Abstract
The protective role of specific antibodies against Paracoccidioides brasiliensis is controversial. In the present study, we analyzed the effects of monoclonal antibodies on the major diagnostic antigen (gp43) using in vitro and in vivo P. brasiliensis infection models. The passive administration of some monoclonal antibodies (MAbs) before and after intratracheal or intravenous infections led to a reduced fungal burden and decreased pulmonary inflammation. The protection mediated by MAb 3E, the most efficient MAb in the reduction of fungal burden, was associated with the enhanced phagocytosis of P. brasiliensis yeast cells by J774.16, MH-S, or primary macrophages. The ingestion of opsonized yeast cells led to an increase in NO production by macrophages. Passive immunization with MAb 3E induced enhanced levels of gamma interferon in the lungs of infected mice. The reactivity of MAb 3E against a panel of gp43-derived peptides suggested that the sequence NHVRIPIGWAV contains the binding epitope. The present work shows that some but not all MAbs against gp43 can reduce the fungal burden and identifies a new peptide candidate for vaccine development.
Paracoccidioides brasiliensis is a thermally dimorphic fungus that is the etiological agent of paracoccidioidomycosis (PCM), the most prevalent systemic mycosis in Latin America that is endemic in areas of Brazil, Argentina, Colombia, and Venezuela. The impact of the disease is demonstrated by Coutinho et al. (14), who reported that 3,181 lethal cases of PCM occurred in the 1980 to 1995 period in Brazil. The acute and subacute forms of PCM affect both genders and primarily involve the reticuloendothelial/lymphatic system. The chronic form affects mainly adult males with predominant pulmonary and/or mucocutaneous involvement (20). The activation of the immune cellular response is the primary effective mechanism to control experimental and human PCM (4, 25). A correlation has been found between the severity of the disease and an impaired delayed-type hypersensitivity response (30).
Antifungal chemotherapy is required for PCM treatment, though even after prolonged administration, there is no assurance of the complete destruction of the fungus. The period of treatment depends on the drug used and disease severity. Standard drugs include sulfonamides, amphotericin B, and azoles (44).
gp43, first described by Puccia et al. (35), is the major diagnostic antigen of P. brasiliensis used in a variety of serological tests (16, 50). Antibody titers to gp43 have been used to monitor the response to treatment in patients (6). gp43 has also proven to be immunodominant in a crude antigenic preparation, eliciting delayed hypersensitivity reactions in guinea pigs (40) and humans (42), which indicated the presence of T-CD4+-reacting epitopes.
The gp43 gene has been cloned and sequenced (13). It encodes a polypeptide of 416 amino acids (Mr, 45,947) with a leader peptide of 35 residues; the mature protein has a single high-mannose N-glycosylated chain (1). The H-2d-restricted T-cell epitope has been mapped to a 15-mer peptide called P10 (48). Different 12-mer sequences, all containing the hexapeptide HTLAIR, induce the proliferation of lymph node cells from mice sensitized to gp43 or infected with P. brasiliensis. Lymphoproliferation induced by either P10 or gp43 involves CD4+ T-helper lymphocytes producing interleukin-2 (IL-2) and gamma interferon (IFN-γ). The immunization of mice with either gp43 or P10 in complete Freund's adjuvant significantly protected them from intratracheal (i.t.) infection with virulent yeasts of P. brasiliensis. After 3 months of infection, the lung CFU were >200-fold fewer than in the untreated control mice.
The role of antibody-mediated immunity in host resistance to P. brasiliensis is less certain (10). In several systems, however, there is considerable evidence that the administration of monoclonal antibodies (MAbs) can modify the course of disease in mice infected with fungi such as Cryptococcus neoformans (28), Candida albicans (15, 24), Histoplasma capsulatum (31), Pneumocystis spp. (51), Fonsecaea pedrosoi (2), Aspergillus spp. (12), and, more recently, P. brasiliensis (17).
The mechanisms of antibody action in combatting an infectious disease include antigen neutralization, cooperative effects with cellular immunity by the enhancement of phagocytosis or mediating-cell cytotoxicity, complement activation, growth inhibition, adherence, and biofilm or direct antimicrobial effects (reviewed in reference 11). With C. neoformans, it is clear that antibody-mediated immunity can be decisive for host defense. The mechanisms involved are complex and dependent on several parameters, such as antibody isotype (26), T-cell function (53), C. neoformans strain (29), antibody quantity (49), expression of inducible NO synthase (39), Fc region, and complement activation (43).
The control of PCM is classically associated with a vigorous Th1 response and granuloma formation. There is evidence, however, that antibody-mediated immunity can contribute to infection clearance. In the present study, a panel of MAbs against gp43 was utilized for evaluating the effect of passive immunization in mice intravenously (i.v.) or i.t. infected with a virulent strain of P. brasiliensis.
MATERIALS AND METHODS
Animals.
BALB/c mice (6- to 8-week-old males) were bred at the University of São Paulo, São Paulo, Brazil, animal facility under specific pathogen-free conditions. The procedures involving animals and their care were conducted according to the local ethics committee and international rules.
Fungal strain.
Virulent P. brasiliensis Pb18 yeast cells were maintained by weekly passage on solid Sabouraud medium at 37°C and were used after 7 to 10 days of growth. Before the experimental infection, the fungus was grown in modified McVeigh-Morton medium at 37°C for 5 to 7 days (38). The fungal cells were washed in phosphate-buffered saline (PBS; pH 7.2) and counted in a hemocytometer. The viability of the fungal suspensions, determined by staining with Janus B (Merck, Darmstadt, Germany), was always higher than 90%.
MAbs.
The MAbs against gp43—19G, 10D, 32H, and 17D (immunoglobulin G2a [IgG2a]) and 21F and 3E (IgG2b)—were previously characterized (36). IgG ascites was generated in BALB/c mice given intraperitoneal (i.p.) injections of MAb hybridomas. IgG MAbs were purified from ascitic fluid by protein A affinity chromatography (Pierce, Rockland, IL) as per the manufacturer's instructions. Endotoxin (lipopolysaccharide) concentration was <1 ng/ml, measured by the Limulus amebocyte test (BioWhittaker, Walkersville, MD). The antibody concentration was determined by enzyme-linked immunosorbent assay (ELISA) relative to isotype-matched standards. Irrelevant IgG MAb was used as a control and was provided by Elaine G. Rodrigues, Universidade Federal de São Paulo.
Endotoxin-free preparations.
Disposable pyrogen-free plasticware and endotoxin-free water or PBS were used in all experiments. Ascitic fluid and isolated MAb were further purified in Detoxi-Gel columns (Pierce, Rockland, IL) to remove any endotoxin contamination.
