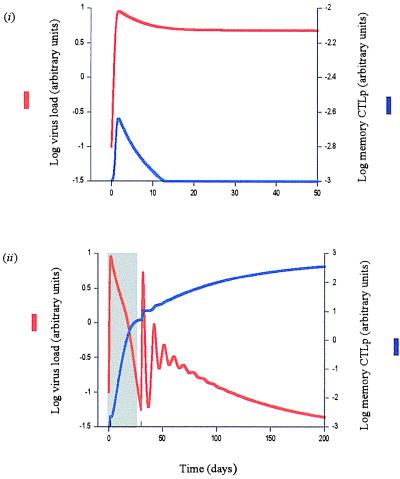
Based on these assumptions, Fig. 1i shows a simulation of primary HIV infection. The virus population replicates to a peak and subsequently approaches a stable equilibrium. The CTL memory response initially expands, but is exhausted because of virus-induced impairment of T helper cell function. We can introduce drug therapy into the model by assuming that treatment reduces the rate of viral replication, expressed as sβxy, where 0 < s < 1. The drugs are 100% efficient if s = 0 and have no effect if s = 1. As shown in Fig. 1ii, drug therapy during the primary phase of the infection may result in the establishment of CTL memory and long-term immunological control of the virus in the absence of the drug, because treatment reduces the amount of T helper cell impairment while the CTL population is expanding in response to the initial viral growth. When treatment is withdrawn, the initial conditions have been shifted so that exhaustion of the established CTL memory becomes impossible. A crucial parameter for successful therapy is the duration of treatment during the primary phase of the infection. As shown in Fig. 2, this success depends on viral, host, and medical factors, such as the time when treatment is started, the replication rate of the virus, the CTL responsiveness of the host, the initial CD4+ T cell count, and the efficacy of the drug.
Figure 1.
Primary HIV infection. Shading indicates drug therapy. (i) Basic dynamics. The virus population replicates up to a peak and subsequently settles to a stable equilibrium. The memory CTLp initially expand, but subsequently are exhausted because of HIV-induced impairment of the T helper cell response. Note that we only take into account an efficient memory CTL response, dependent on CD4 cell help. The model considers a simplified scenario, excluding less efficient CTL responses that may be independent of CD4 cell help and that may not control the virus in the long term. Hence, virus load does not fall by a very large amount after the peak, and the memory CTLp response declines to low levels. The depicted scenario may correspond to fast progressing disease. (ii) Effect of drug therapy. Administration of antiretroviral drugs during the primary phase of the infection minimizes the degree of HIV-induced immune impairment. Consequently, CTL memory becomes established in response to the increased viral load. Once CTL memory has been established, it may control HIV in the long term in the absence of continued therapy. Parameters were chosen as follows: λ = 1; d = 0.1; β = 0.5; a = 0.2; p = 1; c = 0.1; b = 0.01; q = 0.5; h = 0.1; s = 0.0042.
Figure 2.
