Abstract
To infect an animal host, Salmonella enterica serovar Typhimurium must penetrate the intestinal epithelial barrier. This process of invasion requires a type III secretion system encoded within Salmonella pathogenicity island I (SPI1). We found that a mutant with deletions of the acetate kinase and phosphotransacetylase genes (ackA-pta) was deficient in invasion and SPI1 expression but that invasion gene expression was completely restored by supplying medium conditioned by growth of the wild-type strain, suggesting that a signal produced by the wild type, but not by the ackA-pta mutant, was required for invasion. This mutant also excreted 68-fold-less formate into the culture medium, and the addition of sodium formate to cultures restored both the expression of SPI1 and the invasion of cultured epithelial cells by the mutant. The effect of formate was pH dependent, requiring a pH below neutrality, and studies in mice showed that the distal ileum, the preferred site of Salmonella invasion in this species, had the appropriate formate concentration and pH to elicit invasion, while the cecum contained no detectable formate. Furthermore, we found that formate affected the major regulators of SPI1, hilA and hilD, but that the primary routes of formate metabolism played no role in its activity as a signal.
Salmonella enterica serovar Typhimurium has evolved an elaborate mechanism to promote its penetration of the intestinal epithelium, an early step in its pathogenesis. It harbors in its genome Salmonella pathogenicity island 1 (SPI1), a 40-kb region encoding some 40 genes (8, 24, 28, 33, 43, 50). This region produces the components of a type III secretion apparatus and secreted proteins that are exported to the bacterial surface and into adjacent eukaryotic cells. These proteins then signal the eukaryotic cell to induce cytoskeletal changes that lead to bacterial engulfment (11, 23, 29, 38, 66). SPI1 invasion genes are known to be important for both septicemia and enterocolitis caused by Salmonella (24, 33, 49, 62).
The genetic regulation of SPI1 is complex. Much of the response to environmental conditions requires HilA, an SPI1 transcriptional regulator of the OmpR/ToxR family (6, 7). Among the targets of HilA is the SPI1 gene invF, which itself encodes a transcriptional regulator (35). HilA and InvF have overlapping, but not identical, sets of targets, both within and outside SPI1 (1, 14, 17). HilA, in turn, is subject to multiple controls, with two additional SPI1 regulators, HilC and HilD, inducing hilA expression (18, 32, 58). In addition to control by transcriptional regulators within SPI1, invasion genes are also under the control of several regulators outside the island (20, 31, 45, 51, 59, 64). Among these is the two-component regulator BarA/SirA. BarA is a sensor kinase of the phosphorelay type, and SirA is its cognate response regulator, with both being required for invasion gene expression and bacterial penetration of epithelial cells (1, 3, 32, 61). SirA induces invasion through the induction of hilA and hilC and by its control of csrB and csrC (22, 61). The latter two encode untranslated RNAs that oppose the action of the posttranscriptional regulator CsrA, also known to affect invasion (2). Thus, SirA regulates SPI1 genes both directly, through the induction of hilA and hilC, and indirectly, by its control of the csr regulatory system.
The regulation of invasion genes also requires a coordinated response to the varied environmental signals present in the gastrointestinal tract. Environmental conditions control the induction of SPI1 genes through HilA (7) and include pH, oxygen tension, osmolarity, growth phase, and bile (7, 25, 42, 43, 46, 55, 56). In addition, short chain fatty acids (SCFAs) appear to play an important role in regulating invasion genes. We have previously shown that acetate can induce invasion in a barA mutant but requires sirA to do so. This effect also requires ackA-pta, which encodes acetate kinase and phosphate acetyltransferase and mediates the interconversion of acetate to acetyl-phosphate and acetyl-coenzyme A (CoA) (see Fig. 5A), suggesting that at least one of these products is required for the BarA-independent induction of SirA (41). The mammalian gastrointestinal tract contains high levels of SCFAs, including formate, acetate, propionate, and butyrate, as the result of the breakdown of food by digestive processes and the action of resident intestinal bacteria. SCFA types and concentrations vary through the gastrointestinal tract. The levels in the small intestine, the site of Salmonella invasion, are between 20 and 40 mM total SCFA, while the levels in the colon range above 100 mM, depending on the animal species and diet (4, 5, 9, 12, 47). Also varying in the gastrointestinal tract are the proportion and the distribution of these SCFAs. Studies of pigs have shown that acetate and formate predominate in the distal small intestine but that propionate and butyrate are in higher concentrations in the cecum and colon (4, 40). It has been demonstrated that while acetate induces invasion genes, propionate and butyrate can repress them (16, 26, 41), suggesting that these SCFAs provide environmental cues that allow Salmonella to recognize specific regions of the intestinal tract.
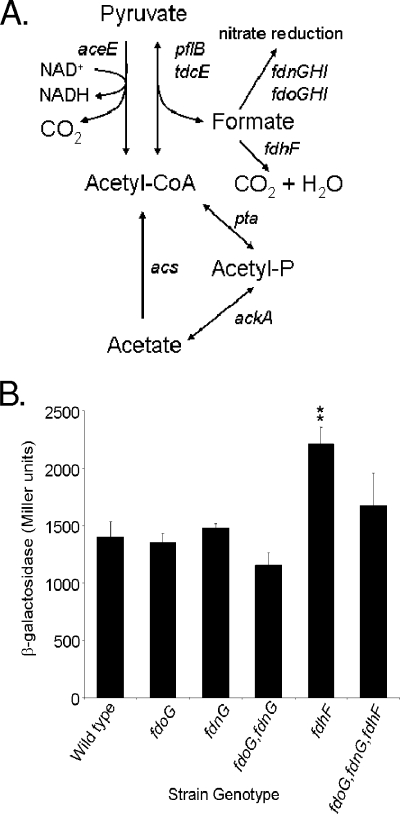
FIG. 5.
Effects of formate metabolism on sipC expression. (A) Pathways for the integration of formate into central carbon metabolism. (B) Strains with the genotype shown and with the sipC::lacZY fusion were grown as standing overnight cultures in LB with 100 mM MOPS, pH 6.7. Triplicate cultures of each strain were assayed for sipC::lacZY expression by using β-galactosidase assays. Double asterisks show a significant difference for the mutant strain compared to the wild type. Error bars show standard deviations.
Although both environmental signals and genetic regulators of Salmonella invasion are known, in most cases the means by which the environment signals Salmonella to invade have not been elucidated. SirA is required for both repression by bile and activation by acetate (41, 56), but the pathways for responses to other signals have not been defined. Here we describe the induction of invasion genes by formate, a common constituent of the mammalian small intestine. We show that formate produced by Salmonella grown in laboratory medium acts as a diffusible signal that induces invasion. This effect is independent of the known induction pathway utilizing BarA/SirA and the csr regulatory system and requires that formate enter the bacterial cytoplasm to have its effect. We also show that formate affects the major regulators of SPI1, hilA and hilD, but that the primary route of formate metabolism, through the formate dehydrogenases, plays no role in its activity as a signal.
MATERIALS AND METHODS
Bacterial strains.
The strains used in this study are shown in Table 1. Precise deletions for all strains described in this study were made by using a one-step inactivation method (15). PCR primers were designed to allow the amplification of the kanamycin resistance marker from plasmid pKD4 or the chloramphenicol resistance marker from pKD3. Each primer had at its 5′ end 40 bases of homology to the regions immediately flanking the initiation or termination codon of the gene to be deleted linked to a 3′ sequence homologous to the plasmid to be amplified. The resulting PCR products were used to transform strain ATCC 14028s carrying pKD46, which encodes λ Red recombinase that provides for allelic exchange. Candidate mutants were tested for the loss of the appropriate region by PCR amplification. The marked disruptions were then moved into ATCC 14028s carrying the sipC::lacZY fusion by P22 transduction. To create unmarked mutants when strains with multiple mutations were required, resistance markers were excised by using FLP recombinase (15). The csrB::lacZ fusion was similarly created by first deleting csrB and then integrating lacZ at the site of the disruption, as described previously (19).