Phagocytosis assay.
Peritoneal and alveolar macrophages from BALB/c mice were harvested by washing the abdominal cavities or the lungs and culturing adherent cells in Dulbecco's modified Eagle's medium with 10% heat-inactivated fetal calf serum and 1% nonessential amino acids (Cultilab, Brazil). Other phagocytosis experiments were carried out with the J774.16 macrophage-like cell line, derived from a reticulum cell sarcoma (37), or with the MH-S line, originated from an SV40-transformed adherent cell-enriched population of alveolar macrophages (23). The protocol for in vitro phagocytosis was that described in earlier studies (46, 47) with minor modifications. Primary cells were plated on 96-well tissue culture plates (Switzerland) at a final density of 1.6 × 105 cells/well, and macrophage-like cells were plated at a density of 0.8 × 105 to 1 × 105 macrophages in order to obtain the same density after overnight incubation. The cells were stimulated with 50 U/ml recombinant murine IFN-γ (PeproTech, Rock Hill, NJ) and incubated at 37°C overnight. Phagocytosis was measured in the presence or absence of purified MAbs (0 to 100 μg/ml). P. brasiliensis cells were added at a ratio of 5:1 macrophages to fungal cells and incubated at 37°C for 6, 12, and 24 h. The cells were then washed several times with sterile PBS, fixed with cold absolute methanol, and stained with a 1/20 solution of Giemsa (Sigma, St. Louis, MO). Phagocytosed yeasts were counted by light microscopy at ×400 magnification. The phagocytic index (PI) is defined by the equation PI = P × F, where P is the percentage of macrophages with internalized yeast and F is the average number of yeast cells per macrophage. Phagocytosis was confirmed by transmission electron microscopy that showed the internalization of yeast cells in macrophages (data not shown). Experiments were carried out in triplicate, and five to eight different fields were counted.
Intratracheal infection of BALB/c mice.
BALB/c mice were inoculated i.t. with 3 × 105 yeast cells of virulent P. brasiliensis Pb18, grown on Sabouraud agar and suspended in sterile saline (0.85% NaCl), per animal. Briefly, the mice were anesthetized i.p. with 200 μl of a solution containing 80 mg/kg ketamine and 10 mg/kg of xylazine (both from União Química Farmacêutica, Brazil). After approximately 10 min, their necks were hyperextended, and the tracheas were exposed at the level of the thyroid and injected with 3 × 105 yeast cells in PBS using a 26-gauge needle. The incisions were sutured with 5-0 silk.
Fungal burden in organs of i.t. infected mice.
For the fungal burden analysis, two protocols were utilized: (i) 1 mg of MAbs was administrated i.p. 24 h before the infections, and the mice were sacrificed 15 and 30 days after i.t. infection, and (ii) 1 mg of MAbs was administered i.p. 30 days after the infections, and the mice were sacrificed 45 and 60 days after i.t. infection. The fungal burden was measured by CFU. Sections of the lungs, livers, and spleens were removed, weighed, homogenized, and then washed three times with PBS. The corresponding pellets were resuspended and homogenized, each in 1 ml of PBS. A 100-μl sample of this suspension was plated on solid brain heart infusion medium supplemented with 4% fetal calf serum (Gibco, NY), 5% spent P. brasiliensis (strain 192) culture supernatant, 10 IU/ml streptomycin/penicillin (Cultilab, Brazil), and 500 μg/ml cycloheximide (Sigma, St. Louis, MO). The petri dishes were incubated at 37°C for at least 10 days, and colonies were counted (1 colony = 1 CFU).
Histopathology.
The lungs of i.t. infected mice were excised, fixed in 10% buffered formalin, and embedded in paraffin for sectioning. The sections were stained with hematoxylin-eosin or silver nitrate and examined microscopically at ×25 magnification (Optiphot-2; Nikon, Tokyo, Japan).
Fungal burden in organs of i.v.-infected mice.
MAbs 3E and 32H and irrelevant MAb were administered (1 mg) i.p. 24 h before i.v. infection with 3 × 105 yeast cells of virulent P. brasiliensis Pb18. A maintenance dose of 100 μg of each MAb was given every week for a month. The mice were sacrificed 30 days after infection and the fungal burden measured as described for the i.t. infection.
Cytokine analysis.
Sections of the lungs (alternating right and left lungs) were homogenized in 2 ml of PBS in the presence of protease inhibitors (Complete Mini; Boehringer Mannheim, Indianapolis, IN). The homogenates were centrifuged, and the supernatants frozen at −80°C until tested. The supernatants were assayed for IL-2, IL-4, IL-10, IL-12, and IFN-γ using ELISA kits (BD PharMingen, San Diego, CA). The detection limits of such assays were as follows: 3.1 pg/ml for IL-2, 7.8 pg/ml for IL-4, 31.25 pg/ml for IL-10 and IFN-γ, and 62.5 pg/ml for IL-12p40, as previously determined by the manufacturer.
Production of nitric oxide.
The levels of nitric oxide metabolite (nitrite) were determined by Griess reaction (33) in the culture supernatant of macrophages challenged with opsonized yeasts. All determinations were performed in triplicate.
Peptide synthesis and purification for screening.
Peptide synthesis and purification were carried out at the Department of Biophysics, Universidade Federal de São Paulo. The amino acid sequence of P. brasiliensis gp43 glycoprotein (GenBank accession number AY005437) was used to synthesize the peptides by solid-phase technology using the 9-fluorenylmethoxy carbonyl strategy on an automated benchtop simultaneous multiple solid-phase peptide synthesizer PSSM8 (Shimadzu, Tokyo, Japan) with 9-fluorenylmethoxy carbonyl-protected amino acid residues and TentaGel Rink resin (Novabiochem, San Diego, CA). Therefore, all peptides were obtained with the C-terminal carboxyl group in an amide form. All peptides were deprotected and cleaved from the resins by treatment with K reagent composed of 80% trifluoroacetic acid, 2.5% triisopropylsilane, 2.5% ethanedithiol, 5.0% anisole, 5.0% water, and 5.0% phenol. The resulting peptides were analyzed by reverse-phase high-performance liquid chromatography (Shimadzu, Tokyo, Japan) on a C18 column eluted at 1 ml/min using a 5% to 95% gradient of 90% acetonitrile in 0.1% trifluoroacetic acid over 30 min. The peptide quality was assessed by a matrix-assisted laser desorption ionization-time of flight instrument (Micromass, Manchester, United Kingdom) using α-cyano-4-hydroxy cinnamic acid as the matrix.