Duration of therapy during primary infection required to successfully establish CTL memory in dependence of host and viral parameters. The same relationships hold true for the duration of the secondary phase of therapy in the asymptomatic period required to re-establish CTL memory (Fig. 3). The arrows with an infinity sign denote parameter thresholds beyond which establishment of CTL memory becomes impossible, regardless of the duration of treatment. (i) Start of therapy in primary infection or after the drug holiday during the asymptomatic period. Treatment should be started when virus load has replicated to a level sufficient to stimulate specific CTLp. Starting too early may result in treatment failure because the immune system has not been boosted enough. On the other hand, if the virus has replicated to sufficiently high levels, delaying the onset of therapy results in an increased duration of treatment required for the establishment of CTL memory. If treatment is started too late, control of the virus becomes impossible. (ii) A fast replication rate of the virus, β, results in a decreased availability of functional T helper cells. Consequently, if β lies above a threshold, immunological control of the virus is impossible. On the other hand, if the virus replicates relatively slowly and β lies below a threshold, virus-induced immune impairment is minimal and the immune system may control the virus without the need for any therapy. For intermediate values of β, immune impairment interferes with the generation of memory, but therapy may restore it, resulting in long-term immunological control of the virus. In this parameter region, the duration of therapy required to establish CTL memory increases with a faster replication rate of the virus (β). (iii) The lower the immune responsiveness of the host (c), the longer the duration of treatment required to establish CTL memory. If the immune responsiveness lies below a threshold, treatment cannot result in the establishment of CTL memory. On the other hand, if the immune responsiveness lies above a threshold and is sufficiently high to overcome virus-induced immune impairment, CTL memory is established and the virus is controlled without the need for therapy. (iv) The rate of CD4+ T cell production (λ), and thus the initial CD4+ T cell count at the start of therapy, is an important parameter for successful treatment. The lower the rate of CD4+ T cell production, the longer the duration of treatment required to establish CTL memory. If the rate of CD4+ cell production has fallen below a threshold, therapy cannot result in the establishment of CTL memory. (v) The lower the efficacy of the drug (1-s), the longer the duration of therapy required to establish CTL memory. If the efficacy of the drugs lies below a threshold, therapy cannot result in the establishment of CTL memory. Baseline parameters were chosen as follows: λ = 1; d = 0.1; β = 0.5; a = 0.2; p = 1; c = 0.1; b = 0.01; q = 0.5; h = 0.1; s = 0.0042.
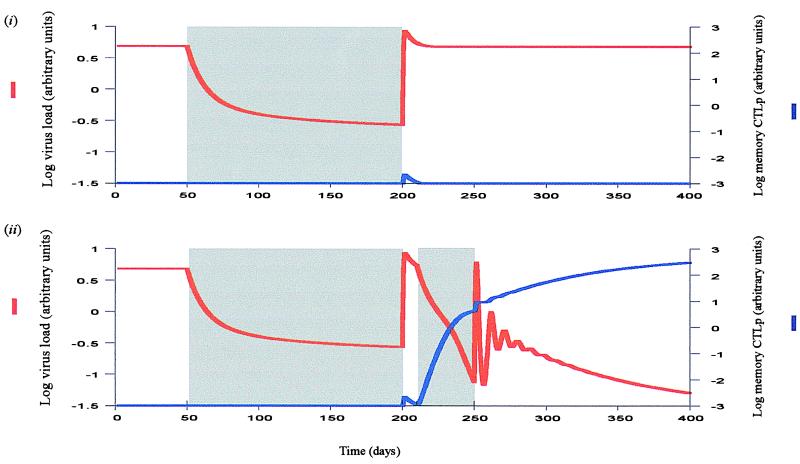
Next we consider HIV infection and drug therapy during the asymptomatic period. Treatment during this phase may reduce virus load below detection limit, but when the drugs are withdrawn, virus load re-emerges to pretreatment levels (Fig. 3i). Long-term immunological control of the infection can be obtained only if the CTL memory response is re-established. According to our models this control can be attained by boosting the immune system with virus while simultaneously treating the patient to minimize the degree of immune impairment. This control can be achieved by a treatment regime that consists of four phases: first treatment, treatment window, secondary treatment, and end of treatment (Fig. 3ii). The first phase of treatment reduces virus load and allows the CD4+ T cell count to increase as far as possible. This phase should continue at least until the virus is below detection limit and preferably longer so that the immune system recovers as much as possible. The treatment window involves simultaneous withdrawal of all drugs in use, minimizing the chances of resistance evolving. It allows virus load to increase for a given period of time. The increased virus load gives the CTL response a boost and is essentially a simulation of events occurring during the primary phase of the infection. As the virus population grows, the secondary treatment phase is initiated. It ensures that the degree of immune impairment is reduced while the CTL response expands, which enables the establishment of CTL memory. Once CTL memory has been generated, therapy can be stopped and the virus is maintained at a low level; the infection is in a state similar to long-term nonprogressors.