TABLE 1.
Strains used in this study
| Strain no. | Genotype | Source or reference |
|---|---|---|
| ATCC 14028s | Wild type | American Type Culture Collection |
| CA412 | sipC::lacZY | 7 |
| CA513 | ΔbarA | 3 |
| CA773 | sirA::cam | 3 |
| CA774 | Δ(ackA-pta) | 41 |
| CA776 | Δ(ackA-pta) ΔbarA | This study |
| CA1282 | Δ(ackA-pta) sirA::cam | This study |
| CA781 | sipC::lacZY ΔbarA | This study |
| CA921 | sipC::lacZY sirA::cam | This study |
| CA925 | sipC::lacZY Δ(ackA-pta) | This study |
| CA1290 | sipC::lacZY ΔfdoG | This study |
| CA1289 | sipC::lacZY ΔfdnG | This study |
| CA1292 | sipC::lacZY ΔfdoG ΔfdnG | This study |
| CA1288 | sipC::lacZY ΔfdhF | This study |
| CA1295 | sipC::lacZY ΔfdoG ΔfdnG ΔfdhF | This study |
| CA1558 | sipC::lacZY ΔpflB | This study |
Conditioned-medium assays.
Conditioned medium was made by growing wild-type, sirA mutant, or ackA-pta mutant strains standing overnight in 1× morpholinepropanesulfonic acid (MOPS) minimal medium (52) with 0.5% glucose and a combination of 18 amino acids in concentrations equivalent to 0.5% tryptone (Difco) and then filter sterilizing the medium. The wild type and the barA and ackA-pta mutants with the sipC::lacZY fusion were grown standing overnight in 50% 2× MOPS minimal medium with 1% glucose and 50% of either fresh or conditioned medium. Fresh medium consisted of 1× MOPS minimal medium with 0.5% glucose and amino acids.
β-Galactosidase assays.
For β-galactosidase assays used to assess the effects of formate, bacteria were grown as standing overnight cultures in LB with the addition of 100 mM MOPS, pH 6.7. Sodium formate was added at a concentration of 10 mM, while control cultures received 10 mM NaCl. To assess the effects of pyruvate, sodium pyruvate or NaCl was added at 50 mM. Triplicate cultures of each bacterial strain were used unless otherwise noted. For assays to assess the role of pH, strains were grown standing overnight in LB with either 100 mM HEPES, pH 8.0, or 100 mM MOPS, pH 6.7. All cultures were assayed for β-galactosidase activity as described previously (48). Each experiment was repeated independently at least twice. Data from a single experiment that is representative of the independent replicates are shown.
Invasion assays.
Bacteria were grown overnight as standing cultures in LB broth buffered with 100 mM MOPS, pH 6.7, and with either 10 mM sodium chloride or 10 mM sodium formate. Bacteria were added to HEp-2 cells grown to confluence in 24-well plates for a multiplicity of infection of approximately 10 bacteria/cell. The plates were incubated for 1 h at 37°C, the medium was removed, the cells were washed three times with phosphate-buffered saline, and the medium was replaced by medium supplemented with gentamicin (20 μg/ml). The cells were incubated for an additional hour, the medium was removed, and monolayers were washed three times with phosphate-buffered saline. The cells were lysed with 1% Triton X-100 for 5 min, and the bacterial titers of the lysates were determined by colony counts. Each strain was tested in quadruplicate for each condition. The assays were performed twice, and the data are the representative results from a single experiment.
Determination of formate concentrations.
The formate concentrations in the media were measured by using a formic acid enzymatic bioanalysis detection kit, following the manufacturer's directions (R-Biopharm). This method measured formate concentration by the enzymatic conversion of formate and NAD+ to bicarbonate and NADH through the action of formate dehydrogenase. The NADH concentration was then measured by the UV light absorbance at a wavelength of 340 nm.
Animal experiments.
Three 7-week-old female C57BL/6 mice were used to determine the pH and the formate concentrations in the intestinal tract. To measure pH, the ileum of the mouse was transected at the junction of the cecum immediately after euthanasia, and an Orion micro combination pH electrode (Thermo Electron Corp., Beverly, MA) was inserted retrograde ∼2 mm into the distal ileum. To determine formate concentrations, cecal and ileal samples were acidified by using a 1% solution of H2SO4, flash frozen in liquid nitrogen, and stored at −80°C. Before analysis, the samples were thawed and the mass of each sample was recorded. The samples were analyzed by high-pressure liquid chromatography as previously described (60), using crotonic acid as the internal standard. Volatile fatty acids (VFA) were separated by using a Supelcogel H (carbohydrate) column (250 by 4.6 mm with 9-μm particles; Supelco, Bellefonte, PA) with a guard column (Supelcogel H; Supelco). Twenty microliters of sample was injected, and a flow rate of 0.17 ml/min was used to adjust for the narrower bore of the Supelcogel H column. All samples were run for 70 min at 39°C using a mobile phase of 0.015 N sulfuric acid and 0.25 mM EDTA. A Beckman System Gold (Beckman Coulter, Fullerton, CA) was used with the UV detector set at 210 nm, and the results were analyzed by using a VFA standard and 32 Karat software (Beckman Coulter).
Reverse transcription-real-time PCR.
The ackA-pta mutant strain was grown for 3 h in LB-100 mM MOPS, pH 6.7, with gentle agitation (60 rpm) to an optical density at 600 nm of 0.5 to 0.55 with either sodium formate or sodium chloride added at a concentration of 10 mM. Two independent cultures were used for each condition. Total RNA was isolated as described at http://derisilab.ucsf.edu/data/microarray/pdfs/Total_RNA_from_Ecoli.pdf with modifications to use 1/10th of all volumes. First-strand cDNA synthesis was performed with 1 μg of RNA using SuperScript III reverse transcriptase (Invitrogen) and 300 ng of random primers. The real-time PCR was performed with B-R Sybr green supermix (Quanta Biosciences) and a MyiQ thermocycler (Bio-Rad), with cycling once at 95°C followed by 40 cycles of 95°C for 10 s and 58°C for 30 s. Each sample was tested in triplicate. The primers used were ATAGCAAACTCCCGACGATG and ATTAAGGCGACAGAGCTGG for hilA; GGTAGTTAACGTGACGCTTG and GATCTTCTGCGCTTTCTCTG for hilD; ATCAGGCTGGTCGATTTACG and GTACGCCGCTACTCAGGAAC for sipC; and GCAAAGTAGTTGTTCCGGTG and CTTTAAGCATGGCTGGAGTG for icdA. The amounts of PCR product present for each invasion gene were normalized to that of icdA, which was previously determined not to be affected by formate (data not shown).
Statistical analysis.
For β-galactosidase and invasion assays, a one-way analysis of variance was used to determine whether the mean of at least one strain differed from that of any of the others. Then, multiple-comparison tests were used to determine which means differed at a P value of ≤0.05 (The SAS system for Windows 8 and MINITAB release 14).
RESULTS
Invasion is induced by a diffusible signal that requires ackA-pta for its production.
