Abstract
Epstein-Barr virus (EBV) infection is associated with many human malignancies. In vitro, EBV transforms primary B lymphocytes into continuously growing lymphoblastoid cell lines. EBV latent membrane protein 1 (LMP-1) is required for EBV transformation processes. Interferon regulatory factor 4 (IRF-4) is a transcription factor and has oncogenic potential. We find that high levels of IRF-4 are associated with EBV transformation of human primary B cells in vitro and with EBV type III latency in which LMP-1 is expressed. We show that EBV LMP-1 stimulates IRF-4 expression in B lymphocytes. The stimulation of IRF-4 by LMP-1 requires signaling from LMP-1 and involves cellular NF-κB. The growth of EBV-transformed cells is inhibited when IRF-4 is specifically down-regulated. We further demonstrate that IRF-4 knockdown cells have lower proliferation but higher apoptotic rates than control cells. Finally, IRF-4 is expressed in significant numbers of specimens of primary central nervous system (CNS) lymphomas (12/27 [44.4%]), an EBV-associated malignancy. The association between the expression levels of LMP-1 and IRF-4 is statistically significant (P = 0.011) in these CNS lymphomas. Our data suggest that IRF-4 may be a critical factor in EBV transformation and a useful target in the therapy of EBV-mediated neoplasia.
Epstein-Barr virus (EBV) infection has been associated with the development of several human malignancies, including nasopharyngeal carcinoma, Burkitt's lymphoma (BL), Hodgkin's lymphoma, T-cell lymphoma, and gastric carcinoma (32, 48). In immunocompromised individuals such as organ transplant recipients and AIDS patients, EBV almost certainly triggers two fatal cancers without the necessity for cofactors: AIDS-associated central nervous system (CNS) lymphoma and posttransplantation lymphoproliferative disorder (46).
EBV establishes several types of latencies in host cells. In type I latency, EBV nuclear antigen 1 (EBNA-1) and small EBV-encoded, nonpolyadenylated nuclear RNAs (EBER-1 and -2) are expressed in host cells. In contrast, six nuclear proteins (EBNA-1, EBNA-2, EBNA-3A, EBNA-3B, EBNA-3C, and EBNA-LP), three membrane proteins (latent membrane protein 1 [LMP-1], LMP-2A, and LMP-2B), plus EBERs are expressed in type III latency (32, 48).
EBV transforms adult primary B cells into continually growing lymphoblastoid cell lines (LCLs) and concomitantly establishes type III latency in vitro. LMP-1 is required for the transformation process: the deletion of LMP-1 prevents the transformation of primary B cells (27, 31), and the inhibition of LMP-1 expression in EBV-transformed cells reverts the transformed phenotypes (33). LMP-1 is an integral membrane protein with transmembrane domains and a C-terminal domain located in the cytoplasm (32, 35). LMP-1 acts as a constitutively active, receptor-like molecule that activates signaling pathways without the binding of a ligand (19). In addition, LMP-1 appears to be a central effector of altered cell growth, survival, adhesive, invasive, and antiviral potential (15, 41, 62, 63, 65, 69, 71).
Interferon (IFN) regulatory factors (IRFs) are a small family of transcription factors with multiple functions. IRFs are apparently associated with viral transformation. IRF-7 is associated with EBV-transformed CNS lymphomas and has oncogenic properties (78). Oncogenic human herpesvirus 8 (HHV-8), also called Kaposi's sarcoma-associated herpesvirus, encodes four IRF-like molecules (viral IRFs [vIRFs]). Rhesus rhadinovirus, another oncogenic herpesvirus, has eight vIRFs in the genome (3, 47). At least human herpesvirus 8 vIRF-1 causes oncogenic transformation (17).
IRF-4, also known as LSIRF, ICSAT, Pip, and Mum1, was cloned independently as a homologous member of the IRF gene family (67) and as an interacting partner of PU.1 (14). IRF-4 is expressed at all stages of B-cell development, in mature T cells, and in macrophages. The analysis of mice lacking IRF-4 (IRF-4−/−) revealed that IRF-4 is essential for the function and homeostasis of both mature B and T lymphocytes (42). IRF-4 is a critical factor for pre-B-to-B transition and the development of certain dendritic cells (37, 58). In addition, IRF-4 is closely associated with the human T-cell leukemia virus transformation process (67), has oncogenic potential in vitro, and may prevent apoptosis (26, 36). IRF-4 is also implicated in the pathogenesis of multiple myeloma: some myeloma cells express high levels of IRF-4 resulting from the chromosome translocation of the IRF-4 gene (26).
In this report, we have examined the role of IRF-4 in the EBV transformation process. The expression of IRF-4 is associated with the EBV transformation of primary B lymphocytes in vitro and with primary CNS lymphomas in vivo. The reduced expression of IRF-4 in EBV-transformed cells decreases the cell proliferation rate and enhances apoptosis. These data suggest that IRF-4 may be a critical factor in the EBV-mediated transformation process.
MATERIALS AND METHODS
Plasmids and antibodies.
Expression plasmids of LMP-1 and its signaling defective mutant, LMP-DM, were described previously (77). An expression plasmid of EBNA-2 (pAG155) was a gift from Paul Ling. The IκB expression plasmid and NF-κB reporter construct were gifts from Albert Baldwin. shLuc (target sequence of 5′-ACTTACGCTGAGTACTTCG-3′), shIRF41 (5′-GCATGAACCTGGAGGGCGG-3′), shIRF42 (5′-GCCACCCCTACACCATGAC-3′), and shIRF43 (5′-AACCTGGACCAGGTCCTGT-3′) were cloned into vector pHP, a small hairpin RNA (shRNA) expression plasmid (66). EBNA-2 (PE2) and LMP-1 (CS1-4) antibodies were purchased from Dako. IRF-4 (H-140), PARP (F-2), caspase-3 (H-277), glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (0411), IRF-1 (C-20), and IRF-2 (C-19) antibodies were purchased from Santa Cruz Biotechnology. Tubulin antibody was purchased from Sigma. IRF-3 antibody was a gift from Peter Howley. IFN-α was obtained from Schering-Plough. The enzyme-linked immunosorbent assay kit for IFN-α was obtained from PBL Biomedical Laboratories (catalog number 41100).
Cell culture, transient transfection, and isolation of transfected cells.
