Abstract
Microsporidia are obligate intracellular pathogens which enter host cells by the discharge of a hollow tube through which the sporoplasma is extruded into the host cell. Since this invasion mechanism is very different from common entry strategies, the formation of the parasitophorous vacuole (PV) in Encephalitozoon species is likely to be distinct from known principles. We investigated the origin of the nascent Encephalitozoon cuniculi PV membrane with the aid of fluorescent lipid probes. When Bodipy 500/510-C12-HPC-labeled spores were used for infection, the emerging PV membrane was unlabeled, suggesting that sporoplasma-derived lipids do not significantly contribute to the formation of the PV membrane. In contrast, when raft and nonraft microdomains of the host cell plasma membrane were selectively labeled with DiIC16 and Speedy DiO, both tracers were detectable in the nascent PV membrane shortly after infection, indicating that the bulk lipids of the PV membrane are host cell derived. Time-lapse fluorescence microscopy revealed that the formation of the PV membrane is a fast event (<1.3 s), which occurred simultaneously with the extrusion of the sporoplasma. The portion of the discharged tube which is in contact with the host cell was found to be coated with labeled host cell lipids, which might be an indication for a plasma membrane invagination at the contact site. To investigate the presence of pores in the E. cuniculi PV membrane, we microinjected fluorescent dyes of different sizes into infected host cells. A 0.5-kDa dextran as well as 0.8- to 1.1-kDa peptides could rapidly enter the PV, while a 10-kDa dextran was stably excluded from the PV lumen, indicating that the PV membrane possesses pores with an exclusion size of <10 kDa, which should allow metabolite exchange.
Microsporidia are obligate intracellular microorganisms which are early branched fungi as revealed by comparative genome analysis (11, 12, 14). They were recognized in the past years as emerging pathogens, particularly for immunocompromised patients. Three out of the four most common human microsporidian pathogens belong to the genus Encephalitozoon, namely, E. cuniculi, E. hellem, and E. intestinalis. While most microsporidia replicate inside the host cell cytosol, the members of this genus reside inside a membrane-bound vacuolar compartment termed the parasitophorous vacuole (PV). E. cuniculi has become a model organism for this genus, since it can be easily cultured in a variety of host cells and its genome is completely sequenced. The E. cuniculi genome is, at 2.9 Mb, the smallest eukaryotic genome sequenced so far and displays a high degree of compactness and an extreme loss of biosynthetic pathways (13, 15).
The intracellular life cycle of E. cuniculi includes the replication of meronts and the subsequent differentiation into sporonts and spores, which are released from the dying host cell (5). The infectious stage is the spore, which contains a unique invasion apparatus to infect host cells. Inside the spore, a long, coiled hollow tubulus, the so-called polar filament, is present, which is explosively extruded due to a sudden rise in osmotic pressure. The sporoplasma, which also contains the nucleus, subsequently is pressed through the polar filament. If the tip of the discharged polar filament has penetrated the plasma membrane of a host cell before, the sporoplasma is directly injected into the host cell cytosol. Shortly after invasion, the meronts are localized inside a PV of unknown origin.
Ultrastructural analysis revealed that the plasma membrane of meronts is closely applied to the PV membrane (2, 5). At a later stage of development, during sporogony, the plasma membrane becomes detached from the PV membrane and the final maturation into spores takes place in nonperipheral areas of the vacuole. The periphery of the E. cuniculi PV is, even at later stages of development, still outlined with a single layer of meronts, as shown by ultrastructural analysis and by immunostaining with meront- and spore-specific monoclonal antibodies (MAbs) (9, 28). The meront stage thus appears to be closely associated with a location near the PV membrane.
The PV membrane forms the interface between the pathogen and the host cell and is thus crucial for any interaction, particularly for transport processes and for metabolite exchange. It has been shown that the PV membrane is tightly associated with host cell mitochondria, which might be an indication that mitochondrion-derived metabolites are preferentially imported into the PV (22). Ultrastructural studies revealed that the PV membrane contains smaller blebs on the cytosolic site and larger protrusions on the luminal site of unknown function (28, 29).
It has been shown that the PV is not part of the host cell's endocytotic network. In earlier ultrastructural analysis of E. cuniculi-infected macrophages, an absence of fusion events between the PV and host cell lysosomes was described (29). Recent immunolocalization studies on E. cuniculi-infected host cells revealed that endosomal and lysosomal marker proteins are absent from the PV membrane throughout the entire intracellular life cycle (9). Furthermore, the PV membrane is also lacking transmembrane marker proteins of the host cell plasma membrane immediately after invasion (9). This raises the question whether the PV membrane is of host cell origin or whether microsporidian-derived phospholipids assemble shortly after injection of the sporoplasma and form the PV membrane.
Based on experiments with fluorescent tracers, we show here that the lipids of the nascent E. cuniculi PV membrane are of host cell and not of parasite origin and that the formation occurs as a coentry process. Furthermore, we demonstrate that the PV membrane contains pores with an exclusion size of <10 kDa, which should allow a metabolite exchange between the host cell cytosol and the PV lumen.
MATERIALS AND METHODS
Cell culture and cultivation of E. cuniculi spores.
E. cuniculi spores were routinely propagated in human foreskin fibroblasts (HFF) as host cells. Adherent cells were maintained in Dulbecco's modified Eagle medium (DMEM) supplemented with 10% fetal calf serum and 1% penicillin-streptomycin. After 5 to 7 days of infection, the host cell monolayer was scraped off and spores were released from vacuoles by passage through a 26-gauge needle in order to disrupt the host cells. Host cell debris was removed by centrifugation at 400 rpm. After a second centrifugation step at 2,500 rpm, the spore pellet was resuspended in phosphate-buffered saline (PBS) at 5 × 107 spores/ml. This spore solution was used to infect new monolayers. Baby hamster kidney (BHK) cells were cultured in DMEM containing 3% fetal calf serum, 10% tryptose phosphate broth, 1% glutamine, and 0.6% penicillin-streptomycin.
