Abstract
Human T-cell leukemia virus (HTLV-1) Env carries a typical disulfide isomerization motif, C225XXC, in the C-terminal domain SU. Here we have tested whether this motif is used for isomerization of the intersubunit disulfide of Env and whether this rearrangement is required for membrane fusion. We introduced the C225A and C228A mutations into Env and found that the former but not the latter mutant matured into covalently linked SU-TM complexes in transfected cells. Next, we constructed a secreted Env ectodomain and showed that it underwent incubation-dependent intersubunit disulfide isomerization on target cells. However, the rearrangement was blocked by the C225A mutation, suggesting that C225 carried the isomerization-active thiol. Still, it was possible to reduce the intersubunit disulfide of the native C225A ectodomain mutant with dithiothreitol (DTT). The importance of the CXXC-mediated disulfide isomerization for infection was studied using murine leukemia virus vectors pseudotyped with wild-type or C225A HTLV-1 Env. We found that the mutant Env blocked infection, but this could be rescued with DTT. The fusion activity was tested in a fusion-from-within assay using a coculture of rat XC target and transfected BHK-21 effector cells. We found that the mutation blocked polykaryon formation, but this could be reversed with DTT. Similar DTT-reversible inhibition of infection and fusion was observed when a membrane-impermeable alkylator was present during the infection/fusion incubation. We conclude that the fusion activity of HTLV-1 Env is controlled by an SU CXXC-mediated isomerization of the intersubunit disulfide. Thus, this extends the applicability of the isomerization model from gammaretroviruses to deltaretroviruses.
Human T-cell leukemia virus 1 (HTLV-1), which belongs to the group of deltaretroviruses, matures by budding at the plasma membrane of an infected cell and enters into a new one by virus-cell membrane fusion (16, 17, 32, 35). The membrane fusion protein or envelope protein (Env) of HTLV-1 is a trimer of a gp46-gp21 subunit pair (9, 15, 43). The fusion activity is carried by the transmembrane (TM) gp21 and the host cell receptor binding activity by the peripheral (surface [SU]) gp46. The two subunits are made as a precursor, gp62, in the rough endoplasmic reticulum of the infected cell (37). The gp62 becomes cleaved into the subunits when it passes through the Golgi complex on its way to budding sites at the cell surface (32). The cell receptor of HTLV-1 is the glucose transporter protein I, which gives the virus a very wide host range (31). The neurophilin-1 and heparan sulfate proteoglycans have also been shown to serve as components of a receptor complex and an attachment factor, respectively (13, 39). We are interested in the way by which the HTLV-1 Env is activated for membrane fusion.
The HTLV-1 SU has been shown to have a modular organization (20). The first 160 residues are folded as a receptor binding N-terminal domain (RBD), which is linked by a 35-residue-long Pro-rich region (PRR) to a C-terminal domain comprising 98 amino acid residues. This SU organization is similar to that of murine leukemia virus (MLV), belonging to the gammaretrovirus group. In the latter case, it has been demonstrated that the RBD of receptor-bound virus transmits a PRR-controlled signal to the C-terminal domain that leads to activation of the fusion function in TM (2, 3, 23, 24, 25). That a similar mechanism also operates in the case of HTLV-1 is suggested by the fact that the MLV TM can also become activated for membrane fusion in a chimeric Env where the RBD and the PRR domains of SU are derived from HTLV-1 (21). The crystal structure of the HTLV-1 TM ectodomain (TMecto), devoid of its N-terminal membrane fusion peptide and fused to the maltose binding protein, has been resolved at 2.5-Å resolution (22). The resolution revealed that the ectodomain fragments formed hairpin-like structures that were joined by an N-terminal coiled coil into trimers. The C-terminal part of the hairpin packed against the grooves of the coiled coil in an extended antiparallel fashion. The overall organization resembled the structure of the activated membrane fusion subunit of influenza virus, HA2 (5, 46). This suggested that the HTLV-1 TM promoted membrane fusion by converting from a prehairpin trimer, with the fusion peptides in the target membrane, into a hairpin trimer with the fusion peptide and the viral membrane anchor in the same orientation. This model was further supported by mutagenesis experiments and in particular by the findings that peptides corresponding to the C-terminal leg of the hairpin could bind to the N-terminal coiled coil of activated Env and inhibit membrane fusion (4, 18, 30, 38). But how is the TM of HTLV-1 activated by the SU?
According to the prevailing model of the so-called class I membrane fusion proteins of enveloped viruses, like influenza virus hemagglutinin and the Envs of MLV and avian leucosis virus (ALV), the TM fusion subunit is kept in a metastable conformation in the native unactivated protein oligomer by its association with the peripheral receptor binding subunit (7, 47, 48). Receptor binding (MLV), low pH (influenza virus), or receptor binding and low pH (ALV) in the endosome decrease the strength of this association (19, 34, 50). This allows the fusion subunit to refold into a stable conformation through fusion-active intermediates. Consequently, MLV fuses at the cell surface, whereas influenza virus and ALV have to enter acidic endosomes for fusion. In all these examples, the subunit association is stabilized by an intersubunit disulfide (26, 46, 49). This will probably help to avoid premature activation of the fusion subunit outside the target cell but also compromise activation in the target cell. The harsh acidic conditions are apparently sufficient to ensure enough subunit dissociation in the influenza virus and ALV despite the intersubunit covalent bond, but acidic conditions are not used by MLV. Instead, the intersubunit disulfide of MLV is linked to a CXXC motif in SU, where the other Cys residue carries a disulfide isomerization-active thiol (49). Thus, upon receptor activation of SU, the thiol becomes deprotonated so that it can attack the intersubunit disulfide and rearrange it into a disulfide isomer within the motif. This results in SU dissociation and TM activation for fusion. Also like MLV Env, the HTLV-1 Env supports membrane fusion at neutral conditions at the cell surface (16, 35). The subunits of the HTLV-1 Env have been shown to be engaged in a covalent linkage that can be rearranged upon membrane solubilization (49). In MLV Env, the intersubunit disulfide links a Cys of the CXXC motif in SU to the last Cys of a conserved CX6CC motif in TM (11, 40). Both motifs are also conserved in deltaretrovirus Envs like those of HTLV, simian T-cell leukemia virus, and bovine leukemia virus (6, 42, 44, 45). Therefore, it is possible that the HTLV-1 Env controls its membrane fusion activity by a CXXC-mediated isomerization of its intersubunit disulfide, like MLV Env does. In the present work, we have studied this possibility.
We used a genetic approach to study the possible involvement of the SU CXXC motif in fusion control. The mechanism for the intersubunit disulfide rearrangement was analyzed with HTLV-1 Env ectodomain (Envecto) fragments bound to target cells, whereas the importance of the reaction for infection was studied with HTLV-1 Env-pseudotyped MLV, and its role in membrane fusion was studied using a cell-to-cell fusion-from-within assay. The results supported a CXXC-mediated intersubunit disulfide isomerization control model for the activation of HTLV-1 Env.
MATERIALS AND METHODS
Cells.