DG75 is an EBV-negative BL cell line (6). BL41 is an EBV-negative BL line; BL41-EBV was generated by in vitro infection of BL41 with EBV strain B95-8 (10). Sav I and Sav III cells have the same cellular background but harbor different strains of EBV with different latencies (44). P3HR1 cells are derived from Jijoye cell line, and both cell lines are EBV positive (2). The Jijoye cell line has all the latency genes in its viral genome, whereas the P3HR1 cell line lacks the EBNA-2 gene and a portion of EBNA-LP (2). As a result of the deletion, P3HR1 cells do not express EBNA-2 and consequently express a very low level of LMP-1 due to the lack of EBNA-2 transactivation of the LMP-1 promoter (1, 18, 61, 64). Akata, Kem I, Daudi, SFC4, and IB4 cells are all EBV-positive cell lines. LCL1 to LCL7 are EBV-transformed B-cell lines collected from the laboratories of K. Izumi and L. Hutt-Flectcher. These cells were maintained in RPMI 1640 plus 10% fetal bovine serum (FBS). Electroporation (320 V; 925 μF) was used for the transfection of DG75 cells as described previously (74, 75, 77). One day after transfection, the cells were used for the isolation of CD4-positive cells with the use of Dynabeads CD4 (Dynal Inc.). Primary B-cell isolation was done as described previously with the use of CD19 antibody-conjugated magnetic beads (Dynal Inc.) (78).
Transfection of IB4 cells.
The transfection of IB4 cells was achieved by using an Amaxa Nucleofector device. Cells (1 × 106) were transfected with 5 μg of DNA in solution B and program U20. Transfected cells were immediately put into 12-well plates with RPMI medium plus FBS. After transfection by use of an Amaxa transfection apparatus, approximately 50% of cells were dead. The growth rates of cells after transfection were slower than those of untransfected cells regardless of the plasmid used. Approximately 70% of the remaining live cells contained transfected plasmids with the protocol. One day later, live cells were isolated by Ficoll-Paque Plus (GE Healthcare) according to the manufacturer's recommendations. The live cells were counted and dispensed in culture flask at 3.5 × 105 cells/ml: this was counted as day 1 after transfection. A small portion of cells were stained daily with trypan blue, and live cells were counted using a hemocytometer. A paired Student's t test was used for statistical analyses.
Cell proliferation assay and Western blot analysis.
The 3-(4,5-methylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) growth assay was used to measure the cell proliferation rate using a CellTiter 96 nonradioactive cell proliferation assay kit (Promega) according to the manufacturer's recommendations. Basically, on day 3, a total 5 × 104 live cells were collected and placed into 85 μl RPMI medium plus 10% FBS in a 96-well plate. Fifteen microliters of dye solution was added to each well. The cells were incubated in 37°C for 4 h. One hundred microliters of solubilization solution/stop mix was added and incubated for another hour. The absorbance at a wavelength of 562 nm was recorded using a 96-well plate reader. The separation of proteins by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and Western blotting were carried out according to standard protocol as described previously (74, 78).
DNA fragmentation assay.
On day 3, the same volumes of cells were pelleted, and DNA isolation was performed under conditions described previously (28). The isolated DNA was separated on an agarose gel and photographed. This method preferentially isolates small DNA molecules.
Clinical samples and immunohistochemical and statistical analysis.
A total of 27 primary CNS lymphomas were collected form the archives of the Pathology Institute, Lausanne, Switzerland, and the Neuropathology Core, Department of Neuroscience, Temple University School of Medicine. Table 1 shows the clinical information for these cases. The methodology for immunohistochemical analysis was described in our previous report (78). The primary antibody used for this study was a polyclonal IRF-4 antibody (H-140) from Santa Cruz (1:50 dilution). For statistical analysis, protein expression levels are defined as follows: 0 represents negative reactivity (−), 1 indicates that 1 to 30% of cells were positive (+), 2 indicates that 31 to 60% of cells were positive (++), and 3 indicates that >60% of cells were positive (+++). Multiple regression analysis was done with the Microsoft Excel program.
TABLE 1.
Expression of IRF-4 in CNS lymphoma specimensa
| Case | Age of patient (yr) | CNS disease | Gender | Expression level
|
|
|---|---|---|---|---|---|
| EBV LMP-1 | IRF-4 | ||||
| 1 | 47 | AIDS | Female | +++ | + |
| 2 | 38 | AIDS | Male | ++ | + |
| 3 | 58 | AIDS | Female | + | + |
| 4 | 62 | AIDS | Female | + | − |
| 5 | 35 | AIDS | Male | ++ | + |
| 6 | 62 | AIDS | Female | ++ | − |
| 7 | 38 | AIDS | Male | + | − |
| 8 | 69 | AIDS | Female | +++ | − |
| 9 | 27 | AIDS | Male | ++ | ++ |
| 10 | 42 | AIDS | Male | +++ | ++ |
| 11 | 39 | AIDS | Male | + | + |
| 12 | 49 | AIDS, CMV | Male | + | − |
| 13 | 77 | AIDS, toxoplasmosis | Female | + | − |
| 14 | 80 | Alzheimer's disease | Male | − | − |
| 15 | 63 | Schizophrenia | Female | − | − |
| 16 | 60 | NR | Male | +++ | + |
| 17 | 82 | NR | Male | − | − |
| 18 | 69 | NR | Male | +++ | + |
| 19 | 67 | NR | Male | ++ | − |
| 20 | 79 | NR | Male | + | ++ |
| 21 | 76 | NR | Male | ++ | ++ |
| 22 | 72 | NR | Female | + | − |
| 23 | 78 | NR | Female | + | − |
| 24 | 65 | NR | Female | + | − |
| 25 | 86 | NR | Male | + | − |
| 26 | 67 | NR | Female | + | − |
| 27 | 69 | NR | Female | ++ | + |
The diagnosis of primary CNS lymphoma was based on the World Health Organization classification of brain tumors. CMV, cytomegalovirus; NR, no report on the clinical history. LMP-1 is used to represent the status of EBV; the data for LMP-1 were described previously (78) and are presented here for the benefit of the readers. “−,” negative reactivity; “+,” 1 to 30% cell positivity; “++,” 31 to 60% cell positivity; “+++,” >60% cell positivity. The association between expression levels of LMP-1 and IRF-4 is statistically significant (R = 0.479; F = 7.444; P = 0.011). Multiple regression analysis was done with Microsoft Excel.
RESULTS
IRF-4 is induced during EBV transformation processes of human B cells in vitro.
IRFs are implicated in the pathogenesis of human cancers (26, 59, 60, 78). IRF-4 was identified as being an LMP-1-inducible gene by microarray techniques (7). Since IRF-7 has been shown to be a potential factor in EBV transformation processes (78), we examined if IRF-4 is potentially involved in EBV transformation processes. Primary B cells were isolated from fresh blood, and the expression levels of IRF-4 for primary B cells and those for EBV-transformed cells (LCLs) were compared. As shown in Fig. 1A, high levels of IRF-4 expression were detected in EBV-transformed cells. In addition, we have shown previously with the same cell lysates that EBV-transformed cells express high levels of IRF-7 and STAT-1 but not IRF-1, -2, and -3 and STAT-2 and -3 (72, 78). Furthermore, primary B cells from two additional individuals were compared with three additional EBV-transformed LCLs, and the same results were obtained (data not shown). Thus, IRF-4 is induced during EBV transformation in vitro.