Peptide containing the reactive epitope of the protective MAb 3H.
The peptide NHVRIPIGYWAV was synthesized at Peptides International (Louisville, KY) with 95% purity, in both the carboxy and amidated forms.
MAb reactivity with peptides.
Antibody reactivity with peptides from gp43 was determined by ELISA. Microtiter plates coated with 100 ng of each peptide/well were incubated with 100 μl of MAb serially diluted, starting at 10 μg/ml, at 37°C for 1 h. The plates were washed three times and incubated with 100 μl of goat anti-mouse IgG peroxidase (Sigma, St. Louis, MO) for 1 h at 37°C. The plates were washed, and the reaction was developed with a solution of o-phenylenediamine (0.5 mg/ml; Sigma, St. Louis, MO) and 0.005% H2O2 (Sigma, St. Louis, MO). The reaction was terminated with 4 N H2SO4 after 8 to 10 min of incubation in the dark. The optical densities were measured at 492 nm in an ELISA reader (Titertek Multiskan EIA reader).
Direct effect of MAbs on P. brasiliensis.
The growth rate of Pb18 in the presence of protective and nonprotective MAbs was compared to that in the presence of irrelevant antibodies or medium alone. Yeast cells were grown in Sabouraud medium at 37°C, and 100 μg/ml of each MAb was added at 96-h intervals. Culture samples were taken at 48-h intervals, and cell numbers were counted with a hemocytometer.
Statistical analysis.
Statistical analysis was done using GraphPad Prism5 software. The results were expressed as the means ± the standard deviations (SD) of the indicated numbers of animals or experiments. The nonparametric Tukey's honestly significant difference test was used. The unpaired Student's t test with Welch's correction (two-tailed) was used for the comparison of the two groups when the data met the assumptions of the t tests. P values of <0.05 indicated statistical significance.
RESULTS
In vitro phagocytosis mediated by MAbs.
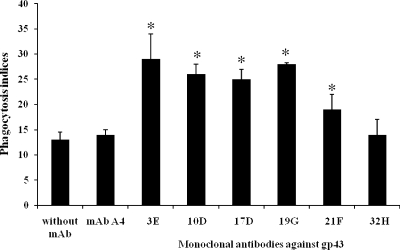
We investigated the effect of MAbs to gp43 on P. brasiliensis phagocytosis in vitro using both primary macrophages (lung and peritoneal) and cell lines J774.16 and MH-S. Yeast cells opsonized with MAbs 19G, 21F, 10D, 17D, and 3E were at least twofold more internalized by J774.16 cells than by nonopsonized or 32H-opsonized yeast cells (Fig. 1). The same result was obtained with primary macrophages. The MH-S cell line and the lung primary macrophages showed similar results. Phagocytosis was observed regardless of whether or not the macrophages were activated with IFN-γ, but the indices were significantly higher with cytokine-stimulated cells (data not shown).
FIG. 1.
Phagocytosis of yeast cells by J774.16 cells after 12 h in the presence of MAbs against gp43 (3E, 10D, 17D, 19G, 21F, and 32H) compared to that of controls of yeast incubated without MAb (PBS) or with an irrelevant MAb (MAb A4). Each bar represents the average of three measurements, and error bars indicate SD. Experiments were done in triplicate, and different fields were counted. *, significant difference (P < 0.05, determined by analysis of variance and Tukey's honestly significant difference test) compared to the control without MAb.
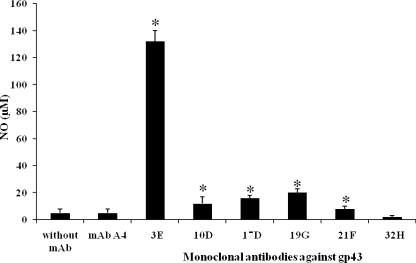
Yeast cells opsonized with MAbs to gp43 promote macrophage fungicidal activity in vitro.
MAbs that increase P. brasiliensis phagocytosis by macrophages also induce an increase in nitric oxide metabolite (nitrite) in the supernatants of the phagocytic cells (Fig. 2).
FIG. 2.
Nitrite measurements in supernatants from phagocytosis assays. P. brasiliensis yeast was cultured with J774.16 cells for 12 h in the presence of MAbs against gp43 (3E, 10D, 17D, 19G, 21F, and 32H), PBS (without MAb), or the irrelevant MAb (A4). Nitrite levels were detected using a Griess assay. *, significant difference (P < 0.05, determined by analysis of variance and Tukey's honestly significant difference test) relative to the PBS control. Error bars denote SD.
Passive immunization reduces fungal burden.
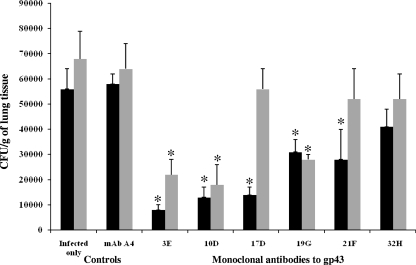
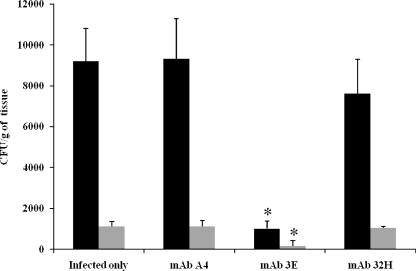
To evaluate whether the administration of MAbs could reduce lung CFU from mice infected with P. brasiliensis, two protocols were followed. In the first protocol, BALB/c mice were immunized with different MAbs against gp43 24 h prior to i.t. infection with 3 × 105 yeast cells of P. brasiliensis. After 15 days of infection, the number of CFU in the lungs of mice that received MAbs 19G, 21F, 10D, 3E, and 17D but not 32H showed a significant reduction compared with that of the controls (Fig. 3). Additionally, MAbs 19G, 10D, and 3E maintained significant CFU reductions after 30 days of infection (Fig. 3). In the second protocol, two MAbs were selected, 32H that did not reduce the lung CFU and 3E that reduced the CFU. BALB/c mice were infected as indicated above, and after 30 days, 1 mg of MAbs 32H or 3E was injected i.p. The CFU were measured after 45 and 60 days of infection, and MAb 3E but not 32H was able to reduce the CFU from the lungs of the mice at day 60 (Fig. 4).
FIG. 3.