BHK-21 cells (American Type Culture Collection, Rockville, MD) were grown in Glasgow minimal essential medium (BHK-21 medium; Gibco BRL, Life Technologies, Paisley, United Kingdom) containing 5% fetal calf serum, 10% tryptose phosphate broth, and 20 mM HEPES. XC cells (CCL-165; American Type Culture Collection) were maintained in Dulbecco's modified Eagle's medium (Gibco BRL) supplemented with 10% fetal calf serum and 20 mM HEPES. HOS and 293T cells were grown in Dulbecco's modified Eagle's medium supplemented with 10% fetal calf serum, 4.5 g/liter d-glucose, 20 mM HEPES, 1 mM sodium pyruvate, and nonessential amino acids.
Reagents.
As thiol alkylators, we used the membrane-permeable N-ethylmaleimide (NEM; Sigma-Aldrich, Stockholm, Sweden) and the membrane-impermeable 4-(N-maleimido)benzyl-α-trimethylammoniumiodide (M135; Toronto Research Chemicals Inc., North York, Canada). Dithiothreitol (DTT; Sigma) was used as the reducing agent.
Antibodies.
A polyclonal antibody (pAb) against the HTLV-1 TM subunit was raised by a commercial service (Innovagen AB, Lund, Sweden) in rabbit by use of keyhole limpet hemocyanin-conjugated TM peptide FLNITNSHVSILQERPPLENRVLTG as the antigen. It immunoprecipitated TMecto but not SU from an uncoupled (isomerized) mixture of Envecto subunits. Anti-HTLV-1 patient sera were obtained from Rigmor Thorstensson (Karolinska Institute, Sweden). These immunoprecipitated free SU but hardly any TMecto.
Mutagenesis and expression vectors.
The Semliki Forest virus (SFV) expression plasmid containing the wild-type (wt) HTLV-1 env gene under the SFV subgenomic promoter was constructed by inserting the env gene, PCR amplified from pSFV1-gp62HTLV-1, into the BamHI and SmaI sites of pSFV1, yielding pSFV1-HTLV1-Env (29, 49). In pSFV1-gp62HTLV-1, the 5′ region of the tax gene precedes the env gene. The corresponding SFV RNA expression replicon was transcribed in vitro from the pSFV1-HTLV1-Env construct and transfected by electroporation into BHK-21 cells. Cys codon mutations (TGC to GCC and TGT to GCT) were introduced into the region encoding the conserved C225XXC motif of the SU part of the env gene to yield the C225A, C228A, and C225A/C228A mutants by fusing PCR using the pSFV1-HTLV1-Env as the template. The numbering is from the first codon of the signal sequence region of the HTLV-1 env gene. The mutated env genes were confirmed by DNA sequencing.
A Trp431-to-stop-codon mutation was introduced into the env gene to direct the synthesis of a secreted form of an ectodomain fragment of Env (8). This Envecto or SU-TMecto lacked the TM and the membrane-internal domains of wt Env. The mutant was constructed by PCR mutagenesis using pSFV1-gp62HTLV-1 as the template. The new construct was called pSFV1-HTLV1-Envecto and it was further used to derive pSFV1-HTLV1-C225AEnvecto.
Moloney MLV (MMLV) vectors carrying the firefly luciferase gene and the HTLV-1 wt or C225A Env were constructed by coelectroporating SFV RNA replicons expressing MMLV gagpol (SFV1-MLVgagpol), MLV-luciferase RNA genome (SFV1-MLV-luc), and HTLV-1 wt Env (SFV1-HTLV1-Env) or C225A Env (SFV1-HTLV1-C225AEnv) into BHK-21 cells (28). Culture medium containing MLV-luc-HTLV1-wtEnv or MLV-luc-HTLV1-C225AEnv vectors was collected after 20 h and stored at −80°C. SFV1-MLV-luc RNA was transcribed in vitro from the corresponding plasmid. This was made by inserting the luciferase gene from pGL3-Basic (Promega Biotech AB, Stockholm, Sweden) into the HindIII and BamHI sites of pEGFP-N3 (Clontech, Mountain View, CA) to make pCMV-Lucif-N3. Then the cytomegalovirus-luciferase gene was excised from pCMV-Lucif-N3 and inserted into the PmeI site of pSFV1-RETRO. The latter plasmid contains a minimal MMLV vector genome and it was derived from pSFV1/LN3i by deleting the Neor gene and part of the U3 region (28). The MLV-luc-HTLV1-wtEnv vectors were purified by centrifugation in a 20/50% (wt/wt) sucrose step gradient and prepared in TN/Ca2+ buffer (14 mM Tris, 12 mM HEPES, 150 mM NaCl, 1.8 mM Ca2+, pH 6.8) by use of an SW28.1 rotor (Beckman Coulter, Fullerton, CA) at 22,000 rpm for 2 h at 4°C.
Analysis of protein biosynthesis.
Transfected cell cultures were metabolically labeled with [35S]Cys (GE Healthcare Biosciences, Buckinghamshire, United Kingdom) for 18 h or for 30 min and subsequently chased in the presence of 2 mM unlabeled Cys for 30 to 120 min as described previously (36). The cultures were lysed in NP-40 lysis buffer (50 mM Tris-HCl, pH 7.5, 150 mM NaCl, 2 mM EDTA, 1 mM phenylmethylsulfonyl fluoride, 1% NP-40) containing 20 mM NEM and Env immunoprecipitated with anti-HTLV-1 pAb (36). Envecto fragments in culture supernatant were captured directly by anti-HTLV-1 pAb. The proteins were analyzed by reducing or nonreducing 12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), which was reproduced as a phosphorimage (BAS-MS2025; Fujifilm, Science Imaging Scandinavia, Nacka, Sweden) or an autoradiograph. For analysis of N-deglycosylated proteins, immunoprecipitates were taken up into 20 μl of buffer containing 50 mM Tris, pH 7.6, 150 mM NaCl, 2 mM EDTA, and 1% SDS by incubation at 70°C for 3 min. Eighty microliters of 1.25% NP-40 lysis buffer containing 0.2 mM phenylmethylsulfonyl fluoride was added and 24-μl samples were digested with 0.5 μl N-glycosidase F (Roche Diagnostics Scandinavia AB, Bromma, Sweden) by incubating at 37°C for 16 h.
Intersubunit disulfide isomerization analysis.
Envectos in culture medium were bound to HOS cells in dishes for 1 h at room temperature and then incubated for different times at 37°C before the cultures (medium and cells) were treated under lysis conditions in the presence of 20 mM NEM. Covalently bound and free Env subunits were immunoprecipitated and analyzed by nonreducing SDS-PAGE. Isomerization of the intersubunit disulfide after different incubation times after incubation was monitored by the decrease of covalently linked SU-TMecto complexes and the increase of free subunits.
Infectivity assays.