Fluorescence labeling of internal E. cuniculi lipids.
Stock solutions of DiIC16/Speedy DiO (MoBiTec) and Bodipy 500/510 C12-HPC (Molecular Probes) were prepared at 1 mM and 10 mM in ethanol, respectively. For labeling of internal membranes, E. cuniculi was cultivated in the presence of the lipid probes. The medium of infected HFF was changed against DMEM containing the lipid probes at 48 h postinfection. DiIC16 labeling was achieved by adding 4 μM DiIC16 to the medium. For Bodipy 500/510 C12-HPC labeling, the medium was supplemented at a final concentration of 100 μM with this compound. After an additional 72 h of infection in the presence of the lipid probes, spores were isolated at 120 h postinfection as described before. To remove extracellular dye, spores were washed with PBS three times. A total of 1.25 × 106 spores were used to infect BHK cells on coverslips.
DiIC16/Speedy DiO labeling of BHK cells.
BHK cells were labeled with DiIC16 and Speedy DiO as described before (6). Briefly, BHK cells on coverslips were rinsed twice for 5 min each with medium 1 (150 mM NaCl, 5 mM KCl, 1 mM CaCl2, 1 mM MgCl2, 20 mM HEPES [pH 7.4], 2 g/liter glucose) (20). Labeling was done at 8°C for 3 min in the dark with 2 μM DiIC16 or Speedy DiO, diluted in 10 mM HEPES, 1 mM CaCl2, and 300 mM sorbitol at pH 7.4. Afterwards, cells were washed briefly in medium 1 and immediately processed for infection or extraction experiments. For infection, freshly harvested spores were added to the coverslips at room temperature. The tissue culture plates were briefly centrifuged at 2,000 rpm to allow a fast and simultaneous invasion. To analyze very early time points after the infection, incubation was stopped after 2 to 4 min by fixing the samples with 4% paraformaldehyde in PBS. For inhibition of a possible phagocytosis of the sporoplasma, cytochalasin D (Sigma) was added at 5 μM to all buffers and solutions used throughout the experiment.
Immunolabeling.
The cells were fixed with 4% paraformaldehyde in PBS for 15 min. Permeabilization and blocking were done by incubation in PBS containing 0.3 mg/ml saponin and 1% bovine serum albumin for 1 h. Samples were then incubated sequentially with the meront-specific MAb 6G2 (9) and a Cy2- or Cy3-conjugated anti-mouse immunoglobulin G (Dianova) diluted 1:150 or 1:250 in PBS-saponin-bovine serum albumin, respectively. Coverslips were finally mounted with nail polish to avoid any interference of glycerol, which is part of many mounting media, with the lipid probes. Microscopic analysis was done with either a Zeiss Axiovert 200 M wide-field fluorescence microscope or a confocal laser scanning Leica TCS SP2 microscope.
Detergent extraction.
Detergent extraction was used to ensure the specificity of the lipid probes toward raft and nonraft microdomains. Following the labeling procedure with DiIC16/Speedy DiO, cells were washed briefly with ice-cold PBS containing 1 mM MgCl2 and 1 mM CaCl2 (PBS+) and then incubated for 30 min with prechilled PBS+ (mock extraction) or PBS+ containing 1% (vol/vol) Triton X-100 on ice (6). The cells were rinsed gently, fixed with prechilled 4% paraformaldehyde in PBS, and mounted with nail polish as described above.
Microinjection.
HFF cells were seeded into petri dishes with thin bottoms for inverted microscopy (Ibidi, catalog no. 80136) and infected with a fresh spore solution. Before microinjection, the medium in the dish was replaced by fresh medium containing 20 mM HEPES (pH 7.4) without phenol red. Microinjection was performed with the micromanipulator 5171 and the transjector 5246 (Eppendorf) attached to a Zeiss Axiovert 200 M time-lapse microscope. The three dyes tetramethylrhodamine-dextran (10,000 molecular weight; 9 to 11 kDa; neutral), Alexa Fluor 488 dextran (3,000 molecular weight; 1.5 to 3 kDa; anionic), and Alexa Fluor 488 hydrazide (0.5 kDa; anionic) (Molecular Probes) were prepared as aqueous 10-mg/ml stock solutions. The 5-carboxyfluorescein (5-FAM)-conjugated peptides RSR (charge, +2) and SGALDVLQ (charge, −1) were obtained from JPT Peptide Technologies. For microinjection, the dyes were diluted with H2O to a final concentration of 0.5 mg/ml, and the dextran conjugate solutions were extracted two times with 1-butanol to remove free dye molecules that were no longer bound to the water-soluble dextran particles. To avoid any clogging effects in the microinjection needle, the dye solutions were centrifuged for 10 min at maximum speed immediately before loading of the capillary. Microinjection needles with an inner diameter of 0.5 μM (Femtotips I; Eppendorf) were filled with the injection solutions with the aid of microloaders (Eppendorf). Individual fibroblasts with one or more PVs were identified by phase-contrast microscopy with a 63× objective, and the fluorophores were then microinjected into the host cell cytoplasm. Dye redistribution across the PV membrane was observed for up to 20 min.
Time-lapse microscopy.