Lung CFU from mice infected i.t. with 3 × 105 yeast cells and treated with MAbs against gp43 (3E, 10D, 17D, 19G, 21F, and 32H) 24 h prior to infection. Mice were sacrificed after 15 (black bars) or 30 (gray bars) days of infection. Control mice were infected and received PBS (infected only) or an irrelevant MAb (A4). Each bar represents the average count of fungi in the lung, and error bars indicate SD. *, significant difference (P < 0.05, determined by analysis of variance and Tukey's honestly significant difference test) relative to the PBS control.
FIG. 4.
Lung CFU from mice infected i.t. with 3 × 105 yeast cells and treated with MAbs against gp43 (3E or 32H) 30 days after infection. Mice were sacrificed after 45 (black bars) or 60 (gray bars) days of infection. Control mice were infected and received PBS (infected only) or an irrelevant MAb (A4). Each bar represents the average count of fungi in the lung, and error bars indicate SD. *, significant difference (P < 0.05) relative to the PBS control.
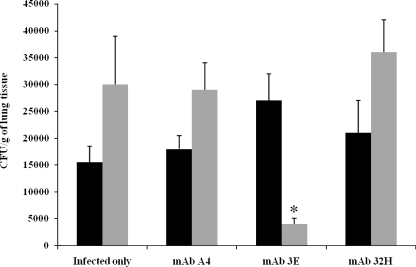
To evaluate the effect of MAbs in mice, an intravenous infection model was used. Groups of animals were passively immunized i.p. with 1 mg of MAb 3E, MAb 32H, or the irrelevant control MAb 24 h prior to infection with 5 × 106 yeast cells and were given 0.1 mg of the MAbs every week for 1 month before the animals were sacrificed. For the CFU determinations, the lung, spleen, and liver tissues were homogenized separately and plated on solid medium. The mice immunized with MAb 3E had significantly lower lung and spleen CFU compared to the CFU of the mice receiving MAb 32H, the irrelevant MAb, or PBS (Fig. 5). Liver CFU were below the detection threshold.
FIG. 5.
CFU from lungs (black bars) or spleens (gray bars) of mice infected i.v. with 3 × 105 yeast cells and treated with 1 mg of MAbs against gp43 (3E or 32H) 24 h prior to infection and with 100 μg every week for a month as a maintenance dose. Mice were sacrificed after 30 days of infection. Control mice were infected and received PBS (infected only) or an irrelevant MAb (A4). Each bar represents the average count of fungi in the organ, and error bars indicate SD. *, significant difference (P < 0.05, determined by analysis of variance and Tukey's honestly significant difference test) relative to the PBS control.
Viability of P. brasiliensis yeast cells in the presence of MAbs 3E and 32H.
The viability of yeast cells incubated with MAbs 3E or 32H or the irrelevant MAb was evaluated during 30 days; no direct inhibitory effects by the MAbs were observed (data not shown).
Lung histopathology of treated, i.t. infected BALB/c mice (30 days).
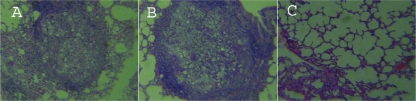
The lungs of the untreated control animals showed intense infiltration, mainly by macrophages, lymphocytes, and epithelioid cells. Around the foci of the epithelioid granulomas, giant cells were also observed. Multiplying fungal cells were seen. Using protocol 1 (for which the mice received MAbs 24 h before i.t. infection), we observed that the animals immunized with MAb 32H were pathologically similar to the infected control mice. In contrast, the animals that received MAb 3E had fewer epithelioid granulomas with a reduced number of yeast cells and large areas of preserved lung tissue (Fig. 6).
FIG. 6.
Histopathology of representative lung sections from mice i.t. infected according to protocol 1 and euthanized at 30 days after infection. (A) Lung section from an untreated infected mouse; (B) lung section from a mouse infected and treated with MAb 32H; (C) lung section from an infected mouse treated with MAb 3E. Hematoxylin-eosin staining, ×10 magnification.
MAb treatment alters cytokine expression.
Cytokine levels were detected in the lung tissue of i.t. infected mice treated with MAb 3E and 32H in both protocols. Groups of 10 mice were used in each protocol; all experiments were repeated twice with similar results. As shown in Table 1, with the first protocol, animals immunized with MAb 3E had higher levels of IFN-γ and reduced levels of IL-4 after 15 and 30 days of infection compared with those of the animals immunized with MAb 32H. The other cytokines (IL-10, IL-12, and TNF-α) showed less variability. With the second protocol, the levels of IL-12 after 45 days of infection and IFN-γ after 45 and 60 days of infection were higher than those of MAb 32H (Table 2).
TABLE 1.
Protocol 1 cytokine levels in the lungs of mice in response to the passive transfer of MAbs 24 h before i.t. infection with P. brasiliensis
| Cytokine | Days of infection | Cytokine level (pg/g of tissue)a
|
||||
|---|---|---|---|---|---|---|
| Sham | Infected only | Irrelevant MAb A4 | Treated with MAb 3E | Treated with MAb 32H | ||
| IL-4 | 15 | 248.2 ± 63.1 | 248.7 ± 43.6 | 327.1 ± 56 | 102.8 ± 52b | 107.7 ± 49.1b |
| 30 | 322.5 ± 70.7 | 380.4 ± 81.9 | 422.4 ± 87.8 | 120.6 ± 67b | 559.5 ± 76.9b | |
| IL-10 | 15 | 4,204 ± 269.4 | 6,786.9 ± 1,250.1 | 8,062.5 ± 926.4 | 11,027.8 ± 1,321.8b | 12,852 ± 1,560b |
| 30 | 3,920 ± 330 | 9,033.3 ± 978.4 | 9,357.1 ± 1,077.8 | 15,804 ± 2,135b | 24,155 ± 1,398b | |
| IL-12 | 15 | 4,682.1 ± 303 | 8,648.6 ± 218.2 | 8,543.7 ± 743 | 12,829.9 ± 1,422.5b | 8,724.3 ± 1,403.4 |
| 30 | 4,880 ± 335.8 | 7,598 ± 838 | 7,050 ± 765 | 6,535.3 ± 834.2 | 10,128.4 ± 988b | |
| IFN-γ | 15 | 101 ± 13 | 300 ± 35.1 | 240 ± 38.3 | 1,100.8 ± 115.6b | 150.4 ± 87.8 |
| 30 | 108 ± 10.1 | 260.9 ± 8.3 | 301.3 ± 50.8 | 780 ± 99.3b | 155.7 ± 93.4 | |
| TNF-α | 15 | 2,142.8 ± 505 | 12,616.7 ± 918.7 | 11,725 ± 1,426.6 | 6,944.4 ± 1,387.5b | 4,217.9 ± 657.5b |
| 30 | 2,680 ± 622.2 | 25,794.8 ± 2,492.2 | 22,857.1 ± 2,412 | 14,456.5 ± 2,224.4b | 11,662.2 ± 2,380.1b | |
Values are means ± standard deviations of measurements from 10 animals per group. The experiment was repeated twice with reproducible results.