MLV-luc vectors in culture medium were bound to suspended 293T cells in Eppendorf tubes for 1 h at 4°C under continuous tilting and then incubated for 50 min at 37°C. The cells with bound vectors were gently sedimented by centrifugation, suspended in TN/Ca2+ buffer, pH 7.4, and incubated for 15 min in the presence of 0 to 8 mM DTT. After this, the DTT was removed and the cells were plated on dishes for incubation in culture medium for 48 h. Eventually, the cells were lysed and assayed for vector-expressed luciferase by use of a luminometer. The activity was expressed as relative light units. In another infectivity assay, purified vectors were bound to suspended 293T cells in TN/Ca2+ buffer for 1 h at 4°C and incubated at 37°C first for 50 min in the presence of 1 mM M135 and then, after removing the alkylator by three washes with the buffer, for an additional 15 min in the presence of 0 to 8 mM DTT. The reducing agent was removed, the cells were plated on dishes, and infectivity was screened as described above.
Fusion-from-within assay.
SFV1-Env RNA replicon-transfected BHK-21 cells were incubated for 18 h and then suspended and layered on top of rat XC cells in serum-free medium in the presence of 8 μg/ml Polybrene. The cell mixtures were incubated for 2.5 h at 37°C in the presence or absence of 4 mM DTT. After this, the cultures were fixed and stained with Giemsa stain for analysis of polykaryons. Mixtures of transfected BHK-21 and XC cells were also incubated in serum-free medium as follows: first for 2.5 h at 37°C in the presence of 1 mM M135; then, after three washes with medium, for 15 min in the presence of 4 mM DTT; and finally, after one more wash with medium, for 2 h at 37°C before staining. A control incubation was done without M135 and DTT.
RESULTS
Mutants of the CXXC motif.
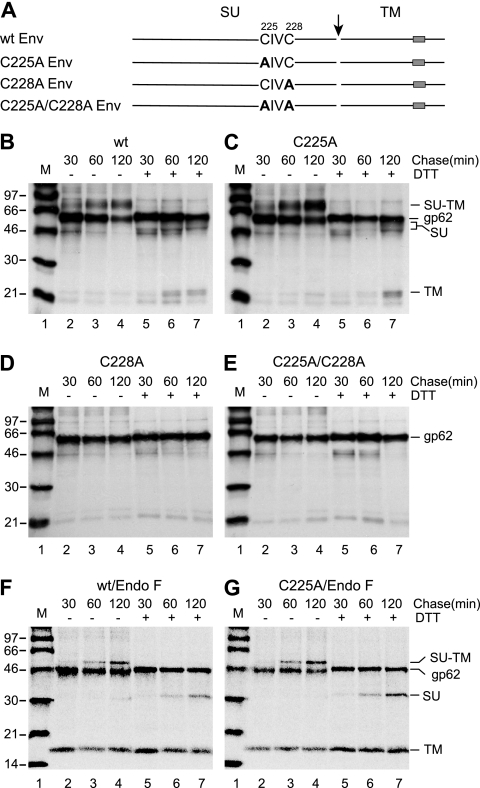
The Cys residues of the C225XXC motif in the SU subunit of HTLV-1 Env were substituted by Ala individually and together and the Env mutants expressed in BHK-21 cells by SFV RNA replicons (Fig. 1A). Env biosynthesis was studied by pulse-labeling with [35S]Cys for 30 min and chases for 30, 60, and 120 min. The cell cultures were lysed in the presence of NEM and Env was captured with the anti-HTLV-1 pAb for SDS-PAGE. Analyses at nonreducing conditions showed that the wt and C225A mutant Envs matured into covalently linked SU-TM complexes (gp46-gp21; molecular mass, about 70 kDa), whereas the C228A and the C225A/C228A Env mutants remained uncleaved as apparent Env precursors (gp62) in the transfected cells (Fig. 1B to E, lanes 2 to 4). Analyses under reducing conditions revealed the intersubunit disulfide in the SU-TM complex of the wt and the C225A mutant (Fig. 1B and C, lanes 5 to 7). The uncoupled SU subunits (gp46) were seen as a fuzzy band in front of the Env precursor and the TM (gp21) was seen just behind two weaker bands. The released SU was detected more clearly when the N-linked sugars were removed by treatment with N-glycosidase F (Fig. 1F and G, lanes 6 and 7). The treatment also resulted in the apparent comigration of the TM and the two bands in front, suggesting that the latter represented underglycosylated TM. The presence of such TM in all samples, including the nonreduced ones, can be explained by the formation of some aberrant Env that lacks the SU-TM disulfide and has incomplete sugar unit processing but is still transport and cleavage competent. When this Env arrives at the cell surface, the SU is shed and only the TM remains cell associated. In contrast to the SU-TM complexes, the precursor remained intact in all samples analyzed under reducing conditions (Fig. 1B to E, lanes 5 to 7). Interestingly, a higher fraction of the C225A mutant Env than of wt Env was found in the mature form. We concluded that C228, but not C225, was required for Env maturation in the biosynthetic transport pathway of the cell and that the intersubunit disulfide did not involve C225 but possibly did involve C228.
FIG. 1.
(A) Schematic representations of the HTLV-1 Env wt and CXXC mutants. The cleavage between SU and TM is indicated by an arrow and the TM region by boxes. Amino acid substitutions in the CXXC motif are shown in bold. (B to G) Biosynthesis of wt and CXXC mutant Env. BHK-21 cell cultures were transfected by electroporation with SFV RNA replicons directing the synthesis of wt, C225A, C228A, and C225A/C228A Env. Eight hours posttransfection, the cultures were pulse-labeled with [35S]Cys for 30 min and chased for 30, 60, and 120 min. After cell lysis in the presence of NEM, Env was captured by anti-HTLV-1 pAb (B to E) for nonreducing and reducing SDS-PAGE. wt and C225A Env were also captured by a mixture of anti-HTLV-1 and anti-TM pAbs and analyzed after the removal of N-linked sugar units with N-glycosidase F (Endo F) (F and G). (B) wt Env. (C) C225A Env. (D) C228A Env. (E) C225A/C228A Env. (F) Endo F-treated wt Env. (G) Endo F-treated C225A Env. Chase times, redox conditions, and viral proteins are indicated. M, molecular mass standards. Panels B to E represent autoradiographs and panels F and G phosphorimages of the gels.
The C225A mutant of Envecto is blocked in isomerization of its intersubunit disulfide.
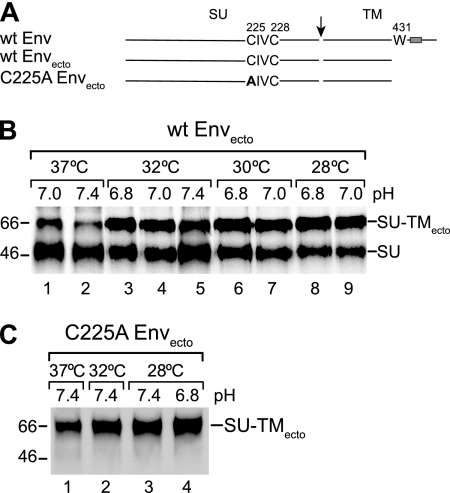
We constructed an HTLV-1 Env gene truncation directing the synthesis of an anchorless Envecto and introduced the C225A mutation into it. Pulse-chase analyses with BHK-21 cells by use of SFV RNA expression replicons showed that under standard culture conditions (37°C, pH 7.4) only a minor amount of the wt ectodomain was released as covalently linked SU-TMecto complexes (Fig. 2B, lane 2). Most of the protein appeared in the medium as separate subunits. In contrast, virtually all of the C225A Envecto was secreted as SU-TMecto complexes (Fig. 2C). Synthesis at lower temperature and pH improved the yield of wt ectodomains with linked subunits significantly and facilitated studies about intersubunit disulfide rearrangement on receptor-positive human HOS cells (Fig. 2B).