Figure 3.
Asymptomatic period of the infection. Shading indicates drug therapy. (i) Efficient drug therapy reduces virus load to low levels. However, if the drugs are withdrawn, virus load re-emerges to pretreatment levels. Although the rise in virus load boosts the immune system, virus-induced immune impairment prevents the development of CTL memory. Note again, that we only consider memory CTLp as defined in the text, dependent on the presence of CD4 cell help. Hence, before therapy, the figure does not show persistence of less efficient CTL at higher levels that may not control the infection in the long term and that may be maintained by continuous viral replication in HIV-infected patients. (ii) Treatment regime required to re-establish CTL memory. It consists of four phases: The first phase of treatment reduces virus load to low levels, which is followed by a drug holiday allowing the virus to replicate, thereby boosting the immune system. While virus load increases, the secondary phase of therapy is initiated. This phase suppresses the amount of virus-induced immune impairment and allows the establishment of CTL memory in response to the increased virus load. Finally, drug therapy can be stopped for good once CTL memory has been generated. The virus now is controlled in the long term by the immune system. Note that after the second phase of therapy virus load transiently rises and oscillates before being controlled by CTL memory, because during the second phase of therapy the CTL effector response will have declined to low levels, allowing the virus to initially attain a positive growth rate. However, this does not indicate failure of the treatment regime. Furthermore, it is important to point out that the secondary phase of treatment reduces virus load to lower levels in a shorter period of time than the primary phase of treatment, because the secondary phase of treatment is associated with a rising CTL memory response that accelerates the death rate and consequently the decay rate of infected cells during therapy. This finding underlines the notion that the effect of drug treatment on virus load is enhanced by the presence of an efficient CTL response (41). Parameters were chosen as follows: λ = 1; d = 0.1; β = 0.5; a = 0.2; p = 1; c = 0.1; b = 0.01; q = 0.5; h = 0.1; s = 0.0042.
Analogous to the primary infection, the secondary phase of treatment shifts conditions in favor of CTL memory. The duration of the secondary phase of therapy is crucial for the success of this regime (Fig. 2). It depends on the length of the treatment window, the replication rate of the virus, the CTL responsiveness, the initial CD4+ T cell count, and the efficacy of the drug.
Note that after drug treatment is withdrawn, the models predict virus load to rise and oscillate before being controlled by CTL memory (Fig. 3), because the CTL effector response is short-lived and decays during drug therapy. This decay allows the virus to initially grow to a peak and oscillate before the dynamics between CTL and HIV have become more stable. A transient rise in virus load after the secondary phase of treatment therefore does not necessarily implicate failure of the treatment regime.
The principle underlying the basic drug window treatment regime is to simulate vaccination of the patient with the infecting virus, resulting in the establishment of CTL memory. Efficient CTL memory controls the virus in the long term. Modifications of this basic treatment schedule should lead to the same outcome. Especially for weak immune responders, or patients who have progressed relatively far in the disease process, repeated phases of therapy and drug windows may be necessary (Fig. 4i). In patients with an inefficient immune response, the drug window followed by the secondary phase of treatment may only partially restore CTL memory and virus load may remain relatively high. Repeating the drug window therapy schedule may result in increased levels of CTL memory, eventually controlling the virus (Fig. 4i).
Figure 4.