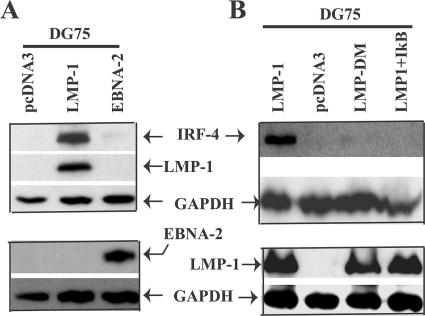
FIG. 1.
Expression of IRF-4 is associated with EBV transformation and type III latency. (A) IRF-4 is associated with EBV transformation of primary B lymphocytes in vitro. Primary B cells were isolated from fresh human blood by the use of CD19 antibody-conjugated magnetic beads. Lysates from primary B cells and EBV-transformed B cells were separated by SDS-PAGE. The expression levels of IRF-4 and tubulin were determined by Western blotting. The same membrane was stripped and reprobed with other antibodies. (B) IRF-4 is highly expressed in cells with EBV type III latency. Cell lysates from the indicated cells lines were separated by SDS-PAGE. The expression levels of IRF-4, LMP-1, EBNA-2, and tubulin proteins were determined by Western blotting. The images in the same box indicate that they are derived from the same membrane. The identities of proteins are shown.
Expression of IRF-4 is associated with EBV type III latency.
To address the mechanism of IRF-4 induction during EBV transformation, we examined if IRF-4 expression is associated with EBV type III latency because type III latency is established during in vitro transformation. Sav I and Sav III (44, 76), Jijoye and P3HR1(2), and BL41 and BL41-EBV (10) are three paired cell lines (see Materials and Methods for details). As shown in Fig. 1B, IRF-4 was expressed at high levels in type III cell lines. The apparent molecular weight of EBNA2 is smaller in Jijoye cells, which is due to the differences in the EBNA-2 gene in EBVs used to generate these cell lines (12).
We also examined IRF-4 expression in other latently EBV-infected cell lines. Daudi cells, like P3HR1 cells, lack EBNA-2 in the EBV genome and have a very low level of LMP-1 expression. Akata and Kem I cells are type I latency BL lines. All these lines express low levels of IRF-4. However, SFC4, an EBV-transformed LCL with type III latency, expresses high levels of IRF-4 (data not shown). All these data indicate that the expression of IRF-4 is associated with EBV type III latency.
LMP-1 stimulates the expression of the IRF-4 protein.
Because EBNA-2 is the primary inducer of LMP-1 mRNA (1, 18, 61, 64), and because of the consistent association between IRF-4 and type III latency (Fig. 1), it is possible that EBNA-2 and/or LMP-1 is responsible for the induction of IRF-4. EBV-negative DG75 cells were used to determine which viral gene could directly induce the expression of IRF-4. LMP-1 or EBNA-2 and a CD4 expression plasmid were transfected into DG75 cells, and the levels of IRF-4 were determined by Western blotting after selection of the transfected cells by use of CD4 antibody-conjugated magnetic beads. As shown in Fig. 2A, LMP-1 expression causes a marked increase in IRF-4 protein levels in DG75 cells; however, EBNA-2 seems to have a limited effect on the expression of IRF-4. The same results were obtained with BL41 cells, an EBV-negative BL line (data not shown). Therefore, LMP-1 appears to directly induce IRF-4 in type III latency. However, EBNA-2 could help induce the expression of IRF-4 indirectly via the induction of LMP-1 in EBV-infected cells.
FIG. 2.
LMP-1 stimulates the expression of IRF-4. (A) LMP-1 induces the expression of the IRF-4 protein. Cell lysates from pcDNA3-, LMP-1-, or EBNA-2-transfected and enriched DG75 cells were used for Western blot analysis with IRF-4, LMP-1, EBNA-2, and GAPDH antibodies. (B) Signaling events from LMP-1 induction of IRF-4. DG75 cells were transfected with pcDNA3, LMP-1, LMP-DM, or LMP-1 plus IκB expression plasmids, and Western blotting was performed as shown. The images in the same box indicate that they are derived from the same membrane. The identities of proteins are shown.
Signaling derived from LMP-1 is required for the induction of IRF-4.
LMP-1 is an integral membrane protein with two C-terminal activating regions (CTARs) that have been shown to initiate signaling processes including the activation of NF-κB and IRF-7. LMP-DM is a mutant of LMP-1 in both CTARs that fails to activate NF-κB and IRF-7 (77). LMP-1 or LMP-DM and a CD4 expression plasmid were transfected into DG75 cells, and the expression of IRF-4 was examined. As shown in Fig. 2B, while wild-type LMP-1 strongly induced IRF-4, LMP-DM failed to induce IRF-4. Thus, signaling from LMP-1 CTARs is required for the induction of IRF-4.
Since NF-κB is a critical mediator of the LMP-1 signaling pathway (20, 54), we examined the involvement of NF-κB in the induction of IRF-4 by LMP-1. LMP-1 alone induced high levels of IRF-4 in DG75 cells. However, the coexpression of LMP-1 and IκB, a repressor of NF-κB, abolished the expression of IRF-4 (Fig. 2B). IκB expression was confirmed because the LMP-1-mediated activation of an NF-κB reporter gene was inhibited (data not shown). All these data suggest that NF-κB activation is required for the LMP-1-mediated induction of IRF-4.
Knockdown of IRF-4 expression inhibits the growth of EBV-transformed cells.
We selected IRF-4 target sequences by comparing all the human IRF sequences and identified three unique sequences of IRF-4. Corresponding oligonucleotides were synthesized and cloned into an shRNA-expressing vector. The three different IRF-4 shRNAs (shIRF4) could individually inhibit the expression of ectopically expressed IRF-4 (data not shown).
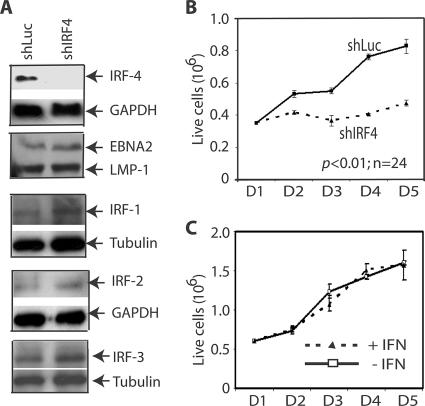
A battery of EBV-transformed cell lines was screened for cells with good transfection efficiencies. IB4, which is a prototypical cell line transformed by EBV in vitro (8, 9, 11, 16, 22, 25), was found to have a relatively high level of efficiency of transfection with Amaxa's technology. The transfection of IB4 cells with shIRF4 expression plasmids dramatically reduced the expression of IRF-4, whereas the expression levels of LMP-1, EBNA-2, IRF-1, IRF-2, IRF-3, tubulin, and GAPDH proteins were not affected (Fig. 3A). In addition, the level of expression of IRF-7 was not reduced by shIRF4 (data not shown). These data suggest that shIRF4 specifically reduced the expression of the IRF-4 protein.
FIG. 3.