In previous work, we showed that either the sensor kinase BarA or acetate was sufficient to induce Salmonella virulence in the mouse model of septicemia and that the ackA-pta operon, encoding acetate kinase and phosphate acetyltransferase, was required for acetate to act as an inducing signal for invasion. In laboratory medium, however, invasion genes were poorly expressed in the ackA-pta mutant, even in the presence of intact barA (41). These contradictory results, that ackA-pta was redundant in vivo but required in vitro, led us to reason that a diffusible signal present in the intestinal tract, other than acetate, was necessary for the induction of invasion genes, but that an ackA-pta mutant failed to express invasion genes when grown in laboratory medium because it was unable to produce that signal. To test this, we examined the effects of culture supernatants obtained from the wild type, a sirA mutant, and an ackA-pta mutant on the expression of a β-galactosidase fusion to sipC, an SPI1 invasion gene that encodes a secreted effector protein and is under the control of known SPI1 regulators (Fig. 1). In the ackA-pta mutant, the expression of sipC was approximately one-ninth that of the wild type when grown without the addition of conditioned medium but was completely restored to the level of the wild type by medium derived from either wild-type or sirA mutant cultures. Medium produced by the growth of the ackA-pta mutant itself, however, failed to significantly increase sipC expression by the ackA-pta mutant. In a barA mutant, the level of sipC expression was approximately 1/11 of that of the wild type and was not significantly induced by conditioned medium from any of the strains, while the level of expression in the wild type was unchanged by the addition of medium conditioned by the growth of any of the three strains. Thus, these results indicate that the induction of SPI1 invasion genes requires a signaling molecule that is secreted into the culture medium and that acetate kinase and/or phosphotransacetylase is necessary for the production of the signal. They also show that barA, encoding the sensor kinase of the BarA/SirA two-component regulator, is required for invasion gene expression even in the presence of this signal but that this two-component regulator is not necessary for signal production.
FIG. 1.
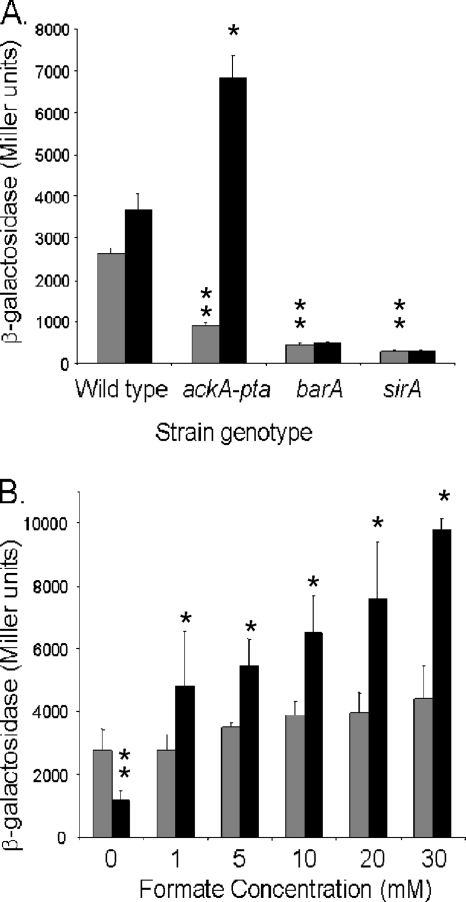
Effects of conditioned medium on sipC expression. The wild type and barA and ackA-pta mutants with a sipC::lacZY fusion were grown standing overnight in 50% 2× MOPS minimal medium with 1% glucose and 50% of either fresh or conditioned medium. Fresh medium (gray bars) consisted of 1× MOPS minimal medium with 0.5% glucose and amino acids. Conditioned medium was made by growing the wild type (striped bars), sirA mutant (white bars), or ackA-pta mutant (black bars) standing overnight in this same medium and then filter sterilizing the culture supernatant. Expression of sipC was assessed by using β-galactosidase assays. Values represent the mean for each condition tested in triplicate. Asterisks show a significant difference (P ≤ 0.05) for the strain with the genotype shown when grown with the addition of conditioned medium compared to the fresh-medium control. Error bars show standard deviations.
Formate induces invasion.
Mutants of ackA and pta have been extensively studied in Escherichia coli, as they are commonly used to optimize culture conditions in the industrial production of recombinant proteins. It is known that in E. coli, ackA-pta mutants fail to produce three metabolic products, acetate, ethanol, and formate, that are normally excreted into the culture medium, the latter two during anaerobic growth (65). We reasoned that a normally excreted product that is not produced by an ackA-pta mutant might be the signal for invasion. When tested, ethanol had no effect on sipC expression (data not shown), and acetate had been shown previously not to restore invasion in an ackA-pta mutant (41). We therefore next considered formate. To verify that the loss of ackA and pta in Salmonella resulted in the reduced excretion of formate into the culture medium, as it does in E. coli, we measured the concentration of formate in culture supernatants by using an enzymatic assay. After overnight growth of the wild-type strain under conditions identical to those used for the conditioned-medium experiments whose results are shown in Fig. 1 (minimal medium supplemented with glucose and amino acids), the formate concentration in the culture supernatant was 8.8 ± 0.23 mM (mean ± standard deviation). The ackA-pta mutant produced only 0.13 ± 0.08 mM formate, indicating that the wild-type strain could secrete 68-fold-more formate into the culture medium than could the mutant. The addition of 10 mM sodium formate to the medium prior to the growth of the ackA-pta mutant restored the formate concentration in the supernatant to 7.6 ± 0.43 mM. This showed that some portion of the added formate was metabolized during bacterial growth. It also demonstrated that the addition of exogenous sodium formate at this concentration created a concentration of formate in the medium for the ackA-pta mutant near to that produced endogenously by the wild type.
We next tested the effects of formate on invasion gene expression. For these and all subsequent experiments, we used pH-buffered LB as the growth medium, as it maintained stable culture pH after overnight growth. We found that the addition of 10 mM sodium formate to an ackA-pta mutant restored sipC expression to a level greater than that of the wild-type strain (Fig. 2A). This same concentration of sodium formate, however, had no significant effect in strains that carried null mutations of either barA or sirA. Similar results were obtained upon the addition of formic acid (data not shown), and the addition of either sodium formate or formic acid did not alter the pH of the medium before or after the growth of the culture. To determine the concentration of formate required to induce invasion genes, we grew the ackA-pta mutant in media containing increasing amounts of sodium formate (Fig. 2B). All of the concentrations tested, 1 mM or higher, significantly induced sipC expression, to the level of the wild type or higher.
FIG. 2.
Effects of formate on sipC expression. (A) Strains were grown as standing overnight cultures in LB with 100 mM MOPS, pH 6.7, and with 10 mM sodium chloride (gray bars) or 10 mM sodium formate (black bars). Triplicate cultures of each strain were assayed for sipC::lacZY expression by using β-galactosidase assays. (B) Overnight standing cultures of the wild type (gray bars) and ackA-pta mutant (black bars) with the sipC::lacZY fusion were grown in LB with 100 mM MOPS, pH 6.7, and with 0, 1, 5, 10, 20, or 30 mM sodium formate. Triplicate cultures for each condition were assayed for sipC::lacZY expression. Single asterisks show a significant difference (P ≤ 0.05) for the strain with the genotype shown when grown with the addition of formate compared to the NaCl control. Double asterisks show a significant difference for the mutant strain compared to the wild type when both were grown without formate. Error bars show standard deviations.