Statistically significant (P < 0.05, determined by analysis of variance and Tukey's honestly significant difference test) relative to the value for the untreated, infected mice.
TABLE 2.
Protocol 2 cytokine levels in the lungs of mice in response to the passive transfer of MAbs 30 days after i.t. infection with P. brasiliensis
| Cytokine | Days of infection | Cytokine level (pg/g of tissue)a
|
||||
|---|---|---|---|---|---|---|
| Sham | Infected only | Irrelevant MAb A4 | Treated with MAb 3E | Treated with MAb 32H | ||
| IL-4 | 45 | 208.2 ± 73.1 | 589.2 ± 81.9 | 342.3 ± 36.2 | 2,151.6 ± 352b | 1,370.6 ± 134b |
| 60 | 254.3 ± 85.7 | 862.8 ± 185.5 | 764.8 ± 107.8 | 1,633.2 ± 188b | 1,832.9 ± 287.6b | |
| IL-10 | 45 | 1,685.7 ± 252.5 | 2,541.9 ± 172.2 | 1,666.7 ± 250 | 3,245.4 ± 318.1b | 1,790.6 ± 240.6b |
| 60 | 2,360 ± 494.7 | 2,760.2 ± 612.3 | 3,238.1 ± 798.4 | 3,583.3 ± 279b | 2,500 ± 839.9 | |
| IL-12 | 45 | 3,257.6 ± 370.4 | 4,912.5 ± 817.4 | 8,362.5 ± 1,969.6 | 21,337.9 ± 3,475.9b | 12,470.1 ± 1,815.5b |
| 60 | 3,243.7 ± 1,885.6 | 6,987.4 ± 891.5 | 9,509.5 ± 3,249.4 | 14,699.3 ± 2,981.1b | 11,630.6 ± 2,350.8b | |
| IFN-γ | 45 | 99.41 ± 33.1 | 280.9 ± 41.2 | 106.6 ± 29.2 | 1,328.6 ± 207.2b | 162.5 ± 42.1b |
| 60 | 90.2 ± 25.4 | 382.4 ± 39.2 | 133.3 ± 33 | 1,229.8 ± 192.3b | 416.4 ± 59.3 | |
| TNF-α | 45 | 1,980.5 ± 359 | 22,870.5 ± 1,057.6 | 22,100 ± 3,225.5 | 16,985.4 ± 1,698.2b | 18,798.5 ± 2,785.2b |
| 60 | 2,010.5 ± 438.8 | 30,021 ± 3,777.8 | 26,789.2 ± 1,469.3 | 14,892.4 ± 2,001b | 21,364.7 ± 3,911.2b | |
Values are means ± standard deviations of measurements from 10 animals per group. The experiment was repeated twice with reproducible results.
Statistically significant (P < 0.05, determined by analysis of variance and Tukey's honestly significant difference test) relative to the value for the untreated, infected mice.
Identification of the epitope of MAb 3E.
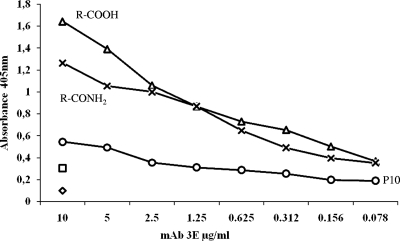
The reactivity of MAb 3E was evaluated against a panel of gp43-derived peptides previously described (48). The analysis of the results suggested that the recognized epitope is within the sequence NHVRIPIGWAV (data not shown). The MAb 3E but not 32H recognized this peptide using ELISA (Fig. 7). This peptide sequence was found to be conserved in the internal sequences of β-1,3-glucanases of Aspergillus fumigatus, Aspergillus oryzae, and Blumeria graminis.
FIG. 7.
Reactivity of peptides NHVRIPIGYWAV(R-CONH2), -(R-COOH), and P10 (QTLAIAHTLAIRYAN) with MAb 3E by ELISA. Microtiter plates were sensitized with 100 ng of each peptide, and the reaction was developed with MAb 3E. The diamond symbol indicates the background measurement with buffer alone, and the square symbol corresponds to the reactivity of the peptides against the irrelevant antibody.
DISCUSSION
The protective role of antibodies against fungal infections is in many cases controversial. Recently, several studies have established that some antibodies are protective against fungi (31, 41, 52). MAbs or fragments of MAbs have been approved for the clinical evaluation of cryptococcosis (21) and candidiasis (32). Murine MAb 18B7 directed against the capsular polysaccharide of C. neoformans was used to evaluate the safety and maximum tolerated dose of a therapeutic MAb. The study used human immunodeficiency virus-infected patients who had successfully been treated for cryptococcal meningitis. The MAb infusion had a half-life in the serum of approximately 53 h and reduced the fungal circulating antigen (21). In another study, a human recombinant MAb to heat shock protein 90 was used in the treatment of patients with invasive candidiasis (32). A consensus has now emerged that the inability of immune sera to mediate protection against fungi reflects inadequate amounts of protective antibody and/or the simultaneous presence of protective and nonprotective antibodies rather than a fundamental inability of antibody to protect against fungal pathogens (11).
The first evidence of an antibody-mediating protection against P. brasiliensis was described by de Mattos Grosso et al. (17). The authors showed that the passive transfer of two murine MAbs against a glycoprotein of 70 kDa, which is recognized in 96% of the sera from PCM patients, led to a significant reduction in the number of CFU in the lungs and fewer granulomas within the organs of experimentally infected mice (17). More recently, another MAb that recognizes a secreted 75-kDa protein with phosphatase activity inhibited P. brasiliensis growth in vitro and reduced lung CFU in vivo (52).
Although the MAbs to gp43 have not directly affected the growth of P. brasiliensis, the protective action of the MAbs was directly associated with their capacity to enhance phagocytosis. Since macrophages are effector cells capable of exerting yeast fungicidal effects, we investigated NO production in macrophages that had phagocytosed MAb-opsonized yeast cells. The NO results showed an increase for all “protective” MAbs, but the response was variable, with MAb 3E demonstrating the most impressive effect. Previous data from our group and others clearly demonstrated that activated macrophages kill the fungus in vitro by the production of nitric oxide and hydrogen peroxide (7, 8).