FIG. 2.
(A) Schematic representations of the HTLV-1 Envecto fragment and the HTLV-1 Envecto C225A mutant. The membrane-proximal Trp (W), the codon of which has been mutated into a stop codon, is shown in full-length Env. Arrow, cleavage between SU and TM; box, TM region. (B and C) Secretion of HTLV-1 Envectos. wt and C225A Envectos were produced and labeled with [35S]Cys for 18 h at indicated pH values and temperatures in transfected BHK-21 cells. The media were collected and released SU-TMecto complexes and free SU was captured for nonreducing SDS-PAGE using anti-HTLV-1 pAb. (B) wt Envectos. (C) C225A Envectos. Covalently linked and free SU subunits are indicated. Note that the anti-HTLV-1 pAb did not react with free TM. The analyses shown represent phosphorimages of the gels. Molecular masses are indicated to the left.
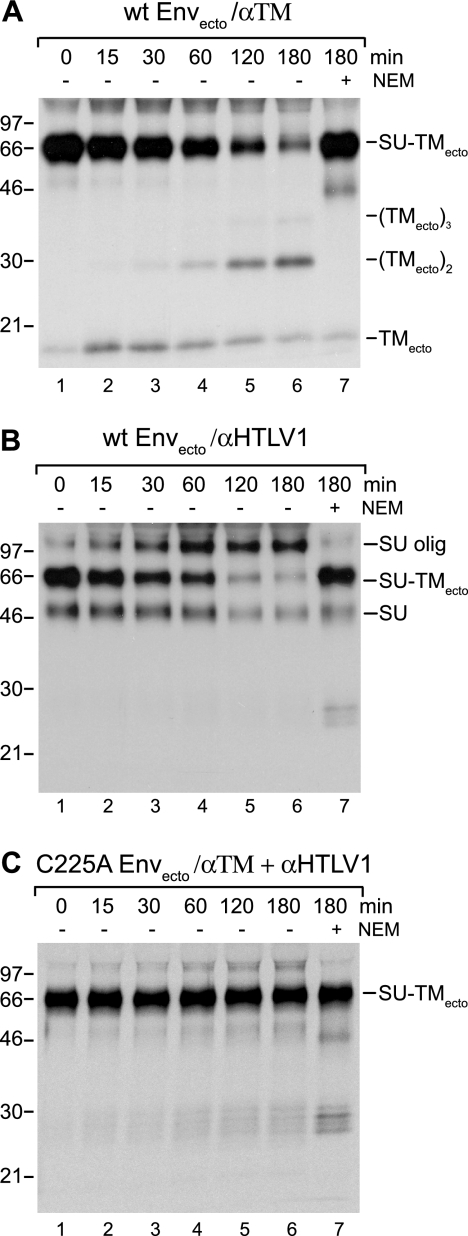
wt ectodomains produced in pH 6.8 medium at 28°C were bound to HOS cells at room temperature for 1 h and then incubated at 37°C for 0 to 180 min. The possible rearrangement of the SU-TMecto disulfide was studied by nonreducing SDS-PAGE after lysing the cell cultures in the presence of NEM and capturing free and disulfide-linked subunits with either anti-TM or anti-HTLV-1 pAb. It should be noted that the presence of NEM in the lysis buffer prevented lysis-induced disulfide rearrangements and thus facilitated specific monitoring of the incubation-induced rearrangement (49). The analyses with anti-TM pAb showed a decreasing amount of SU-TMecto complexes and first an increasing amount of TMecto monomers and then an accumulation of apparent dimers and possibly some trimers at the expense of the TMecto monomers with increasing time of incubation (Fig. 3A). The analyses of the anti-HTLV-1 pAb-immunoprecipitated samples also showed a decreasing amount of the complexes, but the released SU appeared predominantly as SU oligomers (Fig. 3B). This pAb did not react with TM. Thus, the results demonstrated that the bound ectodomain complex rearranged its intersubunit disulfide when incubated at 37°C. The kinetics of the rearrangement increased at higher pH (data not shown) and was completely blocked in the presence of a thiol alkylator, NEM, both indicating disulfide isomerization (Fig. 3A and B, lanes 7) (27, 49). In contrast, analyses of the cell-bound C225A ectodomain showed that it was completely resistant to rearrangement of its intersubunit disulfide, indicating that the isomerization-active thiol belonged to C225 of SU (Fig. 3C). Thus, we concluded that the CXXC motif of HTLV-1 SU carried a thiol (C225) that could attack and isomerize the intersubunit disulfide of Env.
FIG. 3.
Rearrangement and lack of rearrangement of the intersubunit disulfide of wt and C225A Envectos. Radioactively labeled wt and C225A Envectos were bound to HOS cells in medium, pH 6.8, for 1 h and incubated for 0 to 180 min at 37°C. The intersubunit disulfide rearrangement of the SU-TMecto complexes was analyzed by nonreducing SDS-PAGE after cell lysis in the presence of NEM and immunoprecipitation with anti-TM and/or anti-HTLV-1 pAb. (A and B) Analyses of wt Envectos using anti-TM (A) and anti-HTLV-1 pAb (B). (C) Analysis of C225A Envectos by use of anti-TM and anti-HTLV-1 pAbs together. Control samples incubated in the presence of NEM and oligomeric (olig) states of the Env subunits are indicated. All analyses shown represent an autoradiographs of the gels. Molecular masses are indicated to the left. α, anti-.
The intersubunit disulfide of the native C225A SU-TMecto complex is DTT sensitive.
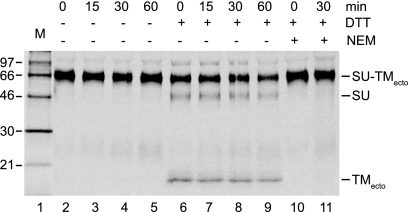
The C225A SU-TMecto complex was bound to HOS cells in culture medium as described above and incubated first for 0 to 60 min at 37°C in the medium and then for an additional 5 min in TN/Ca2+ in the presence or absence of 4 mM DTT to study the resistance of the intersubunit disulfide toward external reduction. After the addition of NEM (20 mM), the entire culture (supernatant and cells) was subjected to lysis conditions and the covalently linked and free subunits were captured by a mixture of anti-HTLV-1 and anti-TM pAbs for nonreducing SDS-PAGE. The analysis showed that the intersubunit disulfide in the C225A SU-TMecto complexes was partially sensitive to the DTT treatment (Fig. 4, lanes 2 to 9). The sensitivity appeared to be independent of the length of the preincubation with cells. However, prolonging the DTT incubation increased the intersubunit disulfide reduction (data not shown). A control experiment where the DTT was mixed with NEM before addition did not show any significant reduction of the disulfide, suggesting that the DTT effect observed in earlier experiments occurred during the incubation of the ectodomain with the cells rather than during sample preparation for the SDS-PAGE (Fig. 4, lanes 10 and 11). We concluded that the intersubunit disulfide of the native form of the ectodomain is exposed for external reduction.