Knockdown of IRF-4 inhibits the growth of EBV-transformed cells. (A) shIRF4 specifically reduced the expression of endogenous IRF-4. An EBV-transformed cell line (IB4) was transfected with shLuc or shIRF4 by use of an Amaxa Nucleofector device. Three shIRF4 plasmids that target different regions of IRF-4 were used together to knock down IRF-4. The expression levels of IRF-4, LMP-1, EBNA-2, IRF-1, IRF-2, IRF-3, tubulin, and GAPDH were examined by Western blots. The identities of the proteins are shown. The images in the same box indicate that they are derived from the same membrane. (B) Knockdown of IRF-4 inhibits the growth of EBV-transformed cells. One day after transfection, live cells were isolated through Ficoll-Paque Plus and seeded. At the indicated days after transfection, surviving cells were enumerated by trypan blue exclusion. Each point represents the number of live cells (means ± standard deviations) from three different counts. One representative from five independent experiments is shown. (C) IFN treatment did not affect growth of IB4 cells. IFN-α (100 U/ml) was used to treat IB4 cells. Trypan blue was used to stain the cells, and live cells were counted. Each point represents the number of live cells (means ± standard deviations) from three different counting. One representative from two independent experiments is shown.
The transfected cells were placed into culture dishes, and live-cell numbers were measured on a daily basis. As shown in Fig. 3B, shIRF4 significantly inhibited cell growth. shIRF4 also caused a severe slowdown in cell growth in medium containing less serum (data not shown). Therefore, these data suggest that IRF-4 is involved in the growth control of EBV-transformed cells.
Certain shRNAs may activate type I IFN pathways (55), which in turn may inhibit cell growth. The effect of IFN-α on the growth property of IB4 cells was examined. As shown in Fig. 3C, IFN had limited effects on cell growth, as expected (4, 29). The IB4 cells were responsive to IFN as determined by the activation of STAT-1 (72; data not shown). Thus, the shIRF4-mediated inhibition of cell growth was not apparently related to the potential activation of the IFN pathway.
Knockdown of IRF-4 expression reduces the proliferation rate but enhances apoptosis.
IRF-4 has oncogenic potential and may also prevent apoptotic processes (36). The IRF-4 knockdown-mediated cell growth inhibition may reflect a decrease in the rate of cell proliferation and/or an increase in the level of apoptosis.
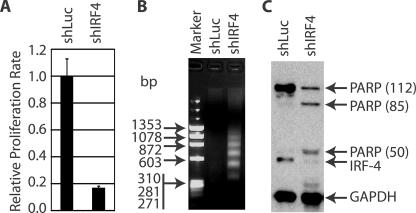
To assess the proliferation rate, the same numbers of cells were placed into a 96-well plate, and MTT assays were carried out at day 3 of transfection. As shown in Fig. 4A, cells expressing IRF-4 shRNA have lower metabolic activities than cells expressing shRNA for luciferase (shLuc). Thus, shIRF4-expressing cells may have a lower proliferation rate than control cells based on the MTT assay.
FIG. 4.
Knockdown of IRF-4 reduces the proliferation rate but enhances apoptosis. (A) Reduction of proliferation rate in IRF-4 knockdown cells. The transfection of IB4 cells were achieved by using an Amaxa Nucleofector device. Three days later, equal amounts of live cells were placed into a 96-well plate, and MTT assays were used for the detection of the proliferation rate. Relative proliferation rates are shown. (B) DNA fragmentation in shIRF4-transfected cells. Total cellular DNAs were isolated on day 3 and separated in agarose gels. The sizes of the DNA markers are as shown on the left in base pairs. (C) PARP cleavage in IRF-4 knockdown cells. Lysates from transfected cells at day 3 were used for Western blot analysis with PARP, IRF-4, and GAPDH antibodies. The molecular masses (kDa) of PARP fragments are indicated in parenthesis. The identities of the proteins are as shown.
To examine if apoptosis contributed to the reduced growth rates of shIRF4-expressing cells, a DNA fragmentation assay was performed. Apoptosis, or programmed cell death, is involved in the regulation of cell number under a wide variety of pathophysiological conditions (13, 45, 51, 56). One of the hallmark features of apoptosis is DNA fragmentation. Cellular DNA was isolated from shLuc- or shIRF4-expressing cells. As shown in Fig. 4B, shIRF4-expressing cells have a high level of fragmented DNA molecules. During apoptosis, poly(ADP-ribose) polymerase (PARP) is cleaved (30, 70). As shown in Fig. 4C, the IRF-4 knockdown resulted in PARP cleavage. Interestingly, an additional 50-kDa PARP fragment was detected (Fig. 4C), which might suggest that necrosis had occurred (53). We also detected the cleavage of caspase-3 in shIRF4-expressing cells (data not shown). All these data suggest that the knockdown of IRF-4 resulted in decreased proliferation and enhanced apoptosis.
IRF-4 is associated with LMP-1 in primary CNS lymphomas in vivo.
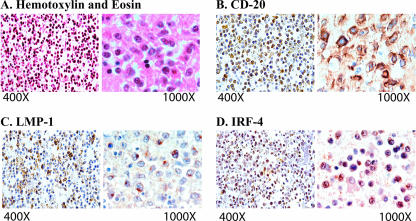
Primary CNS lymphoma occurs almost exclusively in patients with AIDS. EBV is believed to be an important etiological agent of the disease (21, 38, 49, 52, 57). We have 27 human primary CNS lymphoma specimens available (78). The tumors were characterized according to the latest World Health Organization classification of brain tumors (34). These patients had been diagnosed with primary CNS lymphoma without the involvement of any other organ or tissue. Histologically, the tumors were characterized by abundant homogeneous neoplastic lymphocytes, located predominantly within the Virchow-Robin space in a concentric pattern. As shown in Fig. 5, the neoplastic cells had infiltrated the brain parenchyma in the majority of the cases. Pan-B (CD20) and pan-T (CD3) markers were used to determine the origin of the tumors. All of the tumors studied demonstrated a cytoplasmic expression of CD20 and no CD3 expression, indicating the B-cell origin of the lymphoma cells. The EBV status was determined by the expression of LMP-1 (78). IRF-4 is apparently localized in the nuclei predominantly (Fig. 5). IRF-4 was detected in 12 of the total 27 tumor samples examined (44.4%), and all IRF-4-positive specimens also expressed LMP-1 (12/12 [100%]) (Table 1). The association between the expression levels of LMP-1 and IRF-4 was statistically significant (R = 0.479; F = 7.444; P = 0.011) (Table 1). These data strongly suggest that IRF-4 is associated with EBV infection in vivo and LMP-1 expression in particular in these primary CNS lymphomas.
FIG. 5.
Histological and immunohistochemical characterization of IRF-4 in CNS lymphomas. (A) Hematoxylin and eosin staining of a CNS lymphoma demonstrated the perivascular concentric accumulation of neoplastic cells. (B) Immunohistochemistry for the pan-B-cell marker CD20 highlighted the neoplastic lymphocytes in the Virchow-Robin space and confirmed their B-cell origin. (C) The EBV LMP-1 was robustly expressed in the cytoplasm of neoplastic lymphocytes in both the perivascular space and the brain parenchyma. (D) The expression of IRF-4 was detected in numerous neoplastic lymphocytes.