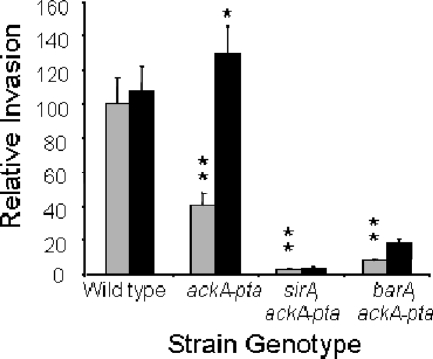
We next directly tested the effect of formate on invasion by using cultured epithelial cells (Fig. 3). The level of invasion of HEp-2 cells by the wild type was 2.5-fold higher than that of the ackA-pta mutant. Similar to the findings for SPI1 gene expression, the addition of 10 mM sodium formate, however, restored invasion threefold in the mutant, to a level comparable to that of the wild type. To assess the importance of BarA/SirA to invasion induced by formate, we tested isogenic ackA-pta mutants that also lacked either sirA or barA. The loss of sirA in the ackA-pta mutant reduced invasion, with the wild type invading at a 33-fold-higher level, and the addition of formate failed to significantly improve invasion. Similarly, the barA null mutation in the same strain background reduced the level of invasion to 1/12 of that of the wild type without a significant increase in invasion with added formate. These results therefore show that formate, or some product of formate, is the signal required for the induction of invasion but that the BarA/SirA two-component regulator remains essential for invasion, even in the presence of formate.
FIG. 3.
Invasion of epithelial cells. Bacteria were grown overnight as standing cultures in LB broth buffered with 100 mM MOPS, pH 6.7, and with either 10 mM sodium chloride (gray bars) or 10 mM sodium formate (black bars). Bacteria were added to HEp-2 cells, and the level of invasion was assessed by using a gentamicin protection assay. Invasion is shown in comparison to that of the wild-type strain set to 100. Each strain and condition was tested in quadruplicate. Single asterisks show a significant difference (P ≤ 0.05) for the strain with the genotype shown when grown with the addition of formate compared to the NaCl control. Double asterisks show a significant difference for the mutant strain compared to the wild type when both were grown without formate. Error bars show standard deviations.
pH affects the signaling activity of formate.
As the intestinal tract of animals, the site of Salmonella invasion, has a pH near or slightly below neutrality, we sought to determine whether the pH of the medium affected the ability of formate to induce invasion. It is also known that weak acids, such as formate, diffuse into the bacterial cytoplasm more readily at a lower pH and so achieve higher concentrations within bacteria. We therefore tested sipC expression in Salmonella strains grown at either pH 6.7, the approximate pH of the mammalian ileum, or pH 8.0 (Fig. 4). At pH 6.7, the level of expression in the ackA-pta mutant was 4.5-fold less than that of the wild type, but the addition of 10 mM formate induced sipC expression in this mutant to the level observed in the wild type when it was grown without the addition of exogenous formate. Formate also increased the level of expression in the wild type to a lesser degree, approximately 67%. At pH 8, the level of sipC expression in the ackA-pta mutant was nearly ninefold less than in the wild type. At this pH, however, formate did not significantly increase sipC expression in either strain. Thus, formate did not affect SPI1 expression under alkaline conditions, but instead required a lower pH, similar to that found within the intestinal tract, suggesting that formate must enter the bacterial cytoplasm to have its effect.
FIG. 4.
Effects of medium pH on sipC expression in response to formate. The wild type and ackA-pta mutants with a sipC::lacZY fusion were grown standing overnight in LB with 100 mM HEPES, pH 8.0, or 100 mM MOPS, pH 6.7, as indicated, and with either 10 mM sodium chloride (gray bars) or 10 mM sodium formate (black bars). Expression of sipC was assessed by using β-galactosidase assays. Values represent the mean for each condition tested in duplicate. Single asterisks show a significant difference (P ≤ 0.05) for the strain with the genotype shown when grown with the addition of formate compared to the NaCl control. Double asterisks show a significant difference for the mutant strain compared to the wild type when both were grown without formate. Error bars show standard deviations.
To confirm that the pH and formate concentrations tested reflect those found in the intestinal tracts of animals used as models of Salmonella infection, we measured these values in mice. We found the pH of the distal ileum of conventionally raised C57BL/6 mice to be 6.80 ± 0.35. Although the level of formate varied among the mice tested, it was detected in this same region of the intestine at a concentration of 8.2 mM ± 6.5 mM, with the lowest concentration detected in any mouse being 3.2 mM. All of these concentrations were therefore well above that required to induce invasion gene expression in vitro (Fig. 2B). In contrast, we could detect no formate in the cecum of these mice (with a limit of detection of less than 0.08 mM), showing that formate is, indeed, present in the distal ileum in a concentration capable of inducing invasion but is absent or in very low concentration in the adjacent cecum.
Effects of formate metabolism on signaling.
Salmonella produces formate as a by-product of anaerobic growth. Formate can then be used to maintain the redox balance during anaerobic respiration by acting as an electron donor for nitrite, as well as a number of other substrates of anaerobic respiration, and can donate electrons to the quinone pool (Fig. 5A). These major routes of formate utilization alternatively employ the formate dehydrogenase O (FdhO) system, encoded by fdoGHI, or the FdhN system, encoded by fdnGHI. To determine whether products of formate oxidation, rather than the molecule itself, were signals for the activation of invasion, we examined mutants with mutations of these known formate degradation pathways for the effects of the mutations on invasion gene expression. We found that the loss of either pathway, through the mutation of either fdoG or fdnG, did not change the expression of sipC (Fig. 5B). The loss of both mechanisms for the production of reduced metabolic intermediates, using an fdoG fdnG double mutant, also failed to alter sipC expression. Alternatively, Salmonella can excrete formate, but under the low-pH conditions induced by fermentation, formate can reenter the bacterium, and there it can be used to stabilize the internal pH by its conversion to CO2 and H2. This reaction requires a membrane-bound formate-hydrogen lyase complex, an essential component of which is formate dehydrogenase H (FdhH), encoded by fdhF. We tested an fdhF null mutant and found it to have a small increase (approximately 63%) in the expression of sipC (Fig. 5B). Thus, as FdhH is required for the production of molecular hydrogen from formate, H2 produced by this pathway cannot be the signal for invasion. It is possible, however, that the small increase in invasion gene expression observed was due to an increase in formate concentration within the bacterium, making it available to other metabolic pathways. It has also been reported that in E. coli, the FdhH system, which normally produces H2 from formate, can play a significant role in the reduction of nitrite by formate (13). We therefore eliminated all of the known routes by which formate can reduce metabolic intermediates by constructing and testing an fdoG fdnG fdhF triple mutant and found that it did not differ from the wild type in the expression of sipC (Fig. 5B). These results thus show that the metabolic products of the known pathways of formate oxidation are not required to induce invasion.