Using a panel of six MAbs against gp43, including IgG2a (10D, 17D, 19G, and 32H) and IgG2b (3E and 21F), we identified the protective and nonprotective MAbs by the passive administration of the antibodies into mice infected i.t. or i.v. with P. brasiliensis. The presence of protective and nonprotective antibodies against the same antigen is a conceptually relevant finding that has previously been described for cryptococcosis (27). The histopathology of lung sections from i.t. infected animals treated with the protective MAb showed a reduction in the number of yeast cells and epithelioid granulomas in comparison with the untreated control mice.
The role of IFN-γ in mediating activated macrophages in a systemic fungal infection has been reported. Murine peritoneal macrophages activated by IFN-γ show enhanced fungicidal activity with both yeast and conidial forms of P. brasiliensis (7-9). The production of IL-10 has been associated with more severe disease in susceptible mice (19). Alveolar macrophages from i.t. infected IL-4-deficient mice more efficiently control fungal growth than wild-type macrophages (34), and also IL-4-deficient mice have increased levels of pulmonary IFN-γ (34). The passive administration of irrelevant MAb, 3E, or 32H in i.t. infected BALB/c mice showed a mixed Th1/Th2 pattern of activation. Nevertheless, the cytokine changes associated with protective MAb administration were more Th1-like, given the increased levels of IFN-γ and IL-12 in the lungs of i.t. infected mice. A discreet but statistically significant increase of IL-10 and a reduction of IL-4 and TNF-α were noted compared with the levels of IL-10, IL-4, and TNF-α in the control infected mice. The passive transfer of MAb 32H induced lower levels of lung IFN-γ and IL-12 than MAb 3E treatment. In fungal disease, the effects of antibody treatment on cytokine production are poorly understood and may depend on several features, including the fungus, target organ, timing of fungal dissemination, and antibody quantity, isotype, and specificity (18, 39, 49). Antibody-mediated protection has been associated with lower levels of IFN-γ in A/J mice infected with C. neoformans (18), but both Th1- and Th2-related cytokines are necessary for antibody protection in cryptococcosis (3). Strong Th1 responses can result in reduced survival and tissue damage (22, 39). In our studies, we found that MAb treatment did not have the polarity of the Th1/Th2 response and that IFN-γ appears to be beneficial.
Attempts at determining the epitope of protective MAb 3E led to the sequence NHVRIPIGYWAV shared with the A. fumigatus, A. oryzae, and B. graminis internal sequences of β-1,3-glucanases. The opsonizing effect of MAb 3E could then involve the recognition of this peptide sequence in the gp43 accumulated on the cell wall of P. brasiliensis. In fact, gp43 is stored inside a vacuole, migrates to the plasma membrane, and spreads into the P. brasiliensis cell wall. The secretion of this antigen occurs at discrete sites along the cell surface (45).
Historically, protection against PCM has been attributed to a vigorous cellular immune response, whereas specific high levels of antibodies have been associated with disease severity, as observed in patients with acute and subacute forms. Here we show that there are protective and nonprotective anti-gp43 antibodies and that the former play an important role in disease control. Antibodies against gp43 are found in almost 100% of patients with PCM (5). Probably, there is a low concentration of protective antibodies in the serum samples of these patients, and they are not sufficient to control the disease. To overcome this, passive immunization with MAbs to gp43 was used and shown to be protective in mouse models of PCM. The present work also raises the potential therapeutic use of the peptide carrying the B epitope of MAb 3E in active immunization adjuvant to chemotherapy and/or in association with a peptide sequence containing the T-cell epitope of gp43, known as P10, that elicits a strong cell-mediated immunity and is protective against experimental infection.
Acknowledgments
The present work was supported by FAPESP grant 05/02776-0. R.P., L.R.T., and C.P.T. are research fellows of CNPq. J.D.N. is supported in part by NIH AI056070-01A2.
Editor: W. A. Petri, Jr.
Footnotes
Published ahead of print on 5 May 2008.
REFERENCES
- 1.Almeida, I. C., D. C. Neville, A. Mehlert, A. Treumann, M. A. Ferguson, J. O. Previato, and L. R. Travassos. 1996. Structure of the N-linked oligosaccharide of the main diagnostic antigen of the pathogenic fungus Paracoccidioides brasiliensis. Glycobiology 6507-515. [DOI] [PubMed] [Google Scholar]
- 2.Alviano, D. S., A. J. Franzen, L. R. Travassos, C. Holandino, S. Rozental, R. Ejzemberg, C. S. Alviano, and M. L. Rodrigues. 2004. Melanin from Fonsecaea pedrosoi induces production of human antifungal antibodies and enhances the antimicrobial efficacy of phagocytes. Infect. Immun. 72229-237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beenhouwer, D. O., S. Shapiro, M. Feldmesser, A. Casadevall, and M. D. Scharff. 2001. Both Th1 and Th2 cytokines affect the ability of monoclonal antibodies to protect mice against Cryptococcus neoformans. Infect. Immun. 696445-6455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Calich, V. L., L. M. Singer-Vermes, M. Russo, C. A. Vaz, and E. Burger. 1994. Immunogenetics in paracoccidioidomycosis, p. 151-173. In M. Franco, C. S. Lacaz, A. Restrepo, and G. del Negro (ed.), Paracoccidioidomycosis. CRC Press, Boca Raton, FL.