DISCUSSION
EBV is able to transform primary human B cells in vitro. LMP-1 is the principal oncoprotein required for EBV transformation. Because transformation in vitro takes place rather rapidly and has a low probability of accumulating genetic mutations, at least initially, LMP-1 must contribute to cellular transformation by altering the expression and activity of cellular genes that are involved in oncogenesis.
In this report, we have provided evidence that cellular IRF-4 may be a key mediator for EBV transformation. First, IRF-4 expression was induced during EBV transformation in vitro and is correlated with LMP-1 expression from EBV-infected cells (Fig. 1). Second, IRF-4 expression was detected in a significant number of primary CNS lymphoma specimens and associated with the expression of LMP-1 in vivo in primary CNS lymphomas (Fig. 5 and Table 1). Third, LMP-1 induced the expression of IRF-4 proteins in transient assays. Furthermore, we have shown that NF-κB, which is a downstream target of the LMP-1 signaling pathway, is required for LMP-1 to induce IRF-4 (Fig. 2). Fourth, the knockdown of IRF-4 by shRNA in EBV-transformed cells resulted in the inhibition of cellular growth, apparently due to the collective effects of a reduced proliferation rate as well as increased apoptosis (Fig. 3 and 4). Because shIRF4 specifically down-regulates IRF-4, but not IRF-1, -2, -3, and -7 (Fig. 3A and data not shown), the potential off-target effects of shIRF4 may be minimal. Fifth, the growth of IB4 cells was not affected in the presence of IFN (Fig. 3C), which is in agreement with data from previous reports (4, 29). In addition, we observed no IFN production due to the expression of shIRF4 by enzyme-linked immunosorbent assays (data not shown). Moreover, EBV EBNA2, which is not affected by shIRF4 (Fig. 3A), could alleviate the IFN-mediated growth arrest (29). Thus, the activation of the IFN pathway is not apparently involved in the alteration of growth phenotypes by IRF-4 knockdown. All these data collectively suggest that IRF-4 is involved in maintaining the growth phenotypes of EBV-transformed cells in vitro.
It is known that IRF-4 interacts with other cellular proteins for its proper functions (39). IRF-4 alone is apparently not sufficient for oncogenesis in the transgenic mouse model (50), suggesting that an additional factor(s) is required for the oncogenic activity of IRF-4 in vivo. We reason that EBV transformation may require functional interactions among IRF-4 and other viral and cellular factors. The depletion of IRF-4 might impair the transformation processes despite the physical presence of other oncogenic genes, such as IRF-7 and LMP-1. However, the direct targets of IRF-4 during EBV-induced transformation are currently unknown.
IRF-2, -4, and -7 are the three IRF members with oncogenic potential. IRF-7, similar to IRF-4, is associated with EBV transformation in vitro and in vivo. IRF-2 is apparently not associated with the EBV transformation process in vitro (78); however, IRF-2 is associated with type III latency and may negatively regulate an important viral latency promoter, Qp (73). Interestingly, IRF-5, which is likely a tumor suppressor (5, 23, 24, 43, 68), is highly expressed in EBV-transformed cells and, together with IRF-4, may be involved in the EBV-mediated regulation of Toll-like receptor 7 activities (40). Clearly, IRFs are important for EBV latency and transformation, potentially by targeting different or overlapping signaling pathways.
IRF-4 may play a role in EBV-associated tumors in vivo. IRF-4 expression was detected in a significant number of clinical samples of primary CNS lymphoma specimens. All IRF-4-positive specimens also expressed EBV LMP-1 (100%). In these LMP-1-negative specimens, no IRF-4 was detected (100%). However, 12/24 (50%) LMP-1-positve specimens had no detectable IRF-4. For those LMP-1-positive, IRF-4-negative specimens, the expression of LMP-1 was apparently lower: 9/12 (75%) specimens expressed low levels of LMP-1 (1 to 30% cell-positive reactivity). However, in those LMP-1-positive, IRF-4-positive specimens, LMP-1 expression was apparently higher: 9/12 (75%) specimens expressed LMP-1 at high levels (>30% cell-positive reactivity) (Table 1). We have found that the IRF-4 antibody used in this study had a moderate sensitivity for immunostaining (data not shown). Thus, the numbers of IRF-4-positve CNS lymphoma specimens might be higher than what was detected. Nevertheless, there is a statistically significant association between expression levels of LMP-1 and those of IRF-4 in these samples (P = 0.011) (Table 1). These data suggest that IRF-4 may be associated with LMP-1 in vivo. CNS lymphoma cells are phenotypically similar to EBV-transformed LCLs in vitro, and EBV is believed to be an etiological agent of AIDS-associated CNS lymphoma in vivo. Because of the critical functions of IRF-4 in the growth control of EBV-transformed cells in vitro (Fig. 3 and 4), it is likely that IRF-4 may play a similar role in vivo in the pathogenesis of CNS lymphoma.
In summary, this report has provided new insights into the EBV transformation process and suggests that IRF-4 may be involved in EBV transformation processes both in vitro and in vivo. In addition, IRF-4 might be a useful target in the therapy of EBV-mediated cellular proliferation.
Acknowledgments
We thank Paul Ling, Kenneth Izumi, Lindsey Hutt-Flectcher, Peter Howley, and Albert Baldwin for providing reagents for this work and Dustin Petrik for the critical reading of the manuscript.
This work was supported in part by grants AI59132 and CA108951 from the NIH and NCRR COBRA grant RR15635 (L. Zhang).
D.X. designed the research, performed experiments, and analyzed data; L. Zhao helped in shRNA technology; and L.D.V. performed the immunohistochemical experiments and analyzed the data from clinical samples. J.M. collected and provided the clinical specimens. L. Zhang designed the research, analyzed data, wrote the paper, provided funding for the research, and supervised all experiments.
Footnotes
Published ahead of print on 16 April 2008.