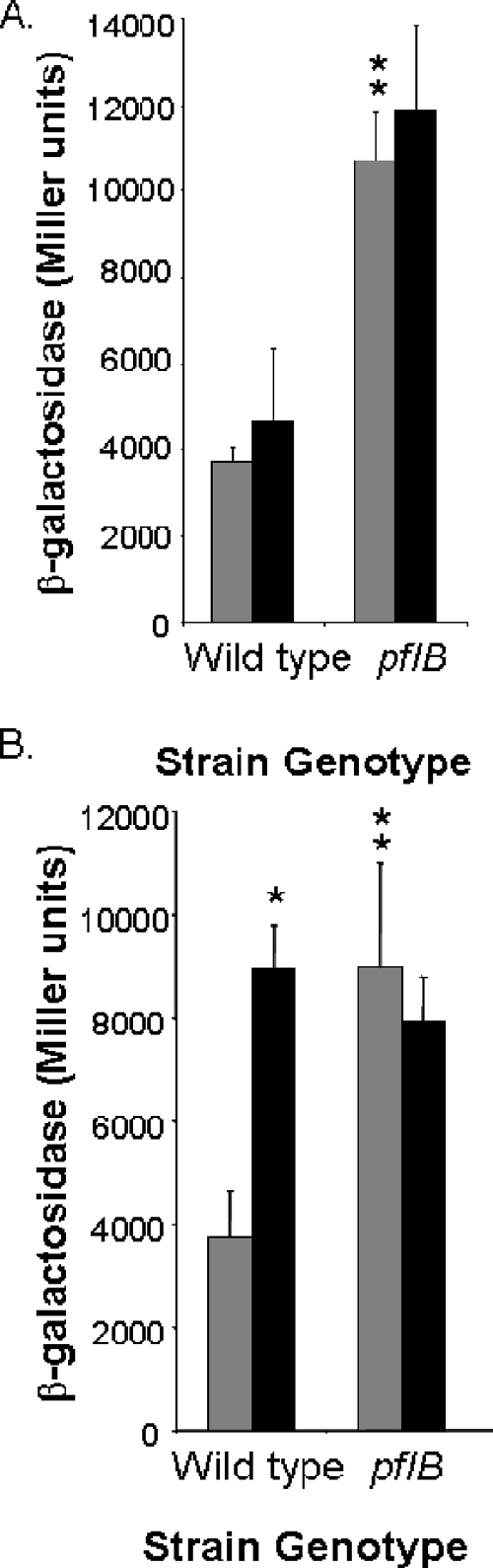
In Salmonella, the major source of formate production is from pyruvate by the action of pyruvate formate-lyase, encoded by pflB, the products of which are formate and acetyl-CoA (Fig. 5A). To determine directly whether endogenous formate production was required for Salmonella invasion gene expression, we tested a pflB null mutant. We first determined the amount of formate produced by this mutant. Unlike the findings for growth in minimal medium with glucose as the carbon source, strains grown in pH-buffered LB did not excrete measurable quantities of formate into the culture medium, presumably due to the lack of glucose as a primary source of formate production. The addition of 10 mM formate to the wild-type strain, however, resulted in 6.8 ± 1.4 mM formate in the medium after overnight growth, while the pflB mutant had no detectable formate remaining in the medium, suggesting that exogenous formate was more completely metabolized due to a reduction of endogenous production. Surprisingly, however, when we tested for the effects of pflB on invasion, we found that in the pflB mutant, the expression of sipC was not reduced but instead was significantly increased, by 2.9-fold (Fig. 6A). A pflB mutant should accumulate pyruvate, as it is unable to efficiently convert pyruvate to formate. It is therefore possible that pyruvate itself is able to stimulate invasion gene expression. To test this directly, we added 50 mM sodium pyruvate to the growth medium and assessed the changes in sipC expression (Fig. 6B). Indeed, we found that the addition of pyruvate increased expression 2.4-fold in the wild-type strain. The pflB mutant had intrinsically higher expression in the absence of exogenous pyruvate, comparable to that of the wild type grown in the presence of pyruvate, and did not show increased expression with the addition of pyruvate. To determine whether pyruvate induced invasion gene expression by a mechanism that was independent of formate, we also tested the effects of the addition of formate to the pflB mutant (Fig. 6A). We found that sipC expression was not significantly increased by formate in this mutant. These findings thus show that an increased level of pyruvate can induce invasion and suggest that this effect may be related to that of formate, as evidenced by the lack of effect of exogenous pyruvate on the pflB mutant. We additionally tested one other known route of pyruvate metabolism. In Salmonella, there also exists a homologue of E. coli tdcE, which shares strong homology with pflB in that organism and has been shown to possess formate pyruvate-lyase activity (30). We found that the mutation of tdcE alone had no effect on sipC expression, both with and without the addition of formate to the culture medium, and that a pflB tdcE double mutant had a phenotype identical to that of the pflB mutant alone (data not shown), indicating that tdcE has no significant role in these effects.
FIG. 6.
Effects of pflB on invasion gene expression in response to formate and pyruvate. Strains were grown as standing overnight cultures in LB with 100 mM MOPS, pH 6.7, and with 10 mM sodium chloride (gray bars) or 10 mM sodium formate (black bars) (A) or 50 mM sodium chloride (gray bars) or 50 mM sodium pyruvate (black bars) (B). Triplicate cultures of each strain were assayed for sipC::lacZY expression by using β-galactosidase assays. Single asterisks show a significant difference (P ≤ 0.05) for the strain with the genotype shown when grown with the addition of formate (A) or pyruvate (B) compared to the NaCl control. Double asterisks show a significant difference for the mutant strain compared to the wild type when both were grown without additions to the medium. Error bars show standard deviations.
Pathways of invasion induction by formate.
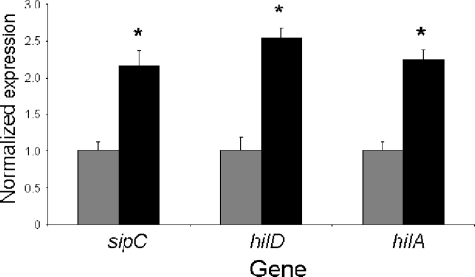
The expression of SPI1 genes is controlled by genetic regulators both within and outside SPI1. We therefore tested the effects of a number of these regulators on invasion gene induction by formate. One possible model for the action of formate is that it induces invasion by activating the sensor kinase BarA. This model is consistent with the genetic requirement for barA and sirA, but not with the proposed role of formate as a cytoplasmic signal, as suggested by its dependence upon pH. As BarA/SirA induces the small untranslated RNAs csrB and csrC, which subsequently control invasion (22, 41, 61), the activation of BarA/SirA by formate would lead to the increased expression of these small RNAs. Using a lacZ fusion to csrB, we found, however, that the ackA-pta mutant did not have reduced csrB expression, nor did the addition of 10 mM sodium formate alter its expression in this mutant strain (not shown), showing that formate does not function by activating BarA/SirA. We similarly tested the regulators phoP, part of the PhoPQ two-component regulator; relA and spoT, which produce ppGpp; and the repressor hilE, all of which are external regulators of SPI1 (8, 21, 54). None of these known regulators was affected by the loss of formate production through the mutation of ackA-pta or by the addition of exogenous formate (not shown). Within SPI1, there exist a number of transcriptional activators that induce invasion gene expression. To determine which of these was involved in the response to formate, we examined their expression levels by using reverse transcription-real-time PCR. HilA is a regulator that is central to the control of SPI1 genes and the integration of environmental signals into that control. We found that the level of hilA message was increased 2.2-fold in the ackA-pta mutant by the addition of exogenous formate (Fig. 7). hilA is itself controlled by HilD, another transcriptional activator within SPI1. We found that the hilD message was similarly elevated by formate, by 2.5-fold, while in the same assay, the sipC message increased 2-fold. Taken together, these results show that formate acts through the SPI1-encoded regulators hilA and hilD, but not through several of the known regulators found outside SPI1.
FIG. 7.
Effects of formate on SPI1 gene expression. The ackA-pta mutant strain was grown in LB broth buffered with 100 mM MOPS, pH 6.7, and with either 10 mM sodium chloride (gray bars) or 10 mM sodium formate (black bars). Total RNA was used to create cDNA and quantified by real-time PCR. Values were normalized by using the housekeeping gene icdA, and levels found in the control cultures were set to 1. Data are shown as the mean expression of two independent cultures with each culture tested in triplicate. Asterisks show a significant difference (P ≤ 0.05) for expression of the gene shown when bacteria were grown with the addition of formate compared to that of the NaCl control. Error bars show standard deviations.