Time-lapse series were taken with an AxioCam MRm camera, attached to a Zeiss Axiovert 200 M microscope, which was controlled by the Axiovision 4.6.3 software. To achieve optimal invasion conditions, the temperature was adjusted to 37°C with an XL-3 incubator in combination with a heating unit (PeCon). The optimal pH was maintained by CO2 supply using a CO2 controller (PeCon).
RESULTS
Fluorescence labeling of internal E. cuniculi lipids.
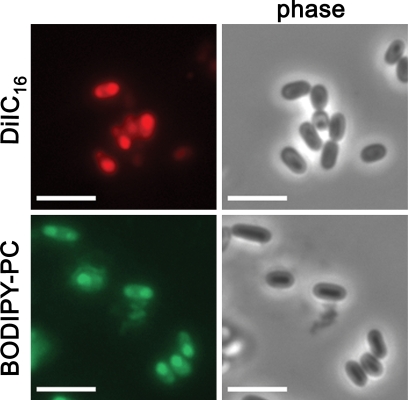
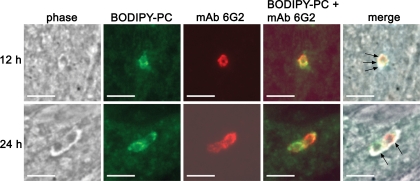
We investigated the origin of the nascent E. cuniculi PV membrane with the aid of fluorescent lipid probes. These probes were used to label either host cell or E. cuniculi lipids, and potential incorporation of the tracers into the PV membrane was monitored by fluorescence microscopy. In order to establish an approach that generates E. cuniculi spores with fluorescent internal membrane compartments, we added the fluorescent lipid derivative DiIC16, Speedy DiO, or Bodipy 500/510 C12-HPC (Bodipy-PC) to the medium of E. cuniculi cultures and investigated whether these dyes became incorporated into developing E. cuniculi spores. The fluorescent probes were supplemented once to the culture medium at 48 h postinfection at concentrations ranging from 4 to 100 μM. Spores were released from their host cells by mechanical force at 120 h postinfection, and fixed extracellular spores were analyzed by fluorescence microscopy. All three fluorescent compounds were found to be incorporated into the mature spore stage. Speedy DiO treatment resulted in a weak fluorescence of spores and was thus not further used for the ongoing experiments. DiIC16 and Bodipy-PC labeling resulted in a heterogeneous staining pattern of the spore, in which internal structures appeared to be labeled (Fig. 1). While Bodipy-PC treatment resulted in the labeling of all spores, a fraction of spores was unstained after DiIC16 treatment. When these spores were used to infect a new host cell monolayer, the meronts, which are derived from DiIC16-labeled spores, did not show any fluorescence. In contrast, we obtained fluorescent meronts with Bodipy-PC-labeled spores, indicating that this lipid can be used to trace the distribution and the fate of sporoplasma membrane compounds which are injected during infection into the host cell (Fig. 2). In order to determine whether lipids from the injected sporoplasma contribute to the formation of the nascent PV membrane, we followed the fate of Bodipy-PC from 1 h up to 24 h postinfection. We confirmed in these experiments the identity of intracellular meronts, by immunostaining with the meront-specific MAb 6G2 (9). The Bodipy-PC fluorescence was restricted at all analyzed time points to the meront itself, while the membrane and the matrix of the PV were completely unlabeled (Fig. 2). Our data thus suggest that the majority of phospholipids which form the PV membrane are not derived from the injected sporoplasma itself. It was not possible to follow the fluorescent tracer for longer than 24 h postinfection, most likely due to the dilution of the signal by meront replication. The Bodipy-PC-labeled spores displayed an infection rate that was reduced to 15% to that of untreated controls. However, once the infection was established, Bodipy-PC-labeled meronts showed an unaltered replication rate and differentiated normally into sporonts and spores (data not shown).
FIG. 1.
Incorporation of the fluorescent lipid probes DiIC16 and Bodipy 500/510 C12-HPC into mature E. cuniculi spores. The culture medium of E. cuniculi-infected HFF was supplemented with either 4 μM DiIC16 or 100 μM Bodipy 500/510 C12-HPC at 48 h postinfection. Spores were released from their host cells by mechanical force at 120 h postinfection, fixed on glass slides, and analyzed by fluorescence microscopy. Both lipid probes were incorporated into mature spores that developed during the incubation time. Bars, 5 μm.
FIG. 2.
Lipids of the injected sporoplasm do not contribute to the nascent PV membrane. Bodipy 500/510 C12-HPC-labeled spores were used to infect an HFF monolayer on coverslips. Cells were fixed at 12 h and 24 h postinfection, and meronts were detected by indirect immunofluorescence staining with the meront-specific MAb 6G2 and a Cy3-conjugated anti-mouse immunoglobulin G. The Bodipy-PC fluorescence (green) colocalizes with the antibody signal (red) but is restricted to the meront itself and is not incorporated into the PV membrane. Bars, 5 μm.
The nascent PV membrane of E. cuniculi is formed by the host cell.