FIG. 4.
DTT sensitivity of the intersubunit disulfide of C225A Envecto fragments. The mutant ectodomains were bound to HOS cells in duplicate and incubated for 0 to 60 min at 37°C. The cultures were then subjected to an additional incubation in TN/Ca2+, pH 7.4, for 5 min at 37°C, one series in the presence of 4 mM DTT and the other series without. An excess of NEM (40 mM) was added and the cells and the supernatants were treated under lysis conditions and subjected to immunoprecipitation using a combination of anti-HTLV-1 and anti-TM pAbs. The captured proteins were analyzed by nonreducing SDS-PAGE. The analysis shown represents a phosphorimage of the gel. Incubation times with cells and DTT addition are indicated. Analyses of control samples where the DTT was mixed with the NEM before addition to the cells are shown in lanes 10 and 11. Molecular masses are indicated to the left.
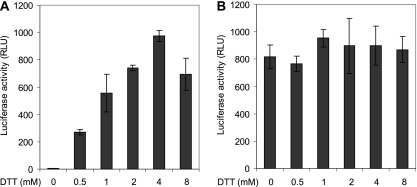
C225A Env-pseudotyped MLV vectors are noninfectious but can be rescued by DTT.
Because of the low infectivity of HTLV-1 particles, we constructed MLV-luc vectors with wt and C225A full-length Envs and tested their infectivity in the presence and absence of DTT on 293T cells by luciferase expression (33, 41, 51). Thus, both vectors were bound to the cells at 4°C in culture medium and then incubated at 37°C first for 50 min in the medium and then for 15 min in TN/Ca2+, pH 7.4, in the presence or absence of DTT. In contrast to wt Env, C225A Env did not support the infectivity of the vector in the absence of DTT (Fig. 5A and B, first columns). However, after DTT treatment, the infectivity of the C225A Env vector was rescued (Fig. 5A, second to sixth columns). The infectivity increased with increasing DTT concentration to 4 mM. There was no corresponding DTT potentiation of infectivity for the wt Env vector, suggesting that the target for the DTT effect was specific to C225A Env (Fig. 5B). The DTT-released infectivity of the mutant Env vectors corresponded to about half of the wt Env vector infectivity when correction was made for the amounts of particles used in the experiments. We concluded that C225 is required for infectivity and that its function can be replaced by the reduction of a disulfide in Env by DTT. Considering the disulfide isomerization activity of C225 in wt Envecto and the DTT sensitivity of the intersubunit disulfide of C225A Envecto, the infectivity of the MLV-luc-C225A Env vector was most likely rescued by DTT reduction of the intersubunit disulfide in C225A Env. Consequently, the CXXC-mediated intersubunit disulfide isomerization controls HTLV-1 infection.
FIG. 5.
DTT rescue of MLV-luc-HTLV1-C225AEnv vector infectivity. Unpurified vectors with wt or C225A Env in culture medium were bound to suspended 293T cells in tubes at 4°C for 1 h and then incubated at 37°C for 50 min. The medium was exchanged for TN/Ca2+ buffer, pH 7.4, and the cultures were incubated for an additional 15 min in the presence of 0 to 8 mM DTT. The reducing agent was then removed, the cultures were plated on dishes, and the infectivity was scored by luciferase activity after a final incubation for 48 h. Two times more C225A Env vectors were used than wt Env vectors. (A) Vectors with C225A Env. (B) Vectors with wt Env. RLU, relative light units.
The infectivity of the wt HTLV Env vector is blocked by Env thiol alkylation but rescued by DTT.
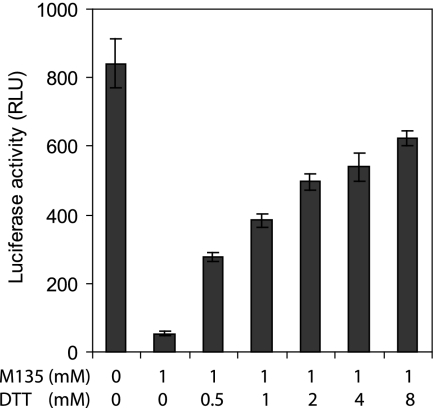
When purified MLV vectors pseudotyped with wt HTLV-1 Env were bound to 293T cells and then incubated at 37°C in TN/Ca2+, pH 7.4, first for 50 min in the presence of 1 mM of the membrane-impermeable alkylator M135 and then for 15 min without DTT, only 6% of the infectivity of the nonalkylated control vector remained (Fig. 6, first and second columns). However, an increasing amount of infectivity could be rescued by including an increasing concentration of DTT in the second incubation for 15 min (Fig. 6, third to seventh columns). At 8 mM DTT, about 75% of the infectivity was rescued. To rule out the possibility that reduction of cellular disulfides was responsible for the rescue, we pretreated the 293T cells with 1 mM M135 for 50 min in TN/Ca2+ at 37°C. The cells were washed five times before infection with the vectors and were found to support more than 50% of the infectivity found with untreated control cells (data not shown). We concluded that the HTLV-1 Env of the vector contains a thiol group that controls infection by isomerization of a disulfide in Env. Thus, this corroborates the role of the SU CXXC motif in HTLV-1 infection.
FIG. 6.
DTT rescue of M135-inhibited MLV-luc-HTLV1-Env infectivity. Purified vectors carrying wt HTLV-1 Env were bound to suspended 293T cells in TN/Ca2+ buffer, pH 7.4, at 4°C for 1 h and then incubated for 50 min at 37°C in the presence of 1 mM M135. The alkylator was washed off and the cultures were further incubated for 15 min in the presence of 0 to 8 mM DTT. After the reducing agent was removed, the cultures were plated on dishes, incubated for 48 h, and then screened for infectivity by luciferase expression (second to seventh columns). A control culture was incubated and washed similarly but without adding M135 or DTT (first column). RLU, relative light units.
The C225A Env-mediated cell-cell fusion from within is inhibited but can be rescued with DTT.