DISCUSSION
To penetrate the intestinal epithelium of an animal host, Salmonella secretes effector proteins by means of the type III secretion apparatus of SPI1. The expression of SPI1 invasion genes must be appropriately timed, and Salmonella relies upon environmental signals from the intestinal tract to induce these genes. We show here that formate is likely to be such a signal. Our results show that formate can act as a diffusible signal for invasion when Salmonella is grown under laboratory conditions. This mechanism of self-induction, however, is almost certainly not required for invasion when Salmonella resides in the intestinal tract of an animal. Indeed, our previous results using the mouse model of septicemia showed that an ackA-pta mutant deficient in the production of formate maintained its virulence (41). Instead, Salmonella likely relies upon the formate produced by the resident microbiota of the intestine as a signal for invasion. For this purpose, formate is particularly well suited, as it is produced in high concentrations in the mammalian intestinal tract. Importantly, the results presented here and those of previous animal studies have shown formate to be present in measurable concentration in the distal ileum but absent or in lower concentration both in more-proximal portions of the intestine and more-distal portions, such as the cecum and colon (39, 40). The distal ileum is the preferred site for Salmonella colonization and penetration in animal models of enteritis and septicemia (10, 34, 53, 62). Thus, formate provides a plausible signal for the induction of invasion determinants at the site most appropriate for productive infection, and signaling by formate can explain, at least in part, the tropism of this pathogen for infection of this region of the intestine.
One finding presented in this work, that formate has its effects only at below-neutral pH, indicates that formate functions as a cytoplasmic signal. When the pH of the surrounding medium is lower than that of the bacterial cytoplasm, exogenous formic acid, a weak acid, can concentrate within bacteria. At equilibrium, the concentration of formate in the cytoplasm is dependent upon the difference between cytoplasmic and external pH and on the external concentration of formate. An external pH of 6.7 with 10 mM formate, as tested here, should allow a cytoplasmic concentration of formate approximately 20-fold greater than that produced at pH 8. The results of our work have shown that this lower pH is necessary for the activity of formate and thus reflect a requirement for high concentrations of cytoplasmic formate.
Formate plays important roles in both anaerobic respiration and fermentation, and thus, much is known about its metabolism. Although it can be degraded to produce both hydrogen and reduced metabolic intermediates, neither of these products appears to be required for the capacity of formate to signal the induction of invasion, as mutations of all known pathways of formate oxidation failed to reduce invasion gene expression. The regulation of formate metabolism may be complex and interconnected, but one possible model for the action of formate is that it shifts the metabolism of pyruvate toward oxidative decarboxylation by pyruvate dehydrogenase and away from that by pyruvate formate-lyase. We have shown that invasion gene expression is elevated in a mutant that cannot make formate due to the loss of pyruvate formate-lyase (Pfl). As this enzyme converts pyruvate to acetyl-CoA and formate, it is likely that this induction of invasion is due to an increase in pyruvate concentration in the mutant strain. It has been shown that in vitro, Pfl is a bidirectional enzyme (36, 37). If Pfl were to function similarly in vivo, it would produce pyruvate when supplied with a high concentration of formate. In support of this, the addition of pyruvate itself caused induction in the wild-type strain, but the addition of formate to the pflB mutant did not. Alternatively, it is possible that formate itself acts as a signal by an unrecognized mechanism or that it is metabolized by a pathway not yet identified. Efforts are ongoing in our laboratory to identify the mechanism by which formate exerts its effects on invasion gene expression.
Although we detected formate in the ileum of mice, its genesis at that site is largely unknown. Presumably, it is produced by the resident microbiota of the small intestine. Recently, there have been great advances in characterizing the microbiota of the large intestine of humans and animals (27, 44, 63). The microbiota of the ileum, however, has not been well described. There is evidence as well that the generation of formate depends upon the complex ecology of the intestinal tract. It has been shown that in the large intestine, the archaeon Methanobrevibacter smithii induces formate production by the bacterium Bacteroides thetaiotaomicron and then uses that formate as a nutrient source (57). Studies have also shown that alteration of the diet of pigs can affect the ileal concentration of formate (40). These findings, along with the fact that very low concentrations of formate are present in the adjacent cecum, suggest that the ileum possesses a microbial ecology that is uniquely suited to the production of this fatty acid.
The induction of Salmonella invasion by formate is but one of several means by which this bacterium senses the environment of the intestine and responds by altering its virulence characteristics. As Salmonella passes through the intestinal tract during the course of infection, it encounters changing environments. In the proximal small intestine, for example, bile is secreted into the intestinal lumen. Bile has been shown to repress invasion, working at or above the level of BarA/SirA (56), and thus prevents invasion at a site at which it would be unproductive. As the bacteria reach the ileum, the most-distal portion of the small intestine, they encounter increased concentrations of formate and the SCFA acetate (4, 5, 12, 39, 40). Both of these constituents induce invasion, with acetate functioning through a mechanism that requires SirA, but not BarA (41). The results of our work suggest that these two environmental cues provide redundant signaling mechanisms, either of which is sufficient to cause disease (41). Yet to be discovered, however, is the signal for BarA, as none of the environmental conditions known have been shown to activate invasion through this sensor kinase. As Salmonella leaves the small intestine and enters the large intestine, it encounters higher concentrations of two other SCFAs, propionate and butyrate, produced by the anaerobic microbiota (4, 5, 12, 47). Both of these repress invasion, acting by an uncharacterized mechanism, again preventing the unproductive expression of invasion determinants (26, 41). Thus, Salmonella uses products of both its animal host and the intestinal microbiota to sense specific regions of the intestine and to promote its virulence.
Acknowledgments
We thank Debbie Ross for invaluable assistance with the high-pressure liquid chromatography analysis and Hélène Marquis for the critical review of the manuscript.
This project was supported by the National Research Initiative of the USDA Cooperative State Research, Education, and Extension Service, award number 2005-35201-16270, and the National Institute of Allergy and Infectious Diseases, National Institutes of Health, Department of Health and Human Services, under contract no. N01-AI-30054.
Footnotes
Published ahead of print on 18 April 2008.