- 5.Camargo, Z. P., C. P. Taborda, E. G. Rodrigues, and L. R. Travassos. 1991. The use of cell-free antigens of Paracoccidioides brasiliensis in serological tests. J. Med. Vet. Mycol. 2931-38. [PubMed] [Google Scholar]
- 6.Camargo, Z. P., C. Unterkircher, and L. R. Travassos. 1989. Identification of antigenic polypeptides of Paracoccidioides brasiliensis by immunoblotting. J. Med. Vet. Mycol. 27407-412. [PubMed] [Google Scholar]
- 7.Cano, L. E., E. Brummer, D. A. Stevens, and A. Restrepo. 1992. Fate of conidia of Paracoccidioides brasiliensis after ingestion by resident macrophages or cytokine-treated macrophages. Infect. Immun. 602096-2100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cano, L. E., B. Gomez, E. Brummer, A. Restrepo, and D. A. Stevens. 1994. Inhibitory effect of deferoxamine or macrophage activation on transformation of Paracoccidioides brasiliensis conidia ingested by macrophages: reversal by holotransferrin. Infect. Immun. 621494-1496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cano, L. E., S. S. Kashino, C. Arruda, D. André, C. F. Xidieh, L. M. Singer-Vermes, C. A. C. Vaz, E. Burger, and V. L. G. Calich. 1998. Protective role of gamma interferon in experimental pulmonary paracoccidioidomycosis. Infect. Immun. 66800-806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Casadevall, A. 1995. Antibody immunity and invasive fungal infections. Infect. Immun. 634211-4218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Casadevall, A., and L. A. Pirofski. 2007. Antibody-mediated protection through cross-reactivity introduces a fungal heresy into immunological dogma. Infect. Immun. 755074-5078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chaturvedi, A. K., A. Kavishwar, G. B. Shiva Keshava, and P. K. Shukla. 2005. Monoclonal immunoglobulin G1 directed against Aspergillus fumigatus cell wall glycoprotein protects against experimental murine aspergillosis. Clin. Diagn. Lab. Immunol. 121063-1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cisalpino, P. S., R. Puccia, L. M. Yamauchi, M. I. Cano, J. F. da Silveira, and L. R. Travassos. 1996. Cloning, characterization, and epitope expression of the major diagnostic antigen of Paracoccidioides brasiliensis. J. Biol. Chem. 2714553-4560. [DOI] [PubMed] [Google Scholar]
- 14.Coutinho, Z. F., D. Silva, M. Lazera, V. Petri, R. M. Oliveira, P. C. Sabroza, and B. Wanke. 2002. Paracoccidioidomycosis mortality in Brazil (1980-1995). Cad. Saude Publica 181441-1454. [DOI] [PubMed] [Google Scholar]
- 15.De Bernardis, F., M. Boccanera, D. Adriani, E. Spreghini, G. Santoni, and A. Cassone. 1997. Protective role of antimannan and anti-aspartyl proteinase antibodies in an experimental model of Candida albicans vaginitis in rats. Infect. Immun. 653399-3405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.De Camargo, Z., C. Unterkircher, S. P. Campoy, and L. R. Travassos. 1988. Production of Paracoccidioides brasiliensis exoantigens for immunodiffusion tests. J. Clin. Microbiol. 262147-2151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.de Mattos Grosso, D., S. R. de Almeida, M. Mariano, and J. D. Lopes. 2003. Characterization of gp70 and anti-gp70 monoclonal antibodies in Paracoccidioides brasiliensis pathogenesis. Infect. Immun. 716534-6542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Feldmesser, M., A. Mednick, and A. Casadevall. 2002. Antibody-mediated protection in murine Cryptococcus neoformans infection is associated with pleotrophic effects on cytokine and leukocyte responses. Infect. Immun. 701571-1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ferreira, K. S., K. R. Bastos, M. Russo, and S. R. Almeida. 2007. Interaction between Paracoccidioides brasiliensis and pulmonary dendritic cells induces interleukin-10 production and toll-like receptor-2 expression: possible mechanisms of susceptibility. J. Infect. Dis. 1961108-1115. [DOI] [PubMed] [Google Scholar]
- 20.Franco, M. 1987. Host-parasite relationships in paracoccidioidomycosis. J. Med. Vet. Mycol. 255-18. [DOI] [PubMed] [Google Scholar]
- 21.Larsen, R. A., P. G. Pappas, J. Perfect, J. A. Aberg, A. Casadevall, G. A. Cloud, R. James, S. Filler, and W. E. Dismukes. 2005. Phase I evaluation of the safety and pharmacokinetics of murine-derived anticryptococcal antibody 18B7 in subjects with treated cryptococcal meningitis. Antimicrob. Agents Chemother. 49952-958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liesenfeld, O., H. Kang, D. Park, T. A. Nguyen, C. V. Parkhe, H. Watanabe, T. Abo, A. Sher, J. S. Remington, and Y. Suzuki. 1999. TNF-alpha, nitric oxide and IFN-gamma are all critical for development of necrosis in the small intestine and early mortality in genetically susceptible mice infected perorally with Toxoplasma gondii. Parasite Immunol. 21365-376. [DOI] [PubMed] [Google Scholar]
- 23.Mbawuike, I. N., and H. B. Herscowitz. 1989. MH-S, a murine alveolar macrophage cell line: morphological, cytochemical, and functional characteristics. J. Leukoc. Biol. 46119-127. [DOI] [PubMed] [Google Scholar]
- 24.Moragues, M. D., M. J. Omaetxebarria, N. Elguezabal, M. J. Sevilla, S. Conti, L. Polonelli, and J. Ponton. 2003. A monoclonal antibody directed against a Candida albicans cell wall mannoprotein exerts three anti-C. albicans activities. Infect. Immun. 715273-5279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mota, N. G., M. T. Rezkallah-Iwasso, M. T. Peracoli, R. C. Audi, R. P. Mendes, J. Marcondes, S. A. Marques, N. L. Dillon, and M. F. Franco. 1985. Correlation between cell-mediated immunity and clinical forms of paracoccidioidomycosis. Trans. R. Soc. Trop. Med. Hyg. 79765-772. [DOI] [PubMed] [Google Scholar]
- 26.Mukherjee, J., W. Cleare, and A. Casadevall. 1995. Monoclonal antibody mediated capsular reactions (Quellung) in Cryptococcus neoformans. J. Immunol. Methods 184139-143. [DOI] [PubMed] [Google Scholar]
- 27.Mukherjee, J., G. Nussbaum, M. D. Scharff, and A. Casadevall. 1995. Protective and nonprotective monoclonal antibodies to Cryptococcus neoformans originating from one B cell. J. Exp. Med. 181405-409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mukherjee, J., M. D. Scharff, and A. Casadevall. 1992. Protective murine monoclonal antibodies to Cryptococcus neoformans. Infect. Immun. 604534-4541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mukherjee, J., M. D. Scharff, and A. Casadevall. 1995. Variable efficacy of passive antibody administration against diverse Cryptococcus neoformans strains. Infect. Immun. 633353-3359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Musatti, C. C., M. T. S. Peracoli, A. M. V. C. Soares, and M. T. Rezkallah-Iwasso. 1994. Cell-mediated immunity in patients with paracoccidioidomycosis, p. 175-186. In M. Franco, C. S. Lacaz, A. Restrepo, and G. del Negro (ed.), Paracoccidioidomycosis. CRC Press, Boca Raton, FL.