REFERENCES
- 1.Abbot, S. D., M. Rowe, K. Cadwallader, A. Ricksten, J. Gordon, F. Wang, L. Rymo, and A. B. Rickinson. 1990. Epstein-Barr virus nuclear antigen 2 induces expression of the virus-encoded latent membrane protein. J. Virol. 642126-2134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Adldinger, H. K., H. Delius, U. K. Freese, J. Clarke, and G. W. Bornkamm. 1985. A putative transforming gene of Jijoye virus differs from that of Epstein-Barr virus prototypes. Virology 141221-234. [DOI] [PubMed] [Google Scholar]
- 3.Alexander, L., L. Denekamp, A. Knapp, M. R. Auerbach, B. Damania, and R. C. Desrosiers. 2000. The primary sequence of rhesus monkey rhadinovirus isolate 26-95: sequence similarities to Kaposi's sarcoma-associated herpesvirus and rhesus monkey rhadinovirus isolate 17577. J. Virol. 743388-3398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aman, P., and A. von Gabain. 1990. An Epstein-Barr virus immortalization associated gene segment interferes specifically with the IFN-induced anti-proliferative response in human B-lymphoid cell lines. EMBO J. 9147-152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barnes, B. J., M. J. Kellum, K. E. Pinder, J. A. Frisancho, and P. M. Pitha. 2003. Interferon regulatory factor 5, a novel mediator of cell cycle arrest and cell death. Cancer Res. 636424-6431. [PubMed] [Google Scholar]
- 6.Ben-Bassat, H., N. Goldblum, S. Mitrani, T. Goldblum, J. M. Yoffey, M. M. Cohen, Z. Bentwith, B. Ramot, E. Klein, and G. Klein. 1977. Establishment in continuous culture of a new type of lymphocyte from a “Burkitt-like” malignant lymphoma (line D.G.-75). Int. J. Cancer 1927-33. [DOI] [PubMed] [Google Scholar]
- 7.Cahir-McFarland, E. D., K. Carter, A. Rosenwald, J. M. Giltnane, S. E. Henrickson, L. M. Staudt, and E. Kieff. 2004. Role of NF-κB in cell survival and transcription of latent membrane protein 1-expressing or Epstein-Barr virus latency III-infected cells. J. Virol. 784108-4119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cahir-McFarland, E. D., D. M. Davidson, S. L. Schauer, J. Duong, and E. Kieff. 2000. NF-kappa B inhibition causes spontaneous apoptosis in Epstein-Barr virus-transformed lymphoblastoid cells. Proc. Natl. Acad. Sci. USA 976055-6060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cahir-McFarland, E. D., K. M. Izumi, and G. Mosialos. 1999. Epstein-Barr virus transformation: involvement of latent membrane protein 1-mediated activation of NF-kappaB. Oncogene 186959-6964. [DOI] [PubMed] [Google Scholar]
- 10.Calender, A., M. Billaud, J. P. Aubry, J. Banchereau, M. Vuillaume, and G. M. Lenoir. 1987. Epstein-Barr virus (EBV) induces expression of B-cell activation markers on in vitro infection of EBV-negative B-lymphoma cells. Proc. Natl. Acad. Sci. USA 848060-8064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carter, K. L., E. Cahir-McFarland, and E. Kieff. 2002. Epstein-Barr virus-induced changes in B-lymphocyte gene expression. J. Virol. 7610427-10436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cohen, J. I., F. Wang, J. Mannick, and E. Kieff. 1989. Epstein-Barr virus nuclear protein 2 is a key determinant of lymphocyte transformation. Proc. Natl. Acad. Sci. USA 869558-9562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Debatin, K. M., and P. H. Krammer. 2004. Death receptors in chemotherapy and cancer. Oncogene 232950-2966. [DOI] [PubMed] [Google Scholar]
- 14.Eisenbeis, C. F., H. Singh, and U. Storb. 1995. Pip, a novel IRF family member, is a lymphoid-specific, PU.1-dependent transcriptional activator. Genes Dev. 91377-1387. [DOI] [PubMed] [Google Scholar]
- 15.Fries, K. L., W. E. Miller, and N. Raab-Traub. 1996. Epstein-Barr virus latent membrane protein 1 blocks p53-mediated apoptosis through the induction of the A20 gene. J. Virol. 708653-8659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Frost, V., S. Delikat, S. Al-Mehairi, and A. J. Sinclair. 2001. Regulation of p27KIP1 in Epstein-Barr virus-immortalized lymphoblastoid cell lines involves non-apoptotic caspase cleavage. J. Gen. Virol. 823057-3066. [DOI] [PubMed] [Google Scholar]
- 17.Gao, S. J., C. Boshoff, S. Jayachandra, R. A. Weiss, Y. Chang, and P. S. Moore. 1997. KSHV ORF K9 (vIRF) is an oncogene which inhibits the interferon signaling pathway. Oncogene 151979-1985. [DOI] [PubMed] [Google Scholar]
- 18.Ghosh, D., and E. Kieff. 1990. cis-acting regulatory elements near the Epstein-Barr virus latent-infection membrane protein transcriptional start site. J. Virol. 641855-1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gires, O., U. Zimber-Strobl, R. Gonnella, M. Ueffing, G. Marschall, R. Zeidler, D. Pich, and W. Hammerschmidt. 1997. Latent membrane protein 1 of Epstein-Barr virus mimics a constitutively active receptor molecule. EMBO J. 166131-6140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grumont, R. J., and S. Gerondakis. 2000. Rel induces interferon regulatory factor 4 (IRF-4) expression in lymphocytes: modulation of interferon-regulated gene expression by rel/nuclear factor kappaB. J. Exp. Med. 1911281-1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guterman, K. S., L. S. Hair, and S. Morgello. 1996. Epstein-Barr virus and AIDS-related primary central nervous system lymphoma. Viral detection by immunohistochemistry, RNA in situ hybridization, and polymerase chain reaction. Clin. Neuropathol. 1579-86. [PubMed] [Google Scholar]
- 22.Henderson, A., S. Ripley, M. Heller, and E. Kieff. 1983. Chromosome site for Epstein-Barr virus DNA in a Burkitt tumor cell line and in lymphocytes growth-transformed in vitro. Proc. Natl. Acad. Sci. USA 801987-1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hu, G., and B. J. Barnes. 2006. Interferon regulatory factor-5-regulated pathways as a target for colorectal cancer therapeutics. Expert Rev. Anticancer Ther. 6775-784. [DOI] [PubMed] [Google Scholar]
- 24.Hu, G., M. E. Mancl, and B. J. Barnes. 2005. Signaling through IFN regulatory factor-5 sensitizes p53-deficient tumors to DNA damage-induced apoptosis and cell death. Cancer Res. 657403-7412. [DOI] [PubMed] [Google Scholar]
- 25.Hurley, E. A., L. D. Klaman, S. Agger, J. B. Lawrence, and D. A. Thorley-Lawson. 1991. The prototypical Epstein-Barr virus-transformed lymphoblastoid cell line IB4 is an unusual variant containing integrated but no episomal viral DNA. J. Virol. 653958-3963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Iida, S., P. H. Rao, M. Butler, P. Corradini, M. Boccadoro, B. Klein, R. S. Chaganti, and R. Dalla-Favera. 1997. Deregulation of MUM1/IRF4 by chromosomal translocation in multiple myeloma. Nat. Genet. 17226-230. [DOI] [PubMed] [Google Scholar]
- 27.Izumi, K. M., K. M. Kaye, and E. D. Kieff. 1997. The Epstein-Barr virus LMP1 amino acid sequence that engages tumor necrosis factor receptor associated factors is critical for primary B lymphocyte growth transformation. Proc. Natl. Acad. Sci. USA 941447-1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jin, L., G. C. Perng, D. J. Brick, J. Naito, A. B. Nesburn, C. Jones, and S. L. Wechsler. 2004. Methods for detecting the HSV-1 LAT anti-apoptosis activity in virus infected tissue culture cells. J. Virol. Methods 1189-13. [DOI] [PubMed] [Google Scholar]
- 29.Kanda, K., T. Decker, P. Aman, M. Wahlström, A. von Gabain, and B. Kallin. 1992. The EBNA2-related resistance towards alpha interferon (IFN-α) in Burkitt's lymphoma cells effects induction of IFN-induced genes but not the activation of transcription factor ISGF-3. Mol. Cell. Biol. 124930-4936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kaufmann, S. H., S. Desnoyers, Y. Ottaviano, N. E. Davidson, and G. G. Poirier. 1993. Specific proteolytic cleavage of poly(ADP-ribose) polymerase: an early marker of chemotherapy-induced apoptosis. Cancer Res. 533976-3985. [PubMed] [Google Scholar]
- 31.Kaye, K. M., K. M. Izumi, and E. Kieff. 1993. Epstein-Barr virus latent membrane protein 1 is essential for B-lymphocyte growth transformation. Proc. Natl. Acad. Sci. USA 909150-9154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kieff, E. 1996. Epstein-Barr virus and its replication, p. 2343-2396. In B. N. Fields, D. M. Knipe, and P. M. Howley (ed.), Fields virology, 3rd ed. Lippincott-Raven Publishers, Philadelphia, PA.