REFERENCES
- 1.Ahmer, B. M., J. van Reeuwijk, P. R. Watson, T. S. Wallis, and F. Heffron. 1999. Salmonella SirA is a global regulator of genes mediating enteropathogenesis. Mol. Microbiol. 31971-982. [DOI] [PubMed] [Google Scholar]
- 2.Altier, C., M. Suyemoto, and S. D. Lawhon. 2000. Regulation of Salmonella enterica serovar Typhimurium invasion genes by csrA. Infect. Immun. 686790-6797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Altier, C., M. Suyemoto, A. I. Ruiz, K. D. Burnham, and R. Maurer. 2000. Characterization of two novel regulatory genes affecting Salmonella invasion gene expression. Mol. Microbiol. 35635-646. [DOI] [PubMed] [Google Scholar]
- 4.Argenzio, R. A., and M. Southworth. 1975. Sites of organic acid production and absorption in gastrointestinal tract of the pig. Am. J. Physiol. 228454-460. [DOI] [PubMed] [Google Scholar]
- 5.Argenzio, R. A., M. Southworth, and C. E. Stevens. 1974. Sites of organic acid production and absorption in the equine gastrointestinal tract. Am. J. Physiol. 2261043-1050. [DOI] [PubMed] [Google Scholar]
- 6.Bajaj, V., C. Hwang, and C. A. Lee. 1995. hilA is a novel ompR/toxR family member that activates the expression of Salmonella typhimurium invasion genes. Mol. Microbiol. 18715-727. [DOI] [PubMed] [Google Scholar]
- 7.Bajaj, V., R. L. Lucas, C. Hwang, and C. A. Lee. 1996. Co-ordinate regulation of Salmonella typhimurium invasion genes by environmental and regulatory factors is mediated by control of hilA expression. Mol. Microbiol. 22703-714. [DOI] [PubMed] [Google Scholar]
- 8.Behlau, I., and S. I. Miller. 1993. A PhoP-repressed gene promotes Salmonella typhimurium invasion of epithelial cells. J. Bacteriol. 1754475-4484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bohnhoff, M., C. P. Miller, and W. R. Martin. 1964. Resistance of the mouse's intestinal tract to experimental salmonella infection. I. Factors which interfere with the initiation of infection by oral inoculation. J. Exp. Med. 120805-816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carter, P. B., and F. M. Collins. 1974. The route of enteric infection in normal mice. J. Exp. Med. 1391189-1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Collazo, C. M., and J. E. Galan. 1997. The invasion-associated type III system of Salmonella typhimurium directs the translocation of Sip proteins into the host cell. Mol. Microbiol. 24747-756. [DOI] [PubMed] [Google Scholar]
- 12.Cummings, J. H., E. W. Pomare, W. J. Branch, C. P. Naylor, and G. T. Macfarlane. 1987. Short chain fatty acids in human large intestine, portal, hepatic and venous blood. Gut 281221-1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Darwin, A., P. Tormay, L. Page, L. Griffiths, and J. Cole. 1993. Identification of the formate dehydrogenases and genetic determinants of formate-dependent nitrite reduction by Escherichia coli K12. J. Gen. Microbiol. 1391829-1840. [DOI] [PubMed] [Google Scholar]
- 14.Darwin, K. H., and V. L. Miller. 1999. InvF is required for expression of genes encoding proteins secreted by the SPI1 type III secretion apparatus in Salmonella typhimurium. J. Bacteriol. 1814949-4954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Datsenko, K. A., and B. L. Wanner. 2000. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc. Natl. Acad. Sci. USA 976640-6645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Durant, J. A., D. E. Corrier, and S. C. Ricke. 2000. Short-chain volatile fatty acids modulate the expression of the hilA and invF genes of Salmonella typhimurium. J. Food Prot. 63573-578. [DOI] [PubMed] [Google Scholar]
- 17.Eichelberg, K., and J. E. Galán. 1999. Differential regulation of Salmonella typhimurium type III secreted proteins by pathogenicity island 1 (SPI-1)-encoded transcriptional activators InvF and HilA. Infect. Immun. 674099-4105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Eichelberg, K., W. D. Hardt, and J. E. Galan. 1999. Characterization of SprA, an AraC-like transcriptional regulator encoded within the Salmonella typhimurium pathogenicity island 1. Mol. Microbiol. 33139-152. [DOI] [PubMed] [Google Scholar]
- 19.Ellermeier, C. D., A. Janakiraman, and J. M. Slauch. 2002. Construction of targeted single copy lac fusions using lambda Red and FLP-mediated site-specific recombination in bacteria. Gene 290153-161. [DOI] [PubMed] [Google Scholar]
- 20.Ellermeier, C. D., and J. M. Slauch. 2003. RtsA and RtsB coordinately regulate expression of the invasion and flagellar genes in Salmonella enterica serovar Typhimurium. J. Bacteriol. 1855096-5108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fahlen, T. F., N. Mathur, and B. D. Jones. 2000. Identification and characterization of mutants with increased expression of hilA, the invasion gene transcriptional activator of Salmonella typhimurium. FEMS Immunol. Med. Microbiol. 2825-35. [DOI] [PubMed] [Google Scholar]
- 22.Fortune, D. R., M. Suyemoto, and C. Altier. 2006. Identification of CsrC and characterization of its role in epithelial cell invasion in Salmonella enterica serovar Typhimurium. Infect. Immun. 74331-339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fu, Y., and J. E. Galan. 1998. Identification of a specific chaperone for SptP, a substrate of the centisome 63 type III secretion system of Salmonella typhimurium. J. Bacteriol. 1803393-3399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Galan, J. E., and R. Curtiss III. 1989. Cloning and molecular characterization of genes whose products allow Salmonella typhimurium to penetrate tissue culture cells. Proc. Natl. Acad. Sci. USA 866383-6387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Galan, J. E., and R. Curtiss III. 1990. Expression of Salmonella typhimurium genes required for invasion is regulated by changes in DNA supercoiling. Infect. Immun. 581879-1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gantois, I., R. Ducatelle, F. Pasmans, F. Haesebrouck, I. Hautefort, A. Thompson, J. C. Hinton, and F. Van Immerseel. 2006. Butyrate specifically down-regulates Salmonella pathogenicity island 1 gene expression. Appl. Environ. Microbiol. 72946-949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gill, S. R., M. Pop, R. T. Deboy, P. B. Eckburg, P. J. Turnbaugh, B. S. Samuel, J. I. Gordon, D. A. Relman, C. M. Fraser-Liggett, and K. E. Nelson. 2006. Metagenomic analysis of the human distal gut microbiome. Science 3121355-1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Groisman, E. A., and H. Ochman. 1993. Cognate gene clusters govern invasion of host epithelial cells by Salmonella typhimurium and Shigella flexneri. EMBO J. 123779-3787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hardt, W. D., H. Urlaub, and J. E. Galan. 1998. A substrate of the centisome 63 type III protein secretion system of Salmonella typhimurium is encoded by a cryptic bacteriophage. Proc. Natl. Acad. Sci. USA 952574-2579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hesslinger, C., S. A. Fairhurst, and G. Sawers. 1998. Novel keto acid formate-lyase and propionate kinase enzymes are components of an anaerobic pathway in Escherichia coli that degrades L-threonine to propionate. Mol. Microbiol. 27477-492. [DOI] [PubMed] [Google Scholar]
- 31.Iyoda, S., T. Kamidoi, K. Hirose, K. Kutsukake, and H. Watanabe. 2001. A flagellar gene fliZ regulates the expression of invasion genes and virulence phenotype in Salmonella enterica serovar Typhimurium. Microb. Pathog. 3081-90. [DOI] [PubMed] [Google Scholar]
- 32.Johnston, C., D. A. Pegues, C. J. Hueck, A. Lee, and S. I. Miller. 1996. Transcriptional activation of Salmonella typhimurium invasion genes by a member of the phosphorylated response-regulator superfamily. Mol. Microbiol. 22715-727. [DOI] [PubMed] [Google Scholar]
- 33.Jones, B. D., and S. Falkow. 1994. Identification and characterization of a Salmonella typhimurium oxygen-regulated gene required for bacterial internalization. Infect. Immun. 623745-3752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jones, B. D., N. Ghori, and S. Falkow. 1994. Salmonella typhimurium initiates murine infection by penetrating and destroying the specialized epithelial M cells of the Peyer's patches. J. Exp. Med. 18015-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kaniga, K., J. C. Bossio, and J. E. Galan. 1994. The Salmonella typhimurium invasion genes invF and invG encode homologues of the AraC and PulD family of proteins. Mol. Microbiol. 13555-568. [DOI] [PubMed] [Google Scholar]
- 36.Kessler, D., and J. Knappe. 1996. Anaerobic dissimilation of pyruvate. In F. C. Neidhardt, R. Curtiss III, L. Ingraham, E. C. Lin, K. B. Low, B. Magasanik, W. S. Reznikoff, M. Riley, M. Schaechter, and H. E. Umbarger (ed.), Escherichia coli and Salmonella: cellular and molecular biology, 2nd ed. ASM Press, Washington, DC.