Modifications of the basic treatment window regime resulting in the re-establishment of CTL memory during the asymptomatic period of the infection. Again, only memory CTL responses generated in the presence of CD4 cell help are considered. Because we assume that CD4 cell help is significantly impaired, these CTL are at low levels before start of therapy. Shading indicates drug therapy. (i) Multiple drug windows. In low immune responders and in patients with advanced HIV disease, the basic drug window treatment regime may result only in partial establishment of CTL memory and failure to control the virus. Further phases of drug therapy separated by treatment windows may successively boost the CTL response, resulting in the eventual generation of efficient CTL memory and long-term control of HIV. Parameters were chosen as follows: λ = 1; d = 0.1; β = 0.5; a = 0.2; p = 1; c = 0.027; b = 0.001; q = 0.5; h = 0.1; s = 0.0042. (ii) Drug therapy in conjunction with vaccination with persisting antigen. Although virus load is kept at low levels because of antiviral therapy, the patient is vaccinated with a mixture of immunogenic HIV peptides. This boost induces the establishment of CTL memory whereas drug treatment keeps HIV-induced immune impairment to a minimum. During the generation of CTL memory, HIV load sharply drops to very low levels, because the CTL response is boosted in the absence of HIV replication, allowing the rising CTL to reduce virus load to ever decreasing values. This process theoretically could clear the infection. However, the presence of latently infected cells and reservoirs inaccessible to CTL renders this goal difficult to achieve. Thus, when drug treatment is stopped, virus load is likely to transiently increase before being controlled in the long term by CTL memory. Vaccination with a recombinant virus vector expressing HIV-specific proteins was modeled according to the basic virus infection model (38). Denoting uninfected target cells for the vector as x2, and vector-infected cells by y2, the model is given by ẋ2 = λ2− d2x2 − β2x2y2; ẏ2 = β2x2y2 − a2y2 − p2y2z. The CTL response is equally stimulated both by HIV and the vaccine. Parameters were chosen as follows: λ = 1; d = 0.1; β = 0.5; a = 0.2; p = 1; c = 0.1; b = 0.001; q = 0.5; h = 0.1; s = 0.0042; λ2 = 1; d2 = 0.1; β2 = 0.5; a2 = 0.2.
Another modification involves a combination of drug therapy and vaccination during the asymptomatic period (Fig. 4ii). Although antiretroviral drugs keep virus load at low or undetectable levels, minimizing the amount of immune impairment, the patient is vaccinated with a mixture of immunogenic HIV peptides, e.g., expressed on a recombinant virus vector. This vaccination again provides a stimulus for the expansion of CTLp, which may result in the generation of CTL memory. Once memory is established, drug therapy can be discontinued and the immune system may control HIV in the long term. This treatment schedule may be preferable, because it decreases the chances of viral resistance evolving, which may be a concern during the presence of a drug window. In addition, a combination of drug treatment and vaccination may be able to reduce virus load to extremely low levels, maximizing the chances of eradicating HIV from the patient (Fig. 4ii), because the immunological boost is given in the absence of HIV replication. The rising memory CTL response therefore may push the number of infected cells to decreasingly low values. Important for success is that the virus antigen mixture used for vaccination induces CTL clones with appropriate specificities (34). Especially advantageous would be the induction of cross-reactive immune responses (34), also including the presence of CD8+ T cells inhibiting virus replication by nonlytic mechanisms (35, 36). As before, early treatment is recommended because a patient with a low CD4+ T cell count is unlikely to re-establish CTL memory. Future research should be directed at elucidating the exact nature of CD4+ T cell help for the establishment of CTL memory. Administration of the relevant factors may greatly facilitate the development of CTL memory in HIV-infected patients.
Although we have analyzed the potential for immunological control of HIV from a modeling perspective, our findings are consistent with empirical results. In long-term nonprogressors controlling viremia in the absence of therapy, extremely low viral loads are associated with a strong CTL response (19, 20) and vigorous HIV-specific CD4+ T cell proliferative responses (13–18). Interestingly, HIV-exposed but uninfected individuals tend to show specific CTL responses detectable 34 months after the last virus exposure (21). Our predictions are also consistent with preliminary data from HIV-infected patients (37), as well as with experiments on drug treatment and rechallenge during the primary phase of simian immunodeficiency virus infection in macaques (unpublished work).
Although our models suggest that the treatment regimes described may result in long-term immunological control of the infection, this result deserves detailed empirical investigation under carefully controlled conditions. An especially important point is the potential evolution of CTL escape mutants which may result in loss of virus control (39, 40). Our models did not take into account antigenic variation and viral evolution, and this requires further detailed analysis. In particular, a comparison of the dynamics of antigenic variation and HIV-induced subversion of the immune system both under a good memory CTL response and under a less efficient response merits further mathematical investigation.