- 33.Kilger, E., A. Kieser, M. Baumann, and W. Hammerschmidt. 1998. Epstein-Barr virus-mediated B-cell proliferation is dependent upon latent membrane protein 1, which simulates an activated CD40 receptor. EMBO J. 171700-1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kleihues, P., D. N. Louis, B. W. Scheithauer, L. B. Rorke, G. Reifenberger, P. C. Burger, and W. K. Cavenee. 2002. The WHO classification of tumors of the nervous system. J. Neuropathol. Exp. Neurol. 61215-225; discussion, 226-229. [DOI] [PubMed] [Google Scholar]
- 35.Liebowitz, D., D. Wang, and E. Kieff. 1986. Orientation and patching of the latent infection membrane protein encoded by Epstein-Barr virus. J. Virol. 58233-237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lohoff, M., H. W. Mittrucker, A. Brustle, F. Sommer, B. Casper, M. Huber, D. A. Ferrick, G. S. Duncan, and T. W. Mak. 2004. Enhanced TCR-induced apoptosis in interferon regulatory factor 4-deficient CD4(+) Th cells. J. Exp. Med. 200247-253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lu, R., K. L. Medina, D. W. Lancki, and H. Singh. 2003. IRF-4,8 orchestrate the pre-B-to-B transition in lymphocyte development. Genes Dev. 171703-1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.MacMahon, E. M., J. D. Glass, S. D. Hayward, R. B. Mann, P. S. Becker, P. Charache, J. C. McArthur, and R. F. Ambinder. 1991. Epstein-Barr virus in AIDS-related primary central nervous system lymphoma. Lancet 338969-973. [DOI] [PubMed] [Google Scholar]
- 39.Marecki, S., and M. J. Fenton. 2002. The role of IRF-4 in transcriptional regulation. J. Interf. Cytok. Res. 22121-133. [DOI] [PubMed] [Google Scholar]
- 40.Martin, H. J., J. M. Lee, D. Walls, and S. D. Hayward. 2007. Manipulation of the Toll-like receptor 7 signaling pathway by Epstein-Barr virus. J. Virol. 819748-9758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Miller, W. E., H. S. Earp, and N. Raab-Traub. 1995. The Epstein-Barr virus latent membrane protein 1 induces expression of the epidermal growth factor receptor. J. Virol. 694390-4398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mittrucker, H. W., T. Matsuyama, A. Grossman, T. M. Kundig, J. Potter, A. Shahinian, A. Wakeham, B. Patterson, P. S. Ohashi, and T. W. Mak. 1997. Requirement for the transcription factor LSIRF/IRF4 for mature B and T lymphocyte function. Science 275540-543. [DOI] [PubMed] [Google Scholar]
- 43.Mori, T., Y. Anazawa, M. Iiizumi, S. Fukuda, Y. Nakamura, and H. Arakawa. 2002. Identification of the interferon regulatory factor 5 gene (IRF-5) as a direct target for p53. Oncogene 212914-2918. [DOI] [PubMed] [Google Scholar]
- 44.Nonkwelo, C., J. Skinner, A. Bell, A. Rickinson, and J. Sample. 1996. Transcription start sites downstream of the Epstein-Barr virus (EBV) Fp promoter in early-passage Burkitt lymphoma cells define a fourth promoter for expression of the EBV EBNA-1 protein. J. Virol. 70623-627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Norbury, C. J., and B. Zhivotovsky. 2004. DNA damage-induced apoptosis. Oncogene 232797-2808. [DOI] [PubMed] [Google Scholar]
- 46.Pagano, J. S. 1999. Epstein-Barr virus: the first human tumor virus and its role in cancer. Proc. Assoc. Am. Physicians 111573-580. [DOI] [PubMed] [Google Scholar]
- 47.Pitha, P. M., W. C. Au, W. Lowther, Y. T. Juang, S. L. Schafer, L. Burysek, J. Hiscott, and P. A. Moore. 1998. Role of the interferon regulatory factors (IRFs) in virus-mediated signaling and regulation of cell growth. Biochimie 80651-658. [DOI] [PubMed] [Google Scholar]
- 48.Rickinson, A. B., and E. Kieff. 1996. Epstein-Barr virus, p. 2397-2446. In B. N. Fields, D. M. Knipe, and P. M. Howley (ed.), Fields virology, 3rd ed. Lippincott-Raven Publishers, Philadelphia, PA.