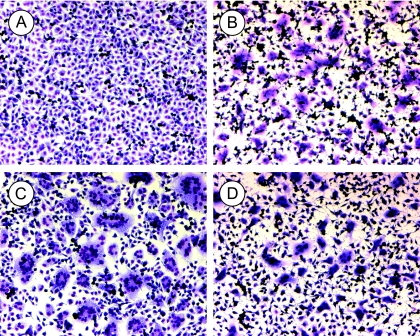
The full-length C225A and wt Envs were expressed in BHK-21 cells and these were layered on top of confluent HTLV-1 receptor-positive rat XC cells to follow effector-target cell fusion. The cultures were incubated at 37°C for 2.5 h with or without 6 mM DTT in serum-free medium and then assayed for polykaryons. We found that the C225A Env (Fig. 7A), in contrast to the wt Env (Fig. 7C), did not support cell-cell fusion into polykaryons. In the presence of DTT, the cells retracted and the wt Env-mediated polykaryons were smaller but still abundant (Fig. 7D). Most significantly, the fusion capacity of the C225A Env was rescued in the presence of DTT to same level as that of wt Env (Fig. 7B). This suggests that the CXXC thiol-directed intersubunit disulfide isomerization reaction controls HTLV-1 Env-mediated membrane fusion. The conclusion was further supported by the DTT alleviation of M135 alkylation-mediated inhibition of wt Env-directed cell-cell fusion (data not shown).
FIG. 7.
DTT rescue of HTLV1-C225A Env-mediated fusion from within. wt or C225A Env was expressed in BHK-21 cells by SFV1 RNA replicon electroporation and 18 h posttransfection the cells were suspended and layered on top of receptor-positive XC cells. The cell mixtures were incubated in serum-free medium at 37°C for 2.5 h in the presence or absence of 6 mM DTT. The cultures were then fixed and stained for analysis of polykaryons. (A) C225A Env without DTT. (B) C225A Env with DTT. (C) wt Env without DTT. (D) wt Env with DTT. Note that cells retract upon the DTT treatment, but this does not prevent the formation of polykaryons.
DISCUSSION
We showed here that HTLV-1 controls its membrane fusion activity by the isomerization of its intersubunit disulfide. The isomerization-active thiol was by mutagenesis mapped to Cys225. A C225A mutation blocked the incubation-dependent rearrangement of the intersubunit disulfide of cell-bound HTLV-1 Envectos and, in the context of the full-length Env, the infection of MLV vectors and cell-cell fusion from within. Significantly, DTT treatment rescued the cell-cell fusion capacity of the mutant Env and the infectivity of vectors carrying the mutant Env. Similar treatment of cell-bound and incubated mutant Envectos was shown to reduce the intersubunit disulfide, suggesting that the DTT rescue of infectivity and fusion was due to the reduction of the intersubunit disulfide of the mutant full-length Env. This implied that the function of the Cys225 is to isomerize the intersubunit disulfide in Env so that TM can be activated for membrane fusion. The model was further corroborated by the facts that the membrane-impermeable alkylating reagent M135 inhibited the infectivity of MLV vectors carrying wt HTLV-1 Envs and the fusion from within of wt Env and that it was possible to reverse the inhibitions by DTT treatments.
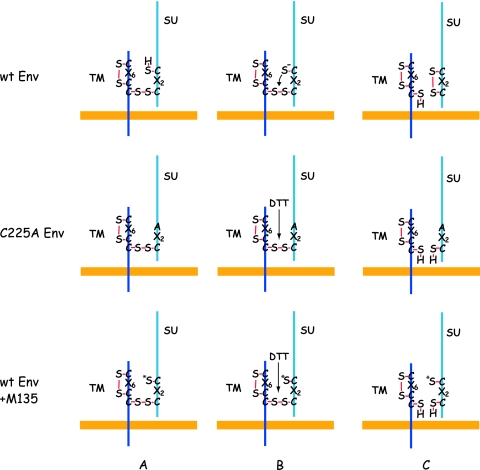
The Cys225 is part of a C225XXC motif in the SU of HTLV-1 Env. This motif forms the active center of redox enzymes like thioredoxin and protein disulfide isomerase (12). In the case of MLV Env, the isomerization-active CXXC motif of SU forms the intersubunit disulfide with Cys563 of a conserved CX6CC563 in TM (11, 40). In the case of HTLV-1 Env, the intersubunit disulfide has not yet been localized, but considering the isomerization activity of the SU CXXC motif and the fact that TM carries a CX6CC motif like MLV TM, it is most likely that Cys228 of the CXXC228 motif is linked to the Cys401 of the CX6C401 motif in TM. This is supported by the fact that the full-length HTLV-1 Env with the C228A mutation did not mature beyond the gp62 precursor stage in transfected cells. A likely interpretation is that Cys228 supported a protein disulfide isomerase-catalyzed disulfide, e.g., with Cys401, which was required for correct folding. Interestingly, in the case of MLV and the MLV-related bird reticuloendotheliosis virus, both Cys residues in the CXXC motif of Env SU have been individually mutated and found to result in maturation-defective Env (14; M. Wallin, unpublished data). This suggests different roles of the thiol-carrying Cys of the motif in HTLV-1 and MLV Envs during Env biosynthesis. In Fig. 8, we show schematically our model for C225 thiol-mediated intersubunit disulfide isomerization during normal Env activation and how the reaction is blocked in the case of the C225A Env mutant and by C225 thiol alkylation but can be rescued by external reduction.
FIG. 8.
Models for intersubunit disulfide activation control of wt Env, C225A Env, and Env with the CXXC thiol modified by alkylation. (Top) How the CXXC thiol mediates wt Env activation through attacking and isomerizing the intersubunit disulfide. (Middle and bottom) How external reduction of the intersubunit disulfide with DTT can replace the lack of a functional thiol in the C225A Env mutant or in M135-alkylated wt Env. In the bottom panels, the alkylation of the CXXC thiol is indicated by an asterisk.
The present results extend the intersubunit disulfide isomerization control model of retrovirus Env activation from gammaretroviruses to deltaretroviruses. In both cases, the intersubunit disulfide suppresses activation, probably by maintaining a tight SU-TM association, which keeps the TM subunit in a metastable fusion-inactive conformation (48). The initiation of the intersubunit disulfide isomerization reaction must involve the stabilization of the active deprotonated (thiolate) form of the thiol group in the CXXC motif of SU (27). This requires a structural change in the locale of the thiol group in the C-terminal domain of SU. In the case of MLV, receptor binding to the RBD results in the release of stabilizing (activation-suppressing) Ca2+ ion(s) from Env and the activation of the C-terminal SU domain via an altered RBD-C-terminal domain interaction (2, 3, 23, 49). The activation involves a His residue which is part of the conserved SPHQ sequence in the N-terminal region of the MLV RBDs (1, 2, 25, 52). We have proposed that the His residue might interact with the locale of the CXXC thiol in the SU C-terminal domain and activate it through hydrogen bond-mediated stabilization of the thiolate form (27). In the case of HTLV-1 Env, there is no SPHQ sequence in the RBD amino acid sequence, but the modular organization of the SU is similar to that of MLV, suggesting similarities in the thiol activation mechanism (20). This is supported by the functional MLV chimera with the RBD and the PRR swapped with those of HTLV-1 (21). However, the thiol activation mechanism of HTLV-1 Env might also show marked differences from that of MLV Env. The latter is characterized by a low spontaneous intersubunit disulfide isomerization activity, which gives the virus a good stability in culture medium (49). In contrast, cell-free HTLV-1 preparations are known to have very low competence for infectivity (33, 41, 51). Although features of the internal viral proteins have been shown to be part of the reason, the low infectivity might also relate to a very high spontaneous intersubunit disulfide isomerization activity and subsequent virus inactivation through TM folding into its stable trimer of hairpins (10). This possibility is supported by the efficient isomerization of wt Envectos produced at 37°C and pH 7.4 (Fig. 3A).