Abbreviations
- CTL
cytotoxic T lymphocyte
- CTLp
CTL precursor
Appendix
The model describing the dynamics between HIV and the CTL response was analyzed as follows. The basic reproductive ratio of the virus is given by R0 = βλ/da. It denotes the average number of newly infected cells produced by one infected cell at the beginning of the infection. If R0 > 1, the system may converge to one of two equilibria. The pathogen may replicate in the absence of an efficient, sustained CTL response, which is described by equilibrium E1:
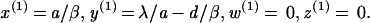 |
On the other hand, an efficient, sustained CTL response may be established, which is described by equilibrium E2:
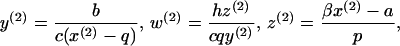 |
where x(2) is given by a solution of a quadratic equation:
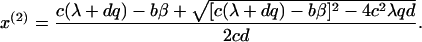 |
If cy(1)(x(1) − q) > b equilibrium E1 loses stability and the system converges to equilibrium E2, i.e., a lasting CTL response invades. If this condition is not fulfilled equilibrium E1 is stable. However, equilibrium E2 may or may not be stable depending on host and viral parameters. If equilibrium E2 is complex ([c(λ + dq) − bβ]2 < 4c2λqd) or negative (x(2) < q or βx(2) < a), a persisting CTL response can never be established. On the other hand, if equilibrium E2 is positive and real, both equilibria E1 and E2 are stable and the outcome depends on the initial conditions. A low initial number of CTLp, a high initial virus load, as well as a low initial CD4+ T cell count promote the absence of an efficient, sustained CTL response. Overall, a high replication rate of the virus as well as a low immune responsiveness of the host shift the dynamics between HIV and the immune system in favor of the virus.
References
- 1.Larder B A, Darby G, Richman D D. Science. 1989;243:1731–1734. doi: 10.1126/science.2467383. [DOI] [PubMed] [Google Scholar]
- 2.Richman D D. Trends Microbiol. 1994;2:401–407. doi: 10.1016/0966-842x(94)90619-x. [DOI] [PubMed] [Google Scholar]
- 3.Condra J H, Schleif W A, Blahy O M, Gabryelski L J, Graham D J, Quintero J C, Rhodes A, Robbins H L, Roth E, Shivaprakash M, et al. Nature (London) 1995;374:569–571. doi: 10.1038/374569a0. [DOI] [PubMed] [Google Scholar]
- 4.Frost S D, McLean A R. AIDS. 1994;8:323–332. doi: 10.1097/00002030-199403000-00005. [DOI] [PubMed] [Google Scholar]
- 5.Chun T W, Stuyver L, Mizell S B, Ehler L A, Mican J A, Baseler M, Lloyd A L, Nowak M A, Fauci A S. Proc Natl Acad Sci USA. 1997;94:13193–13197. doi: 10.1073/pnas.94.24.13193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Finzi D, Hermankova M, Pierson T, Carruth L M, Buck C, Chaisson R E, Quinn T C, Chadwick K, Margolick J, Brookmeyer R, et al. Science. 1997;278:1295–1300. doi: 10.1126/science.278.5341.1295. [DOI] [PubMed] [Google Scholar]
- 7.Perelson A S, Essunger P, Ho D D. AIDS. 1997;11:S17–S24. [PubMed] [Google Scholar]