- 49.Rodriguez, M. M., P. I. Delgado, and C. K. Petito. 1997. Epstein-Barr virus-associated primary central nervous system lymphoma in a child with the acquired immunodeficiency syndrome. A case report and review of the literature. Arch. Pathol. Lab. Med. 1211287-1291. [PubMed] [Google Scholar]
- 50.Saito, T., T. Yamagata, T. Takahashi, H. Honda, and H. Hirai. 1999. ICSAT overexpression is not sufficient to cause adult T-cell leukemia or multiple myeloma. Biochem. Biophys. Res. Commun. 260329-331. [DOI] [PubMed] [Google Scholar]
- 51.Salvesen, G. S., and J. M. Abrams. 2004. Caspase activation—stepping on the gas or releasing the brakes? Lessons from humans and flies. Oncogene 232774-2784. [DOI] [PubMed] [Google Scholar]
- 52.Scadden, D. T. 2000. Epstein-Barr virus, the CNS, and AIDS-related lymphomas: as close as flame to smoke. J. Clin. Oncol. 183323-3324. [DOI] [PubMed] [Google Scholar]
- 53.Shah, G. M., R. G. Shah, and G. G. Poirier. 1996. Different cleavage pattern for poly(ADP-ribose) polymerase during necrosis and apoptosis in HL-60 cells. Biochem. Biophys. Res. Commun. 229838-844. [DOI] [PubMed] [Google Scholar]
- 54.Sharma, S., N. Grandvaux, Y. Mamane, P. Genin, N. Azimi, T. Waldmann, and J. Hiscott. 2002. Regulation of IFN regulatory factor 4 expression in human T cell leukemia virus-I-transformed T cells. J. Immunol. 1693120-3130. [DOI] [PubMed] [Google Scholar]
- 55.Sledz, C. A., M. Holko, M. J. de Veer, R. H. Silverman, and B. R. Williams. 2003. Activation of the interferon system by short-interfering RNAs. Nat. Cell Biol. 5834-839. [DOI] [PubMed] [Google Scholar]
- 56.Sun, S. Y., N. Hail, Jr., and R. Lotan. 2004. Apoptosis as a novel target for cancer chemoprevention. J. Natl. Cancer Inst. 96662-672. [DOI] [PubMed] [Google Scholar]
- 57.Taiwo, B. O. 2000. AIDS-related primary CNS lymphoma: a brief review. AIDS Read. 10486-491. [PubMed] [Google Scholar]
- 58.Tamura, T., P. Tailor, K. Yamaoka, H. J. Kong, H. Tsujimura, J. J. O'Shea, H. Singh, and K. Ozato. 2005. IFN regulatory factor-4 and -8 govern dendritic cell subset development and their functional diversity. J. Immunol. 1742573-2581. [DOI] [PubMed] [Google Scholar]
- 59.Taniguchi, T., H. Harada, and M. Lamphier. 1995. Regulation of the interferon system and cell growth by the IRF transcription factors. J. Cancer Res. Clin. Oncol. 121516-520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Taniguchi, T., K. Ogasawara, A. Takaoka, and N. Tanaka. 2001. IRF family of transcription factors as regulators of host defense. Annu. Rev. Immunol. 19623-655. [DOI] [PubMed] [Google Scholar]
- 61.Tsang, S. F., F. Wang, K. M. Izumi, and E. Kieff. 1991. Delineation of the cis-acting element mediating EBNA-2 transactivation of latent infection membrane protein expression. J. Virol. 656765-6771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wang, D., D. Leibowitz, and E. Kieff. 1985. An EBV membrane protein expressed in immortalized lymphocytes transforms established rodent cells. Cell 43831-840. [DOI] [PubMed] [Google Scholar]
- 63.Wang, F., C. Gregory, C. Sample, M. Rowe, D. Liebowitz, R. Murray, A. Rickinson, and E. Kieff. 1990. Epstein-Barr virus latent membrane protein (LMP1) and nuclear proteins 2 and 3C are effectors of phenotypic changes in B lymphocytes: EBNA-2 and LMP1 cooperatively induce CD23. J. Virol. 642309-2318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wang, F., S. F. Tsang, M. G. Kurilla, J. I. Cohen, and E. Kieff. 1990. Epstein-Barr virus nuclear antigen 2 transactivates latent membrane protein LMP1. J. Virol. 643407-3416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Xu, D., K. Brumm, and L. Zhang. 2006. The latent membrane protein 1 of Epstein-Barr virus primes EBV latency cells for type I interferon production. J. Biol. Chem. 2819163-9169. [DOI] [PubMed] [Google Scholar]
- 66.Xu, D., T. Coleman, J. Zhang, A. Fagot, C. Kotalik, L. Zhao, P. Trivedi, C. Jones, and L. Zhang. 2007. Epstein-Barr virus inhibits Kaposi's sarcoma-associated herpesvirus lytic replication in primary effusion lymphomas. J. Virol. 816068-6078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yamagata, T., J. Nishida, S. Tanaka, R. Sakai, K. Mitani, M. Yoshida, T. Taniguchi, Y. Yazaki, and H. Hirai. 1996. A novel interferon regulatory factor family transcription factor, ICSAT/Pip/LSIRF, that negatively regulates the activity of interferon-regulated genes. Mol. Cell. Biol. 161283-1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yanai, H., H. M. Chen, T. Inuzuka, S. Kondo, T. W. Mak, A. Takaoka, K. Honda, and T. Taniguchi. 2007. Role of IFN regulatory factor 5 transcription factor in antiviral immunity and tumor suppression. Proc. Natl. Acad. Sci. USA 1043402-3407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yoshizaki, T., H. Sato, M. Furukawa, and J. S. Pagano. 1998. The expression of matrix metalloproteinase 9 is enhanced by Epstein-Barr virus latent membrane protein 1. Proc. Natl. Acad. Sci. USA 953621-3626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yuan, J., S. Shaham, S. Ledoux, H. M. Ellis, and H. R. Horvitz. 1993. The C. elegans cell death gene ced-3 encodes a protein similar to mammalian interleukin-1 beta-converting enzyme. Cell 75641-652. [DOI] [PubMed] [Google Scholar]
- 71.Zhang, J., S. C. Das, C. Kotalik, A. K. Pattnaik, and L. Zhang. 2004. The latent membrane protein 1 of Epstein-Barr virus establishes an antiviral state via induction of interferon-stimulated genes. J. Biol. Chem. 27946335-46342. [DOI] [PubMed] [Google Scholar]
- 72.Zhang, L., K. Hong, J. Zhang, and J. Pagano. 2004. Multiple signal transducers and activators of transcription (STATs) are induced by EBV LMP-1. Virology 323141-152. [DOI] [PubMed] [Google Scholar]
- 73.Zhang, L., and J. S. Pagano. 1999. Interferon regulatory factor 2 represses the Epstein-Barr virus BamHI Q latency promoter in type III latency. Mol. Cell. Biol. 193216-3223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zhang, L., and J. S. Pagano. 2000. Interferon regulatory factor 7 is induced by Epstein-Barr virus latent membrane protein 1. J. Virol. 741061-1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhang, L., and J. S. Pagano. 2001. Interferon regulatory factor 7 mediates the activation of Tap-2 by Epstein-Barr virus latent membrane protein 1. J. Virol. 75341-350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zhang, L., and J. S. Pagano. 1997. IRF-7, a new interferon regulatory factor associated with Epstein-Barr virus latency. Mol. Cell. Biol. 175748-5757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zhang, L., L. H. Wu, K. Hong, and J. S. Pagano. 2001. Intracellular signaling molecules activated by Epstein-Barr virus for induction of interferon regulatory factor 7. J. Virol. 7512393-12401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zhang, L., J. Zhang, Q. Lambert, C. J. Der, L. Del Valle, J. Miklossy, K. Khalili, Y. Zhou, and J. S. Pagano. 2004. Interferon regulatory factor 7 is associated with Epstein-Barr virus-transformed central nervous system lymphoma and has oncogenic properties. J. Virol. 7812987-12995. [DOI] [PMC free article] [PubMed] [Google Scholar]