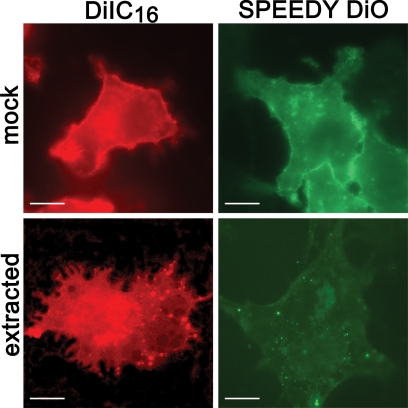
The last experiment indirectly suggests that the majority of phospholipids which form the nascent PV membrane are host cell derived. To gain positive experimental support for this hypothesis, we created host cells with fluorescently labeled membranes and traced the signal after infection with E. cuniculi. Two different fluorescent lipid probes were used in order to discriminate between lipid raft and nonraft microdomains. DiIC16 is a compound that is specifically integrated into packed, rigid (raft) membrane subdomains, while Speedy DiO integrates into disordered, fluid (nonraft) membrane subdomains (6, 19, 24, 27). To confirm the specificity of these lipid probes for raft and nonraft microdomains in our experimental setup, we applied cold Triton X-100 extraction after labeling of BHK cells with these compounds (Fig. 3). Speedy DiO was accessible to detergent extraction, which is typical for the integration into nonraft membrane subdomains. In contrast, Triton X-100 extraction in DiIC16-labeled cells resulted in a “Swiss cheese” pattern, which is characteristic for an association with lipid rafts after extraction of the nonraft subdomains (10).
FIG. 3.
Differential susceptibility of DiIC16 and Speedy DiO to cold Triton X-100 extraction. Live BHK cells were stained with either DiIC16 or Speedy DiO and subjected to extraction with cold PBS+ (mock) or cold PBS+ containing 1% Triton X-100 (extracted). Images were captured after fixation with paraformaldehyde. Speedy DiO-labeled membrane domains were susceptible to detergent extraction, which demonstrates the specificity of Speedy DiO for nonraft domains. In contrast, lipid rafts, which can be labeled by DiIC16, are detergent resistant. This results in a “Swiss cheese” phenotype due to extraction of nonraft microdomains (DiIC16 panel). Bars, 10 μm.
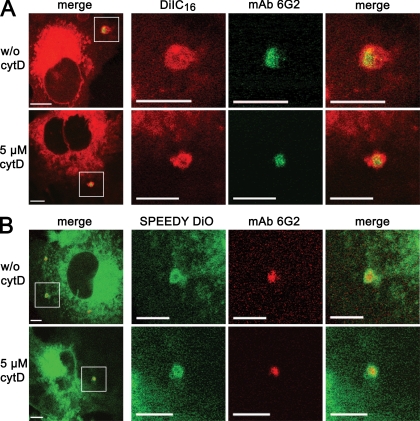
To investigate the fate of DiIC16 and Speedy DiO during infection, BHK cells were labeled with these tracers for 3 min at 8°C, infected with E. cuniculi spores, and fixed after a 2- to 4-min incubation period at room temperature. Intracellular meronts were detected by immunostaining with the meront-specific MAb 6G2. Confocal immunofluorescence microscopy revealed that meronts were surrounded by a fluorescent rim, which was derived from the lipid probes (Fig. 4). This strongly suggests that host cell lipids contribute to the nascent E. cuniculi PV membrane. Control experiments, in which E. cuniculi spores were allowed to infect BHK cells 1 h before the addition of DiIC16 and Speedy DiO, revealed that meronts were not surrounded by a fluorescent rim (data not shown). This excludes the possibility that the fluorescent tracers are incorporated in an unspecific postentry event after the formation of the vacuole. Furthermore, we used cytochalasin D treatment of BHK cells to exclude the possibility that sporoplasm internalized by phagocytosis contributes to the staining pattern. BHK cells were pretreated for 10 min with 5 μM cytochalasin D and cytochalasin D treatment was continued throughout the whole experiment in order to inhibit actin-mediated phagocytosis. Cytochalasin-D-treated cells displayed the same DiIC16- or Speedy DiO-derived fluorescent rim around internalized meronts as untreated cells, demonstrating that the internalized sporoplasm is indeed derived from the natural infection (Fig. 4).
FIG. 4.
The nascent E. cuniculi PV membrane is formed by host cell lipids. BHK cells were labeled either with DiIC16 (A) for raft domain staining or with Speedy DiO (B) for nonraft domain membrane staining. The cells were infected with spores immediately after the staining procedure in the presence or absence of the phagocytosis inhibitor cytochalasin D (cytD). Samples were fixed at 3 min postinfection and stained with the meront-specific MAb 6G2 and a Cy2- or Cy3-conjugated secondary antibody. Confocal imaging was applied for sample analysis. All left panels (overview) show a low-power micrograph of an infected cell. The following three columns show the area of the indicated square in a higher magnification (bars, 5 μm). The meronts were surrounded immediately after infection by a rim of DiIC16 or Speedy DiO fluorescence, suggesting that both raft and nonraft host cell membrane domains contribute to the nascent PV membrane. Note that the fluorescent rim emerges independently of the cytochalasin D treatment.
The described experiments clearly indicate that host cell raft and nonraft lipid microdomains both contribute to the nascent PV membrane. However, it was not possible to accurately discriminate whether the PV membrane lipids were derived from the host cell plasma membrane or from internal host cell membrane compartments, since the fluorescent probes were partly internalized and became integrated into internal host cell membrane systems during the time course of the experiment. Furthermore, it was not possible to determine from fixed samples whether PV membrane formation occurred as a coentry event or whether the sporoplasm is first injected into the cytosol and vacuole formation takes place as a postentry event in the first minutes after invasion.
The PV membrane is immediately formed during entry of the sporoplasm.