- 31.Nosanchuk, J. D., J. N. Steenbergen, L. Shi, G. S. Deepe, Jr., and A. Casadevall. 2003. Antibodies to a cell surface histone-like protein protect against Histoplasma capsulatum. J. Clin. Investig. 1121164-1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pachl, J., P. Svoboda, F. Jacobs, K. Vandewoude, B. van der Hoven, P. Spronk, G. Masterson, M. Malbrain, M. Aoun, J. Garbino, J. Takala, L. Drgona, J. Burnie, and R. Matthews. 2006. A randomized, blinded, multicenter trial of lipid-associated amphotericin B alone versus in combination with an antibody-based inhibitor of heat shock protein 90 in patients with invasive candidiasis. Clin. Infect. Dis. 421404-1413. [DOI] [PubMed] [Google Scholar]
- 33.Pick, E., and D. Mizel. 1981. Rapid microassays for the measurement of superoxide and hydrogen peroxide production by macrophages in culture using an automatic enzyme immunoassay reader. J. Immunol. Methods 46211-226. [DOI] [PubMed] [Google Scholar]
- 34.Pina, A., R. C. Valente-Ferreira, E. E. Molinari-Madlum, C. A. Vaz, A. C. Keller, and V. L. Calich. 2004. Absence of interleukin-4 determines less severe pulmonary paracoccidioidomycosis associated with impaired Th2 response. Infect. Immun. 722369-2378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Puccia, R., S. Schenkman, P. A. Gorin, and L. R. Travassos. 1986. Exocellular components of Paracoccidioides brasiliensis: identification of a specific antigen. Infect. Immun. 53199-206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Puccia, R., and L. R. Travassos. 1991. The 43-kDa glycoprotein from the human pathogen Paracoccidioides brasiliensis and its deglycosylated form: excretion and susceptibility to proteolysis. Arch. Biochem. Biophys. 289298-302. [DOI] [PubMed] [Google Scholar]
- 37.Ralph, P., J. Prichard, and M. Cohn. 1975. Reticulum cell sarcoma: an effector cell in antibody-dependent cell-mediated immunity. J. Immunol. 114898-905. [PubMed] [Google Scholar]
- 38.Restrepo, A., and M. D. Arango. 1980. In vitro susceptibility testing of Paracoccidioides brasiliensis to sulfonamides. Antimicrob. Agents Chemother. 18190-194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rivera, J., J. Mukherjee, L. M. Weiss, and A. Casadevall. 2002. Antibody efficacy in murine pulmonary Cryptococcus neoformans infection: a role for nitric oxide. J. Immunol. 1683419-3427. [DOI] [PubMed] [Google Scholar]
- 40.Rodrigues, E. G., and L. R. Travassos. 1994. Nature of the reactive epitopes in Paracoccidioides brasiliensis polysaccharide antigen. J. Med. Vet. Mycol. 3277-81. [PubMed] [Google Scholar]
- 41.Rodrigues, M. L., L. R. Travassos, K. R. Miranda, A. J. Franzen, S. Rozental, W. de Souza, C. S. Alviano, and E. Barreto-Bergter. 2000. Human antibodies against a purified glucosylceramide from Cryptococcus neoformans inhibit cell budding and fungal growth. Infect. Immun. 687049-7060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Saraiva, E. C., A. Altemani, M. F. Franco, C. S. Unterkircher, and Z. P. Camargo. 1996. Paracoccidioides brasiliensis-gp43 used as paracoccidioidin. J. Med. Vet. Mycol. 34155-161. [DOI] [PubMed] [Google Scholar]
- 43.Shapiro, S., D. O. Beenhouwer, M. Feldmesser, C. Taborda, M. C. Carroll, A. Casadevall, and M. D. Scharff. 2002. Immunoglobulin G monoclonal antibodies to Cryptococcus neoformans protect mice deficient in complement component C3. Infect. Immun. 702598-2604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shikanai-Yasuda, M. A., Q. Telles Filho Fde, R. P. Mendes, A. L. Colombo, and M. L. Moretti. 2006. Guidelines in paracoccidioidomycosis. Rev. Soc. Bras. Med. Trop. 39297-310. (In Portuguese.) [DOI] [PubMed] [Google Scholar]
- 45.Straus, A. H., E. Freymuller, L. R. Travassos, and H. K. Takahashi. 1996. Immunochemical and subcellular localization of the 43 kDa glycoprotein antigen of Paracoccidioides brasiliensis with monoclonal antibodies. J. Med. Vet. Mycol. 34181-186. [DOI] [PubMed] [Google Scholar]
- 46.Taborda, C. P., and A. Casadevall. 2002. CR3 (CD11b/CD18) and CR4 (CD11c/CD18) are involved in complement-independent antibody-mediated phagocytosis of Cryptococcus neoformans. Immunity 16791-802. [DOI] [PubMed] [Google Scholar]
- 47.Taborda, C. P., and A. Casadevall. 2001. Immunoglobulin M efficacy against Cryptococcus neoformans: mechanism, dose dependence, and prozone-like effects in passive protection experiments. J. Immunol. 1662100-2107. [DOI] [PubMed] [Google Scholar]
- 48.Taborda, C. P., M. A. Juliano, R. Puccia, M. Franco, and L. R. Travassos. 1998. Mapping of the T-cell epitope in the major 43-kilodalton glycoprotein of Paracoccidioides brasiliensis which induces a Th-1 response protective against fungal infection in BALB/c mice. Infect. Immun. 66786-793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Taborda, C. P., J. Rivera, O. Zaragoza, and A. Casadevall. 2003. More is not necessarily better: prozone-like effects in passive immunization with IgG. J. Immunol. 1703621-3630. [DOI] [PubMed] [Google Scholar]
- 50.Travassos, L. R., C. P. Taborda, L. K. Iwai, E. Cunha Neto, and R. Puccia. 2004. The gp43 from Paracoccidioides brasiliensis: a major diagnostic antigen and vaccine candidate, p. 279-296. In J. E. Domer and G. S. Kobayashi (ed.), The mycota, vol. 12. Human fungal pathogens. Springer-Verlag, Berlin, Germany. [Google Scholar]
- 51.Wells, J., C. G. Haidaris, T. W. Wright, and F. Gigliotti. 2006. Active immunization against Pneumocystis carinii with a recombinant P. carinii antigen. Infect. Immun. 742446-2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Xander, P., A. F. Vigna, S. Feitosa Ldos, L. Pugliese, A. M. Bailao, C. M. Soares, R. A. Mortara, M. Mariano, and J. D. Lopes. 2007. A surface 75-kDa protein with acid phosphatase activity recognized by monoclonal antibodies that inhibit Paracoccidioides brasiliensis growth. Microbes Infect. 91484-1492. [DOI] [PubMed] [Google Scholar]
- 53.Yuan, R. R., A. Casadevall, J. Oh, and M. D. Scharff. 1997. T cells cooperate with passive antibody to modify Cryptococcus neoformans infection in mice. Proc. Natl. Acad. Sci. USA 942483-2488. [DOI] [PMC free article] [PubMed] [Google Scholar]