- 8.Wong J K, Hezareh M, Gunthard H F, Havlir D V, Ignacio C C, Spina C A, Richman D D. Science. 1997;278:1291–1295. doi: 10.1126/science.278.5341.1291. [DOI] [PubMed] [Google Scholar]
- 9.McMichael A J, Phillips R E. Annu Rev Immunol. 1997;15:271–296. doi: 10.1146/annurev.immunol.15.1.271. [DOI] [PubMed] [Google Scholar]
- 10.Ogg G S, Jin X, Bonhoeffer S, Dunbar P R, Nowak M A, Monard S, Segal J P, Cao Y, Rowland-Jones S L, Cerundolo V, et al. Science. 1998;279:2103–2106. doi: 10.1126/science.279.5359.2103. [DOI] [PubMed] [Google Scholar]
- 11.Saah A J, Hoover D R, Weng S, Carrington M, Mellors J, Rinaldo C R, Jr, Mann D, Apple R, Phair J P, Detels R, et al. AIDS. 1998;12:2107–2113. doi: 10.1097/00002030-199816000-00005. [DOI] [PubMed] [Google Scholar]
- 12.Jeffery K J, Usuku K, Hall S E, Matsumoto W, Taylor G P, Procter J, Bunce M, Ogg G S, Welsh K I, Weber J N, et al. Proc Natl Acad Sci USA. 1999;96:3848–3853. doi: 10.1073/pnas.96.7.3848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rosenberg E S, Billingsley J M, Caliendo A M, Boswell S L, Sax P E, Kalams S A, Walker B D. Science. 1997;278:1447–1450. doi: 10.1126/science.278.5342.1447. [DOI] [PubMed] [Google Scholar]
- 14.Rosenberg E S, Walker B D. AIDS Res Hum Retroviruses. 1998;14, Suppl. 2:S143–S147. [PubMed] [Google Scholar]
- 15.Rosenberg E S, LaRosa L, Flynn T, Robbins G, Walker B D. Immunol Lett. 1999;66:89–93. doi: 10.1016/s0165-2478(98)00165-5. [DOI] [PubMed] [Google Scholar]
- 16.Kalams S A, Walker B D. J Exp Med. 1998;188:2199–2204. doi: 10.1084/jem.188.12.2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kalams S A, Buchbinder S P, Rosenberg E S, Billingsley J M, Colbert D S, Jones N G, Shea A K, Trocha A K, Walker B D. J Virol. 1999;73:6715–6720. doi: 10.1128/jvi.73.8.6715-6720.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brander C, Walker B D. Curr Opin Immunol. 1999;11:451–459. doi: 10.1016/S0952-7915(99)80076-4. [DOI] [PubMed] [Google Scholar]
- 19.Harrer T, Harrer E, Kalams S A, Barbosa P, Trocha A, Johnson R P, Elbeik T, Feinberg M B, Buchbinder S P, Walker B D. J Immunol. 1996;156:2616–2623. [PubMed] [Google Scholar]
- 20.Harrer T, Harrer E, Kalams S A, Elbeik T, Staprans S I, Feinberg M B, Cao Y, Ho D D, Yilma T, Caliendo A M, et al. AIDS Res Hum Retroviruses. 1996;12:585–592. doi: 10.1089/aid.1996.12.585. [DOI] [PubMed] [Google Scholar]
- 21.Bernard N F, Yannakis C M, Lee J S, Tsoukas C M. J Infect Dis. 1999;179:538–547. doi: 10.1086/314621. [DOI] [PubMed] [Google Scholar]
- 22.Schmitz J E, Kuroda M J, Santra S, Sasseville V G, Simon M A, Lifton M A, Racz P, Tenner-Racz K, Dalesandro M, Scallon B J, et al. Science. 1999;283:857–860. doi: 10.1126/science.283.5403.857. [DOI] [PubMed] [Google Scholar]
- 23.Borrow P, Tishon A, Lee S, Xu J, Grewal I S, Oldstone M B, Flavell R A. J Exp Med. 1996;183:2129–2142. doi: 10.1084/jem.183.5.2129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Borrow P, Tough D F, Eto D, Tishon A, Grewal I S, Sprent J, Flavell R A, Oldstone M B. J Virol. 1998;72:7440–7449. doi: 10.1128/jvi.72.9.7440-7449.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thomsen A R, Johansen J, Marker O, Christensen J P. J Immunol. 1996;157:3074–3080. [PubMed] [Google Scholar]