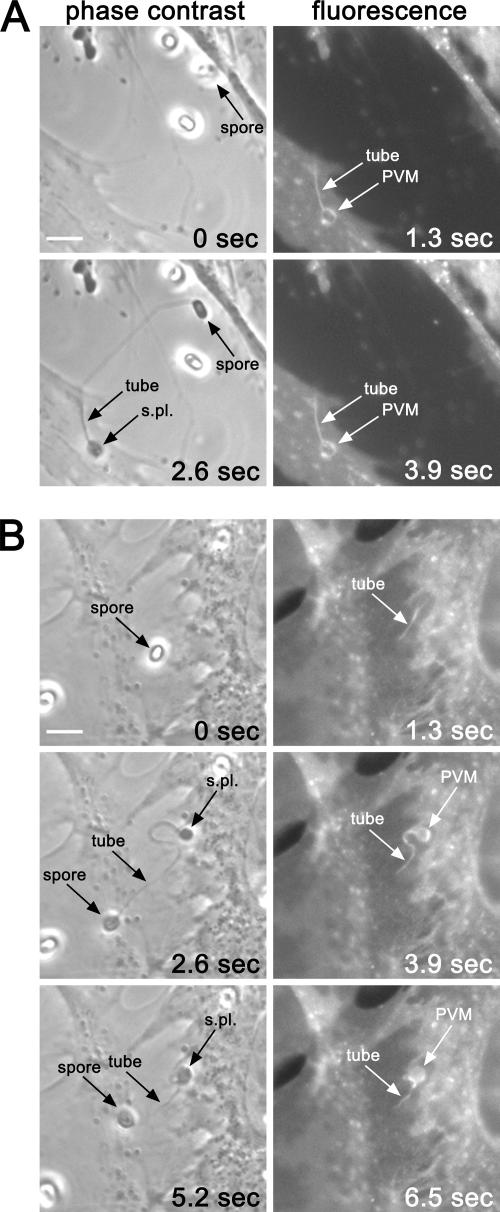
In order to distinguish a coentry from a postentry formation, we followed the kinetics of PV membrane formation on viable, DiIC16-labeled human fibroblasts by time-lapse fluorescence microscopy. Phase-contrast and fluorescence images were consecutively taken at intervals of 1.3 s (Fig. 5). A discharge event for a spore was identified from subsequently taken phase-contrast images by a change of the spore contrast from a brighter appearance before extrusion to a darker appearance of the empty spore shell after extrusion (Fig. 5A and 5B, compare 0 s with 2.6 s). The fluorescence images (Fig. 5A, 1.3 s and 3.9 s) show the immediate appearance of the fluorescently labeled PV membrane after extrusion, demonstrating that the formation of this vacuole is a very fast process, which is completed in less than 1.3 s. Surprisingly, the distal portion of the polar tube, which interacts with the host cell, was consistently labeled with the fluorescent DiIC16 probe (Fig. 5A, 1.3 s and 3.9 s). This suggests that the portion of the polar tube which is in contact with the host cell is coated with host cell lipids. Figure 5B shows that this coating occurs very fast after extrusion, even before the sporoplasm is completely ejected from the polar tube (Fig. 5B, 1.3 s).
FIG. 5.
Invasion followed by time-lapse fluorescence microscopy. HFF cells with DiIC16-labeled plasma membranes were infected with E. cuniculi spores. Phase-contrast and fluorescence images were consecutively taken at intervals of 1.3 s by time-lapse microscopy. Arrows in phase-contrast images indicate spores before (0 s) and after (2.6 and 5.2 s) discharge of the polar filament. The polar filament (tube) and the extruded sporoplasma (S.pl.) are also indicated. Arrows in fluorescence images indicate the fluorescent parts of the tube and of the nascent PV membrane (PVM). (A) The PV membrane is formed immediately after discharge of the tube (1.3 s). Additionally, the distal portion of the tube, which makes contact with the host cell, becomes labeled (1.3 s and 3.9 s). (B) Coating of the tube with fluorescent lipids is very fast even before the extrusion of the sporoplasm is complete (1.3 s). Bars, 5 μm.
The E. cuniculi PV membrane contains pores.
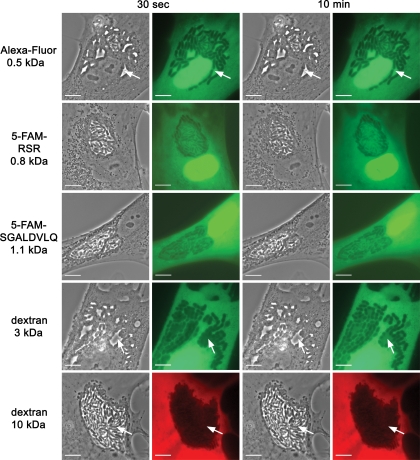
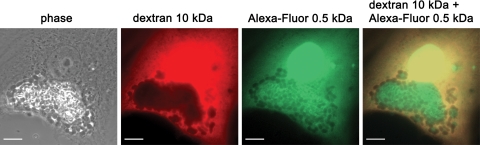
Microinjection of membrane-impermeable fluorescent dyes into infected host cells is a suitable approach to test for the presence of pores in PVs of pathogens (1, 25, 30). We used tetramethylrhodamine-conjugated dextrans of 10 kDa, Alexa Fluor 488-conjugated dextrans of 3 kDa, and Alexa Fluor 488 hydrazide (0.5 kDa) for microinjection into E. cuniculi-infected HFF. The dyes were microinjected into the cytosol of PV-containing HFF, which were infected 36 h before. The 10-kDa dextran was stably excluded from the lumen of the PV (Fig. 6). In contrast, after the injection of the 0.5-kDa Alexa Fluor 488 hydrazide into the host cell cytosol, individual microsporidia inside the PV were immediately surrounded by a bright fluorescence, indicating that this dye has entered the lumen of the PV (Fig. 6). In addition, two peptides which were conjugated with the fluorescent dye 5-FAM were also used for microinjection. The positively charged peptide 5-FAM-RSR (0.8 kDa) and the negatively charged peptide 5-FAM-SGALDVLQ (1.1 kDa), like Alexa Fluor 488, rapidly entered the PV lumen (Fig. 6). Microinjection of a 3-kDa Alexa Fluor 488-conjugated dextran displayed an intermediate phenotype. The dye was excluded 30 s after injection from parts of the PV lumen; however, this exclusion was not stable as for the 10-kDa dextran, but the 3-kDa dextran slowly entered all regions of the PV lumen within 10 min. We also injected a mixture composed of the 10-kDa dextran and the 0.5-kDa Alexa Fluor 488 hydrazide simultaneously into E. cuniculi-infected host cells. This experiment confirmed that only the smaller Alexa Fluor 488 hydrazide and not the larger 10-kDa dextran can enter the lumen of PV (Fig. 7). To examine whether pore expression changes during intracellular E. cuniculi development, PV-containing host cells were injected with the fluorescent dyes at different time points from 24 to 96 h postinfection. We found that the exclusion size of the PV membrane was independent of the vacuole size and remained unchanged in the investigated time frame. Together, these data provide evidence that the PV membrane contains pores with an exclusion size of <10 kDa.