Acknowledgments
We thank Rigmor Thorstensson for the anti-HTLV-1 antisera and Mathilda Sjöberg for comments and discussions.
Swedish Science Foundation grant 2778 and Swedish Cancer Foundation grant 0525 to H.G. supported this work.
Footnotes
Published ahead of print on 14 May 2008.
REFERENCES
- 1.Bae, Y., S. M. Kingsman, and A. J. Kingsman. 1997. Functional dissection of the Moloney murine leukemia virus envelope protein gp70. J. Virol. 712092-2099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barnett, A. L., and J. M. Cunningham. 2001. Receptor binding transforms the surface subunit of the mammalian C-type retrovirus envelope protein from an inhibitor to an activator of fusion. J. Virol. 759096-9105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barnett, A. L., R. A. Davey, and J. M. Cunningham. 2001. Modular organization of the Friend murine leukemia virus envelope protein underlies the mechanism of infection. Proc. Natl. Acad. Sci. USA 984113-4118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brighty, D. W., and S. R. Jassal. 2001. The synthetic peptide P-197 inhibits human T-cell leukemia virus type 1 envelope-mediated syncytium formation by a mechanism that is independent of Hsc70. J. Virol. 7510472-10478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bullough, P. A., F. M. Hughson, J. J. Skehel, and D. C. Wiley. 1994. Structure of influenza haemagglutinin at the pH of membrane fusion. Nature 37137-43. [DOI] [PubMed] [Google Scholar]
- 6.Calattini, S., S. A. Chevalier, R. Duprez, P. Afonso, A. Froment, A. Gessain, and R. Mahieux. 2006. Human T-cell lymphotropic virus type 3: complete nucleotide sequence and characterization of the human tax3 protein. J. Virol. 809876-9888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carr, C. M., C. Chaudhry, and P. S. Kim. 1997. Influenza hemagglutinin is spring-loaded by a metastable native conformation. Proc. Natl. Acad. Sci. USA 9414306-14313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carrington, C. V., R. A. Weiss, and T. F. Schulz. 1994. A truncated HTLV-I envelope protein, lacking the hydrophobic membrane anchor domain, is associated with cellular membranes and virions. Virology 20261-69. [DOI] [PubMed] [Google Scholar]
- 9.Center, R. J., B. Kobe, K. A. Wilson, T. Teh, G. J. Howlett, B. E. Kemp, and P. Poumbourios. 1998. Crystallization of a trimeric human T cell leukemia virus type 1 gp21 ectodomain fragment as a chimera with maltose-binding protein. Protein Sci. 71612-1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Derse, D., S. A. Hill, P. A. Lloyd, H. Chung, and B. A. Morse. 2001. Examining human T-lymphotropic virus type 1 infection and replication by cell-free infection with recombinant virus vectors. J. Virol. 758461-8468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fass, D., and P. S. Kim. 1995. Dissection of a retrovirus envelope protein reveals structural similarity to influenza hemagglutinin. Curr. Biol. 51377-1383. [DOI] [PubMed] [Google Scholar]
- 12.Ferrari, D. M., and H. D. Soling. 1999. The protein disulphide-isomerase family: unravelling a string of folds. Biochem. J. 3391-10. [PMC free article] [PubMed] [Google Scholar]
- 13.Ghez, D., Y. Lepelletier, S. Lambert, J. M. Fourneau, V. Blot, S. Janvier, B. Arnulf, P. M. van Endert, N. Heveker, C. Pique, and O. Hermine. 2006. Neuropilin-1 is involved in human T-cell lymphotropic virus type 1 entry. J. Virol. 806844-6854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gu, J., S. Parthasarathi, A. Varela-Echavarria, Y. Ron, and J. P. Dougherty. 1995. Mutations of conserved cysteine residues in the CWLC motif of the oncoretrovirus SU protein affect maturation and translocation. Virology 206885-893. [DOI] [PubMed] [Google Scholar]
- 15.Hattori, S., T. Kiyokawa, K. Imagawa, F. Shimizu, E. Hashimura, M. Seiki, and M. Yoshida. 1984. Identification of gag and env gene products of human T-cell leukemia virus (HTLV). Virology 136338-347. [DOI] [PubMed] [Google Scholar]
- 16.Hoshino, H., M. Shimoyama, M. Miwa, and T. Sugimura. 1983. Detection of lymphocytes producing a human retrovirus associated with adult T-cell leukemia by syncytia induction assay. Proc. Natl. Acad. Sci. USA 807337-7341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hunter, E. 1997. Viral entry and receptors, p. 71-119. In J. M. Coffin, S. H. Hughes, and H. E. Varmus (ed.), Retroviruses. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY. [PubMed]
- 18.Jinno, A., Y. Haraguchi, H. Shiraki, and H. Hoshino. 1999. Inhibition of cell-free human T-cell leukemia virus type 1 infection at a postbinding step by the synthetic peptide derived from an ectodomain of the gp21 transmembrane glycoprotein. J. Virol. 739683-9689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jones, J. S., and R. Risser. 1993. Cell fusion induced by the murine leukemia virus envelope glycoprotein. J. Virol. 6767-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim, F. J., N. Manel, E. N. Garrido, C. Valle, M. Sitbon, and J. L. Battini. 2004. HTLV-1 and -2 envelope SU subdomains and critical determinants in receptor binding. Retrovirology 141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim, F. J., I. Seiliez, C. Denesvre, D. Lavillette, F. L. Cosset, and M. Sitbon. 2000. Definition of an amino-terminal domain of the human T-cell leukemia virus type 1 envelope surface unit that extends the fusogenic range of an ecotropic murine leukemia virus. J. Biol. Chem. 27523417-23420. [DOI] [PubMed] [Google Scholar]
- 22.Kobe, B., R. J. Center, B. E. Kemp, and P. Poumbourios. 1999. Crystal structure of human T cell leukemia virus type 1 gp21 ectodomain crystallized as a maltose-binding protein chimera reveals structural evolution of retroviral transmembrane proteins. Proc. Natl. Acad. Sci. USA 964319-4324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lavillette, D., B. Boson, S. J. Russell, and F. L. Cosset. 2001. Activation of membrane fusion by murine leukemia viruses is controlled in cis or in trans by interactions between the receptor-binding domain and a conserved disulfide loop of the carboxy terminus of the surface glycoprotein. J. Virol. 753685-3695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lavillette, D., M. Maurice, C. Roche, S. J. Russell, M. Sitbon, and F. L. Cosset. 1998. A proline-rich motif downstream of the receptor binding domain modulates conformation and fusogenicity of murine retroviral envelopes. J. Virol. 729955-9965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lavillette, D., A. Ruggieri, S. J. Russell, and F. L. Cosset. 2000. Activation of a cell entry pathway common to type C mammalian retroviruses by soluble envelope fragments. J. Virol. 74295-304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Leamnson, R. N., and M. S. Halpern. 1976. Subunit structure of the glycoprotein complex of avian tumor virus. J. Virol. 18956-968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li, K., S. Zhang, M. Kronqvist, M. Ekström, M. Wallin, and H. Garoff. 2007. The conserved His8 of the Moloney murine leukemia virus Env SU subunit directs the activity of the SU-TM disulphide bond isomerase. Virology 361149-160. [DOI] [PubMed] [Google Scholar]