- 26.Thomsen A R, Nansen A, Christensen J P, Andreasen S O, Marker O. J Immunol. 1998;161:4583–4590. [PubMed] [Google Scholar]
- 27.Ehl S, Klenerman P, Aichele P, Hengartner H, Zinkernagel R M. Eur J Immunol. 1997;27:3404–3413. doi: 10.1002/eji.1830271240. [DOI] [PubMed] [Google Scholar]
- 28.Bachmann M F, Kundig T M, Hengartner H, Zinkernagel R M. Proc Natl Acad Sci USA. 1997;94:640–645. doi: 10.1073/pnas.94.2.640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kundig T M, Bachmann M F, Ohashi P S, Pircher H, Hengartner H, Zinkernagel R M. Immunol Rev. 1996;150:63–90. doi: 10.1111/j.1600-065x.1996.tb00696.x. [DOI] [PubMed] [Google Scholar]
- 30.Kundig T M, Bachmann M F, Oehen S, Hoffmann U W, Simard J J, Kalberer C P, Pircher H, Ohashi P S, Hengartner H, Zinkernagel R M. Proc Natl Acad Sci USA. 1996;93:9716–9723. doi: 10.1073/pnas.93.18.9716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zinkernagel R M, Bachmann M F, Kundig T M, Oehen S, Pirchet H, Hengartner H. Annu Rev Immunol. 1996;14:333–367. doi: 10.1146/annurev.immunol.14.1.333. [DOI] [PubMed] [Google Scholar]
- 32.Gray C M, Lawrence J, Schapiro J M, Altman J D, Winters M A, Crompton M, Loi M, Kundu S K, Davis M M, Merigan T C. J Immunol. 1999;162:1780–1788. [PubMed] [Google Scholar]
- 33.Kalams S A, Goulder P J, Shea A K, Jones N G, Trocha A K, Ogg G S, Walker B D. J Virol. 1999;73:6721–6728. doi: 10.1128/jvi.73.8.6721-6728.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Letvin N L. Science. 1998;280:1875–1880. doi: 10.1126/science.280.5371.1875. [DOI] [PubMed] [Google Scholar]
- 35.Cocchi F, DeVico A L, Garzino-Demo A, Arya S K, Gallo R C, Lusso P. Science. 1995;270:1811–1815. doi: 10.1126/science.270.5243.1811. [DOI] [PubMed] [Google Scholar]
- 36.Levy J A, Mackewicz C E, Barker E. Immunol Today. 1996;17:217–224. doi: 10.1016/0167-5699(96)10011-6. [DOI] [PubMed] [Google Scholar]
- 37.Lisziewicz J, Rosenberg E, Lieberman J, Jessen H, Lopalco L, Siliciano R, Walker B, Lori F. N Engl J Med. 1999;340:1683–1684. doi: 10.1056/NEJM199905273402114. [DOI] [PubMed] [Google Scholar]
- 38.Nowak M A, Bangham C R. Science. 1996;272:74–79. doi: 10.1126/science.272.5258.74. [DOI] [PubMed] [Google Scholar]
- 39.Nowak M A, Anderson R M, McLean A R, Wolfs T F W, Goudsmit J, May R M. Science. 1991;254:963–969. doi: 10.1126/science.1683006. [DOI] [PubMed] [Google Scholar]
- 40.Nowak M A, May R M, Phillips R E, Rowlandjones S, Lalloo D G, McAdam S, Klenerman P, Koppe B, Sigmund K, Bangham C R M, McMichael A J. Nature (London) 1995;375:606–611. doi: 10.1038/375606a0. [DOI] [PubMed] [Google Scholar]
- 41.Bonhoeffer S, May R M, Shaw G M, Nowak M A. Proc Natl Acad Sci USA. 1997;94:6971–6976. doi: 10.1073/pnas.94.13.6971. [DOI] [PMC free article] [PubMed] [Google Scholar]