FIG. 6.
Size-related entry of fluorescent probes into the PV after cytoplasmic microinjection. Alexa Fluor 488 hydrazide (0.5 kDa), fluorescently labeled peptides (5-FAM-RSR [0.8 kDa] and 5-FAM-SGALDVLQ [1.1 kDa]), an Alexa Fluor 488-labeled dextran molecule (3 kDa), and a tetramethylrhodamine-dextran molecule (10 kDa) were microinjected into the cytosol of E. cuniculi-infected HFF. Phase-contrast and corresponding fluorescence images of vacuole-containing fibroblasts are shown at 30 s and 10 min after microinjection. The 0.5-kDa Alexa Fluor molecule as well as the fluorescent peptides of 0.8 and 1.1 kDa entered the vacuole quickly after injection (30-s panels), resulting in a silhouetting of the parasites inside the vacuole. The arrows show dye-filled free space inside the vacuole. The equilibration of the 3-kDa molecule across the PV membrane shows a delayed phenotype. The dye is partly excluded from the vacuole at 30 s after injection (arrows), but parasites are completely silhouetted after 10 min. The 10-kDa dextran is stably excluded from the vacuole (30-s and 10-min panels). Bars, 10 μm.
FIG. 7.
Simultaneous microinjection of a 0.5-kDa fluorescent dye and a 10-kDa fluorescent dye within the same E. cuniculi-infected host cell. Phase-contrast and corresponding fluorescence images of an infected HFF cell at 30 s after microinjection of a mixture of the 10-kDa tetramethylrhodamine-conjugated dextran and the Alexa Fluor 488 hydrazide (0.5-kDa) molecule are shown. The 10-kDa dextran molecule is excluded from the vacuole, while the 0.5-kDa molecule rapidly enters the vacuole. Bars, 10 μm.
DISCUSSION
Based on the incorporation of fluorescent lipid probes, we provide in this study evidence that the phospholipids of the nascent E. cuniculi PV membrane are of host cell and not of parasite origin. The term PV has long been used to designate the vacuolar compartments of Encephalitozoon species and implies by definition a vacuole, which is host cell derived (3, 4, 5). The morphology of the Encephalitozoon PV was thoroughly studied by ultrastructural analysis in previous studies (2, 21, 26); however, to our knowledge, direct evidence for the origin of the vacuolar membrane had not been provided so far. This report thus describes for the first time an experimental design that allows a clear distinction as to whether the vacuolar membrane is host cell or pathogen derived.
The fluorescent lipid derivatives DiIC16 and Speedy DiO were used to selectively label either raft or nonraft host cell membrane microdomains. Both tracers were detectable in the nascent PV membrane shortly after infection (3 min), suggesting that both host cell raft and nonraft lipid microdomains contribute to the formation of the PV membrane. The reverse experiment was performed with spores which had incorporated the fluorescent lipid probe Bodipy 500/510 C12-HPC in their sporoplasma. No fluorescence signal was obtained in the nascent PV membrane, when these spores were used to infect unlabeled host cells. Under the assumption that the tracer Bodipy-PC was not selectively excluded from certain lipid structures inside the sporoplasma, this indicates that the majority of the nascent PV membrane phospholipids are unlikely to be derived from sporoplasma-derived lipids. Although the lipids for the nascent PV membrane appear to be exclusively host cell derived, it is conceivable that the phospholipids which are used for the enlargement of the vacuole at later stages of the development are at least in part pathogen derived. E. cuniculi possesses several phospholipid-modifying enzymes involved in polar head group synthesis (8); however, it lacks the type I or type II FAS complex and thus needs to import bulk lipids from the host cell.
An intriguing question is the exact mechanism which leads to the formation of the PV membrane. The formation of PVs in other intracellular pathogens is closely linked to the invasion mechanism. Bacteria enter host cells by phagocytosis, by induced phagocytosis, or via clustered lipid rafts (7, 31). Apicomplexan parasites enter host cells by a motility-based invasion mechanism (18). The resulting PV membrane is in all cases derived from the plasma membrane of the host cell. The injection-based invasion mechanism of microsporidia is, however, fundamentally different from phagocytosis and motility-based invasion. We could demonstrate by time-lapse fluorescence microscopy in conjunction with fluorescently labeled host cell plasma membranes that the formation of the PV membrane is a fast process, which takes less than 1.3 s and occurs simultaneously with the extrusion of the sporoplasm. The time-lapse images also revealed that immediately after discharge of the polar filament, the portion of the tube which interacts with the host cell becomes coated with host cell lipids and that this coating occurred even before the sporoplasma was extruded from the tube.