- 37.Knappe, J., H. P. Blaschkowski, P. Grobner, and T. Schmitt. 1974. Pyruvate formate-lyase of Escherichia coli: the acetyl-enzyme intermediate. Eur. J. Biochem. 50253-263. [DOI] [PubMed] [Google Scholar]
- 38.Kubori, T., Y. Matsushima, D. Nakamura, J. Uralil, M. Lara-Tejero, A. Sukhan, J. E. Galan, and S. I. Aizawa. 1998. Supramolecular structure of the Salmonella typhimurium type III protein secretion system. Science 280602-605. [DOI] [PubMed] [Google Scholar]
- 39.Laerke, H. N., and B. B. Jensen. 1999. D-Tagatose has low small intestinal digestibility but high large intestinal fermentability in pigs. J. Nutr. 1291002-1009. [DOI] [PubMed] [Google Scholar]
- 40.Laerke, H. N., B. B. Jensen, and S. Hojsgaard. 2000. In vitro fermentation pattern of D-tagatose is affected by adaptation of the microbiota from the gastrointestinal tract of pigs. J. Nutr. 1301772-1779. [DOI] [PubMed] [Google Scholar]
- 41.Lawhon, S. D., R. Maurer, M. Suyemoto, and C. Altier. 2002. Intestinal short-chain fatty acids alter Salmonella typhimurium invasion gene expression and virulence through BarA/SirA. Mol. Microbiol. 461451-1464. [DOI] [PubMed] [Google Scholar]
- 42.Lee, C. A., and S. Falkow. 1990. The ability of Salmonella to enter mammalian cells is affected by bacterial growth state. Proc. Natl. Acad. Sci. USA 874304-4308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lee, C. A., B. D. Jones, and S. Falkow. 1992. Identification of a Salmonella typhimurium invasion locus by selection for hyperinvasive mutants. Proc. Natl. Acad. Sci. USA 891847-1851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ley, R. E., F. Backhed, P. Turnbaugh, C. A. Lozupone, R. D. Knight, and J. I. Gordon. 2005. Obesity alters gut microbial ecology. Proc. Natl. Acad. Sci. USA 10211070-11075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lucas, R. L., C. P. Lostroh, C. C. DiRusso, M. P. Spector, B. L. Wanner, and C. A. Lee. 2000. Multiple factors independently regulate hilA and invasion gene expression in Salmonella enterica serovar Typhimurium. J. Bacteriol. 1821872-1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lundberg, U., U. Vinatzer, D. Berdnik, A. von Gabain, and M. Baccarini. 1999. Growth phase-regulated induction of Salmonella-induced macrophage apoptosis correlates with transient expression of SPI-1 genes. J. Bacteriol. 1813433-3437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Macfarlane, G. T., G. R. Gibson, and J. H. Cummings. 1992. Comparison of fermentation reactions in different regions of the human colon. J. Appl. Bacteriol. 7257-64. [DOI] [PubMed] [Google Scholar]
- 48.Miller, J. H. 1992. A short course in bacterial genetics: a laboratory manual and handbook for Escherichia coli and related bacteria. Cold Spring Harbor Laboratory, Cold Spring Harbor, NY.
- 49.Miller, S. I., and J. J. Mekalanos. 1990. Constitutive expression of the phoP regulon attenuates Salmonella virulence and survival within macrophages. J. Bacteriol. 1722485-2490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mills, D. M., V. Bajaj, and C. A. Lee. 1995. A 40 kb chromosomal fragment encoding Salmonella typhimurium invasion genes is absent from the corresponding region of the Escherichia coli K-12 chromosome. Mol. Microbiol. 15749-759. [DOI] [PubMed] [Google Scholar]
- 51.Nakayama, S., A. Kushiro, T. Asahara, R. Tanaka, L. Hu, D. J. Kopecko, and H. Watanabe. 2003. Activation of hilA expression at low pH requires the signal sensor CpxA, but not the cognate response regulator CpxR, in Salmonella enterica serovar Typhimurium. Microbiology 1492809-2817. [DOI] [PubMed] [Google Scholar]
- 52.Neidhardt, F. C., P. L. Bloch, and D. F. Smith. 1974. Culture medium for enterobacteria. J. Bacteriol. 119736-747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Penheiter, K. L., N. Mathur, D. Giles, T. Fahlen, and B. D. Jones. 1997. Non-invasive Salmonella typhimurium mutants are avirulent because of an inability to enter and destroy M cells of ileal Peyer's patches. Mol. Microbiol. 24697-709. [DOI] [PubMed] [Google Scholar]
- 54.Pizarro-Cerda, J., and K. Tedin. 2004. The bacterial signal molecule, ppGpp, regulates Salmonella virulence gene expression. Mol. Microbiol. 521827-1844. [DOI] [PubMed] [Google Scholar]
- 55.Prouty, A. M., I. E. Brodsky, J. Manos, R. Belas, S. Falkow, and J. S. Gunn. 2004. Transcriptional regulation of Salmonella enterica serovar Typhimurium genes by bile. FEMS Immunol. Med. Microbiol. 41177-185. [DOI] [PubMed] [Google Scholar]
- 56.Prouty, A. M., and J. S. Gunn. 2000. Salmonella enterica serovar Typhimurium invasion is repressed in the presence of bile. Infect. Immun. 686763-6769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Samuel, B. S., and J. I. Gordon. 2006. A humanized gnotobiotic mouse model of host-archaeal-bacterial mutualism. Proc. Natl. Acad. Sci. USA 10310011-10016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Schechter, L. M., S. M. Damrauer, and C. A. Lee. 1999. Two AraC/XylS family members can independently counteract the effect of repressing sequences upstream of the hilA promoter. Mol. Microbiol. 32629-642. [DOI] [PubMed] [Google Scholar]
- 59.Schechter, L. M., S. Jain, S. Akbar, and C. A. Lee. 2003. The small nucleoid-binding proteins H-NS, HU, and Fis affect hilA expression in Salmonella enterica serovar Typhimurium. Infect. Immun. 715432-5435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Siegfried, V., H. Ruckemann, and G. Stumpf. 1984. Eine HPLC-methode zur bestimmung organishcer sauren in silagen. Landwirtsch. Forschung. 37298-304. [Google Scholar]
- 61.Teplitski, M., R. I. Goodier, and B. M. Ahmer. 2003. Pathways leading from BarA/SirA to motility and virulence gene expression in Salmonella. J. Bacteriol. 1857257-7265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tsolis, R. M., L. G. Adams, T. A. Ficht, and A. J. Baumler. 1999. Contribution of Salmonella typhimurium virulence factors to diarrheal disease in calves. Infect. Immun. 674879-4885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Turnbaugh, P. J., R. E. Ley, M. A. Mahowald, V. Magrini, E. R. Mardis, and J. I. Gordon. 2006. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature 4441027-1031. [DOI] [PubMed] [Google Scholar]
- 64.Wilson, R. L., S. J. Libby, A. M. Freet, J. D. Boddicker, T. F. Fahlen, and B. D. Jones. 2001. Fis, a DNA nucleoid-associated protein, is involved in Salmonella typhimurium SPI-1 invasion gene expression. Mol. Microbiol. 3979-88. [DOI] [PubMed] [Google Scholar]
- 65.Yang, Y. T., A. A. Aristidou, K. Y. San, and G. N. Bennett. 1999. Metabolic flux analysis of Escherichia coli deficient in the acetate production pathway and expressing the Bacillus subtilis acetolactate synthase. Metab. Eng. 126-34. [DOI] [PubMed] [Google Scholar]
- 66.Zhou, D., M. S. Mooseker, and J. E. Galan. 1999. Role of the S. typhimurium actin-binding protein SipA in bacterial internalization. Science 2832092-2095. [DOI] [PubMed] [Google Scholar]