- 28.Li, K. J., and H. Garoff. 1996. Production of infectious recombinant Moloney murine leukemia virus particles in BHK cells using Semliki Forest virus-derived RNA expression vectors. Proc. Natl. Acad. Sci. USA 9311658-11663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liljestrom, P., and H. Garoff. 1991. A new generation of animal cell expression vectors based on the Semliki Forest virus replicon. Biotechnology 91356-1361. [DOI] [PubMed] [Google Scholar]
- 30.Maerz, A. L., R. J. Center, B. E. Kemp, B. Kobe, and P. Poumbourios. 2000. Functional implications of the human T-lymphotropic virus type 1 transmembrane glycoprotein helical hairpin structure. J. Virol. 746614-6621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Manel, N., F. J. Kim, S. Kinet, N. Taylor, M. Sitbon, and J. L. Battini. 2003. The ubiquitous glucose transporter GLUT-1 is a receptor for HTLV. Cell 115449-459. [DOI] [PubMed] [Google Scholar]
- 32.Mazurov, D., G. Heidecker, and D. Derse. 2006. HTLV-1 Gag protein associates with CD82 tetraspanin microdomains at the plasma membrane. Virology 346194-204. [DOI] [PubMed] [Google Scholar]
- 33.Miyamoto, K., N. Tomita, A. Ishii, T. Nishizaki, K. Kitajima, T. Tanaka, T. Nakamura, S. Watanabe, and T. Oda. 1984. Transformation of ATLA-negative leukocytes by blood components from anti-ATLA-positive donors in vitro. Int. J. Cancer 33721-725. [DOI] [PubMed] [Google Scholar]
- 34.Mothes, W., A. L. Boerger, S. Narayan, J. M. Cunningham, and J. A. Young. 2000. Retroviral entry mediated by receptor priming and low pH triggering of an envelope glycoprotein. Cell 103679-689. [DOI] [PubMed] [Google Scholar]
- 35.Nagy, K., P. Clapham, R. Cheingsong-Popov, and R. A. Weiss. 1983. Human T-cell leukemia virus type I: induction of syncytia and inhibition by patients’ sera. Int. J. Cancer 32321-328. [DOI] [PubMed] [Google Scholar]
- 36.Opstelten, D. J., M. Wallin, and H. Garoff. 1998. Moloney murine leukemia virus envelope protein subunits, gp70 and Pr15E, form a stable disulfide-linked complex. J. Virol. 726537-6545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Paine, E., R. Gu, and L. Ratner. 1994. Structure and expression of the human T-cell leukemia virus type 1 envelope protein. Virology 199331-338. [DOI] [PubMed] [Google Scholar]
- 38.Pinon, J. D., S. M. Kelly, N. C. Price, J. U. Flanagan, and D. W. Brighty. 2003. An antiviral peptide targets a coiled-coil domain of the human T-cell leukemia virus envelope glycoprotein. J. Virol. 773281-3290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pinon, J. D., P. J. Klasse, S. R. Jassal, S. Welson, J. Weber, D. W. Brighty, and Q. J. Sattentau. 2003. Human T-cell leukemia virus type 1 envelope glycoprotein gp46 interacts with cell surface heparan sulfate proteoglycans. J. Virol. 779922-9930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pinter, A., R. Kopelman, Z. Li, S. C. Kayman, and D. A. Sanders. 1997. Localization of the labile disulfide bond between SU and TM of the murine leukemia virus envelope protein complex to a highly conserved CWLC motif in SU that resembles the active-site sequence of thiol-disulfide exchange enzymes. J. Virol. 718073-8077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Popovic, M., P. S. Sarin, M. Robert-Gurroff, V. S. Kalyanaraman, D. Mann, J. Minowada, and R. C. Gallo. 1983. Isolation and transmission of human retrovirus (human T-cell leukemia virus). Science 219856-859. [DOI] [PubMed] [Google Scholar]
- 42.Rice, N. R., R. M. Stephens, D. Couez, J. Deschamps, R. Kettmann, A. Burny, and R. V. Gilden. 1984. The nucleotide sequence of the env gene and post-env region of bovine leukemia virus. Virology 13882-93. [DOI] [PubMed] [Google Scholar]
- 43.Schneider, J., N. Yamamoto, Y. Hinuma, and G. Hunsmann. 1984. Sera from adult T-cell leukemia patients react with envelope and core polypeptides of adult T-cell leukemia virus. Virology 1321-11. [DOI] [PubMed] [Google Scholar]
- 44.Seiki, M., S. Hattori, Y. Hirayama, and M. Yoshida. 1983. Human adult T-cell leukemia virus: complete nucleotide sequence of the provirus genome integrated in leukemia cell DNA. Proc. Natl. Acad. Sci. USA 803618-3622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Seiki, M., T. Watanabe, A. Komuro, I. Miyoshi, M. Hayami, and M. Yoshida. 1984. Characterization of simian retrovirus genome related to human T-cell leukemia virus type I. Princess Takamatsu Symp. 15241-249. [PubMed] [Google Scholar]
- 46.Skehel, J. J., and D. C. Wiley. 2000. Receptor binding and membrane fusion in virus entry: the influenza hemagglutinin. Annu. Rev. Biochem. 69531-569. [DOI] [PubMed] [Google Scholar]
- 47.Smith, J. G., W. Mothes, S. C. Blacklow, and J. M. Cunningham. 2004. The mature avian leukosis virus subgroup A envelope glycoprotein is metastable, and refolding induced by the synergistic effects of receptor binding and low pH is coupled to infection. J. Virol. 781403-1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wallin, M., M. Ekstrom, and H. Garoff. 2005. The fusion-controlling disulfide bond isomerase in retrovirus env is triggered by protein destabilization. J. Virol. 791678-1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wallin, M., M. Ekstrom, and H. Garoff. 2004. Isomerization of the intersubunit disulphide-bond in Env controls retrovirus fusion. EMBO J. 2354-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.White, J., K. Matlin, and A. Helenius. 1981. Cell fusion by Semliki Forest, influenza, and vesicular stomatitis viruses. J. Cell Biol. 89674-679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yamamoto, N., M. Okada, Y. Koyanagi, M. Kannagi, and Y. Hinuma. 1982. Transformation of human leukocytes by cocultivation with an adult T cell leukemia virus producer cell line. Science 217737-739. [DOI] [PubMed] [Google Scholar]
- 52.Zavorotinskaya, T., Z. Qian, J. Franks, and L. M. Albritton. 2004. A point mutation in the binding subunit of a retroviral envelope protein arrests virus entry at hemifusion. J. Virol. 78473-481. [DOI] [PMC free article] [PubMed] [Google Scholar]