A hypothetical explanation for this staining pattern is that after discharge of the polar filament, the tip of the tube is not penetrating the host cell membrane but instead forces the host cell plasma membrane to form a long, channel-like invagination. The sporoplasma is then extruded into this plasma membrane invagination, thereby forming the PV, which is finally released from the tube. According to this model, the PV membrane is derived from the plasma membrane of the host cell. Alternatively, if the tube is not invaginating but is penetrating the plasma membrane, a very fast association of the tube surface with intracellular, DiIC16-labeled host cell vesicles has to be postulated. Although we can currently not completely exclude this mechanism, it appears less likely than the invagination model, since the majority of the DiIC16 fluorescence was still on the cell surface and not within vesicles and the time for the coating process is very short (<1.3 s).
Previous ultrastructural studies on the invasion of Encephalitozoon species described discharged polar tubes that were found to be localized inside host cell membrane invaginations (17, 23). However, these invaginations were believed to be the result of a phagocytic process, which involved the rearrangement of cytoskeletal elements (17, 23). The putative plasma membrane invaginations suggested by our experiments are unlikely to result from host cell cytoskeletal rearrangements, since those processes are much slower than the observed <1.3 s. In the context of our results, an alternative explanation of the invaginations observed by Magaud et al. (17) and Schottelius et al. (23) is their generation by the mechanical force of the discharged tube itself.
The E. cuniculi genome displays an extreme loss of own metabolic pathways, which requires an extensive import of various metabolites into the cytosol of the pathogen. We demonstrated by microinjection of fluorescent conjugates that the PV membrane contains pores which allow a diffusion of small molecules, including positively and negatively charged peptides, from the cytosol into the PV lumen. The exclusion size was determined to be in the range between 3 to 10 kDa, revealing that the PV membrane represents no diffusion barrier for metabolites such as ATP, carbohydrates, amino acids, and small peptides. The presence of pores in the PV is in agreement with earlier findings by Leitch et al., who demonstrated that the PV of E. hellem displays a pH and calcium concentration similar to those of the host cell cytosol (16). The expression of pores in E. cuniculi and their exclusion size are stable throughout the intracellular life cycle and allow the pathogen to have permanent access to the cytosolic metabolite pool of the host cell. Pores were also described in the PVs of apicomplexan parasites, e.g., Toxoplasma, Plasmodium, and Eimeria (1, 25, 30). Their appearance in phylogenetically completely unrelated organisms suggests that they were invented independently in evolution as an adaptation to intracellular parasitism.
Acknowledgments
This study has been supported by a grant (GR906/11-2) from the Deutsche Forschungsgemeinschaft (DFG).
Footnotes
Published ahead of print on 11 April 2008.
REFERENCES
- 1.Bano, N., J. D. Romano, B. Jayabalasingham, and I. Coppens. 2007. Cellular interactions of Plasmodium liver stage with its host mammalian cell. Int. J. Parasitol. 371329-1341. [DOI] [PubMed] [Google Scholar]
- 2.Barker, R. J. 1975. Ultrastructural observation on Encephalitozoon cuniculi Levaditi, Nicolau et Schoen, 1923, from mouse peritoneal macrophages. Fol. Parasitol. 221-9. [Google Scholar]
- 3.Cali, A. 1986. Comparison of the biology of Microsporidia from different host groups, p. 356-359. In R. A. Samson, J. M. Black, and D. Peters (ed.), Fundamental and applied aspects of invertebrate pathology. Foundation for the 4th International Colloquium on Invertebrate Pathology, Wageningen, The Netherlands.
- 4.Cali, A. 1971. Morphogenesis in the genus Nosema. In Proceedings of the IVth International Colloquium in Insect Pathology, College Park, Md.
- 5.Cali, A., and P. M. Takvorian. 1999. Developmental morphology and life cycles of the microsporidia, p. 85-128. In M. Wittner and L. M. Weiss (ed.), The microsporidia and microsporidiosis. American Society for Microbiology, Washington, DC.
- 6.Charron, A. J., and L. D. Sibley. 2004. Molecular partitioning during host cell penetration by Toxoplasma gondii. Traffic 5855-867. [DOI] [PubMed] [Google Scholar]
- 7.Cossart, P., and P. J. Sansonetti. 2004. Bacterial invasion: the paradigms of enteroinvasive pathogens. Science 304242-248. [DOI] [PubMed] [Google Scholar]
- 8.El Alaoui, H., J. Bata, P. Peyret, and C. P. Vivares. 2001. Encephalitozoon cuniculi (Microspora): characterization of a phospholipid metabolic pathway potentially linked to therapeutics. Exp. Parasitol. 98171-179. [DOI] [PubMed] [Google Scholar]
- 9.Fasshauer, V., U. Gross, and W. Bohne. 2005. The parasitophorous vacuole membrane of Encephalitozoon cuniculi lacks host cell membrane proteins immediately after invasion. Eukaryot. Cell 4221-224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hao, M., S. Mukherjee, and F. R. Maxfield. 2001. Cholesterol depletion induces large scale domain segregation in living cell membranes. Proc. Natl. Acad. Sci. USA 9813072-13077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hirt, R. P., J. M. Logsdon, Jr., B. Healy, M. W. Dorey, W. F. Doolittle, and T. M. Embley. 1999. Microsporidia are related to fungi: evidence from the largest subunit of RNA polymerase II and other proteins. Proc. Natl. Acad. Sci. USA 96580-585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.James, T. Y., et al. 2006. Reconstructing the early evolution of fungi using a six-gene phylogeny. Nature 443818-822. [DOI] [PubMed] [Google Scholar]
- 13.Katinka, M. D., S. Duprat, E. Cornillot, G. Metenier, F. Thomarat, G. Prensier, V. Barbe, E. Peyretaillade, P. Brottier, P. Wincker, F. Delbac, H. El Alaoui, P. Peyret, W. Saurin, M. Gouy, J. Weissenbach, and C. P. Vivares. 2001. Genome sequence and gene compaction of the eukaryote parasite Encephalitozoon cuniculi. Nature 414450-453. [DOI] [PubMed] [Google Scholar]
- 14.Keeling, P. J., M. A. Luker, and J. D. Palmer. 2000. Evidence from beta-tubulin phylogeny that microsporidia evolved from within the fungi. Mol. Biol. Evol. 1723-31. [DOI] [PubMed] [Google Scholar]
- 15.Keeling, P. J. 2001. Parasites go the full monty. Nature 414401-402. [DOI] [PubMed] [Google Scholar]
- 16.Leitch, G. J., M. Scanlon, G. S. Visvesvara, and S. Wallace. 1995. Calcium and hydrogen ion concentrations in the parasitophorous vacuoles of epithelial cells infected with the microsporidian Encephalitozoon hellem. J. Eukaryot. Microbiol. 42445-451. [DOI] [PubMed] [Google Scholar]
- 17.Magaud, A., A. Achbarou, and I. Desportes-Livage. 1997. Cell invasion by the microsporidium Encephalitozoon intestinalis. J. Eukaryot. Microbiol. 4481S. [DOI] [PubMed] [Google Scholar]
- 18.Morisaki, J. H., J. E. Heuser, and L. D. Sibley. 1995. Invasion of Toxoplasma gondii occurs by active penetration of the host cell. J. Cell Sci. 1082457-2464. [DOI] [PubMed] [Google Scholar]
- 19.Mouritsen, O. G., and K. Jørgensen. 1995. Micro-, nano- and meso-scale heterogeneity of lipid bilayers and its influence on macroscopic membrane properties. Mol. Membr. Biol. 1215-20. [DOI] [PubMed] [Google Scholar]
- 20.Mukherjee, S., T. T. Soe, and F. R. Maxfield. 1999. Endocytic sorting of lipid analogues differing solely in the chemistry of their hydrophobic tails. J. Cell Biol. 1441271-1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pakes, S. P., J. A. Shadduck, and A. Cali. 1975. Fine structure of Encephalitozoon cuniculi from rabbits, mice and hamsters. J. Protozool. 22481-488. [DOI] [PubMed] [Google Scholar]
- 22.Scanlon, M., G. J. Leitch, G. S. Visvesvara, and A. P. Shaw. 2004. Relationship between the host cell mitochondria and the parasitophorous vacuole in cells infected with Encephalitozoon microsporidia. J. Eukaryot. Microbiol. 5181-87. [DOI] [PubMed] [Google Scholar]
- 23.Schottelius, J., C. Schmetz, N. P. Kock, T. Schüler, I. Sobottka, and B. Fleischer. 2000. Presentation by scanning electron microscopy of the life cycle of microsporidia of the genus Encephalitozoon. Microbes Infect. 21401-1406. [DOI] [PubMed] [Google Scholar]
- 24.Schroeder, R., E. London, and D. Brown. 1994. Interactions between saturated acyl chains confer detergent resistance on lipids and glycosylphosphatidylinositol (GPI)-anchored proteins: GPI-anchored proteins in liposomes and cells show similar behavior. Proc. Natl. Acad. Sci. USA 9112130-12134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schwab, J. C., C. J. Beckers, and K. A. Joiner. 1994. The parasitophorous vacuole membrane surrounding intracellular Toxoplasma gondii functions as a molecular sieve. Proc. Natl. Acad. Sci. USA 91509-513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sprague, V., and S. H. Vernick. 1971. The ultrastructure of Encephalitozoon cuniculi (Microsporida, Nosematidae) and its taxonomic significance. J. Protozool. 18560-569. [DOI] [PubMed] [Google Scholar]
- 27.Thomas, J. L., D. Holowka, B. Baird, and W. W. Webb. 1994. Large-scale co-aggregation of fluorescent lipid probes with cell surface proteins. J. Cell Biol. 125795-802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vavra, J., and J. I. R. Larsson. 1999. Structure of the microsporidia, p. 7-84. In M. Wittner and L. M. Weiss (ed.). The microsporidia and microsporidiosis. American Society for Microbiology, Washington, DC.
- 29.Weidner, E. 1975. Interactions between Encephalitozoon cuniculi and macrophages. Parasitophorous vacuole growth and the absence of lysosomal fusion. Z. Parasitenkd. 471-9. [DOI] [PubMed] [Google Scholar]
- 30.Werner-Meier, R., and R. Entzeroth. 1997. Diffusion of microinjected markers across the parasitophorous vacuole membrane in cells infected with Eimeria nieschulzi (Coccidia, Apicomplexa). Parasitol. Res. 83611-613. [DOI] [PubMed] [Google Scholar]
- 31.Zaas, D. W., M. Duncan, J. Rae Wright, and S. N. Abraham. 2005. The role of lipid rafts in the pathogenesis of bacterial infections. Biochim. Biophys. Acta 1746305-313. [DOI] [PubMed] [Google Scholar]