Abstract
Apoptosis is a host defense mechanism against viruses that can be subverted by viral gene products. Human cytomegalovirus encodes viral mitochondria-localized inhibitor of apoptosis (vMIA; also known as pUL37x1), which is targeted to mitochondria and functions as a potent cell death suppressor by binding to and inhibiting proapoptotic Bcl-2 family members Bax and Bak. vMIA expression also dramatically alters mitochondrial morphology, causing the fragmentation of these organelles. A potential ortholog of vMIA, m38.5, which was identified in murine cytomegalovirus, has been shown to localize to mitochondria and protect against chemically induced apoptosis by unknown mechanisms. Despite sharing negligible homology with vMIA and no region detectably corresponding to the vMIA Bax-binding domain, we find that m38.5, like vMIA, binds to Bax and recruits Bax to mitochondria. Interestingly, m38.5 and vMIA appear to block Bax downstream of translocation to mitochondria and after an initial stage of Bax conformational change. In contrast to vMIA, m38.5 neither binds to Bak nor causes mitochondrial fragmentation. Consistently with Bax-selective inactivation by m38.5, m38.5 fragments mitochondria in Bak knockout (KO) cells and protects Bak KO cells from apoptosis better than Bax KO cells. Thus, vMIA and m38.5 share some, but not all, features of apoptosis regulation through Bcl-2 family interaction and allow the dissection of Bax translocation into discrete steps.
Programmed cell death plays key roles in development, the maintenance of tissue homeostasis, and the elimination of virus-infected cells. Many viruses circumvent this host defense mechanism by expressing proteins that inhibit apoptosis (5, 9, 10, 23, 24, 40, 43). Human cytomegalovirus (HCMV), a prototypic betaherpesvirus, expresses the first exon of the UL37 gene (UL37x1) (18, 51) encoding vMIA (viral mitochondria-localized inhibitor of apoptosis; also known as pUL37x1), which prevents apoptosis by blocking mitochondrial membrane permeabilization and the release of cytochrome c (16).
The initiation and execution of apoptosis is regulated by the Bcl-2 family of proteins (50). vMIA interacts with two proapoptotic Bcl-2 family members, Bax (5, 39) and Bak (27), and localizes to mitochondria to exert its antiapoptotic function. Paradoxically, vMIA expression leads to mitochondrial association and the oligomerization of Bax (5, 40), two processes typically associated with the early stages of apoptosis (3, 20, 46, 49). While primate CMVs have been shown to encode homologs of vMIA based on sequence similarity, murine CMV (MCMV) lacks a predicted protein homolog of vMIA (15, 31). However, Bax and Bak are sequestered in oligomers at the mitochondria in cells infected with MCMV (1), indicating that MCMV encodes a functional homolog of vMIA. Recently, the gene product m38.5, encoded by MCMV in a genomic position analogous to that of the UL37x1 open reading frame (6, 25), has been found to localize to mitochondria and protect cells from apoptosis induced by proteasome inhibition (30). With little sequence similarity but analogous activity, m38.5 was proposed to be a functional ortholog of vMIA (2, 30).
The expression of vMIA disrupts the interconnected network of mitochondria, resulting in a fragmented mitochondrial phenotype (32). The fragmentation is due to a decrease in the rate of organelle fusion (27), possibly due to the sequestration of Bax and Bak. Recent work has shown that Bcl-2 family members, in addition to their apoptosis-regulating roles, function in healthy cells to maintain normal interconnected mitochondrial morphology (12, 27). The loss of Bax and Bak by genetic knockout results in the fragmentation of the mitochondrial network that can be reversed by the permanent reexpression of Bak. Either Bax or Bak alone may maintain mitochondrial elongation, as seen in single Bax or Bak knockout (KO) cells. Ectopic Bax expression also was shown to reverse the induction of mitochondrial fragmentation induced by vMIA (27). This reversal of vMIA-mediated fragmentation by Bax is due to an increase in the rate of mitochondrial fusion, as the inhibition of fission using a dominant-negative fission protein (Drp1) is unable to reverse the mitochondrial fragmentation activity of vMIA. Recently, the phosphate carrier protein in the mitochondrial inner membrane, inorganic phosphate carrier (PiC), has been shown to mediate both vMIA and Mfn2 knockdown-mediated mitochondrial fragmentation (36). Whether Bax inhibits vMIA or PiC activity or functions independently to mediate mitochondrial elongation remains unclear.
Cellular binding partners of vMIA interacting with a region overlapping the Bax-binding site (amino acids 115 to 147) have been identified by yeast two-hybrid screens (41). One protein found to interact with vMIA is growth arrest and DNA damage 45 (GADD45α), a cell cycle-regulatory protein that is induced by p53 upon genotoxic stress. GADD45α was shown to be required for cell death suppression by vMIA. Interestingly, GADD45α also was found to bind and enhance cellular protection mediated by Bcl-xL.
In order to explore how m38.5 and vMIA inhibit apoptosis, we compared their effects on mitochondria as well as their effects on apoptotic stimuli in cells lacking Bax or Bak. Our studies reveal that, despite a lack of detectable homology between any region of m38.5 and the Bax-binding domain of vMIA, Bax binds to m38.5 and accumulates on mitochondria in response to m38.5 expression in both human- and mouse-derived cell lines. However, m38.5 does not detectably bind human or mouse Bak, in contrast to vMIA. Consistently with Bax-selective inactivation, m38.5 alters mitochondrial morphology only in the absence of Bak and functions to selectively protect murine cells through Bax-dependent pathways. The species-specific nature of the CMVs, considered in the context of the interactions of vMIA and m38.5 with Bax and Bak, sheds light on the differential importance of the two proapoptotic Bcl-2 family members in viral infection-induced cell death in each organism.
MATERIALS AND METHODS
Cells, virus, constructs, and antibodies (Abs).
HeLa human cervical carcinoma cells, WT/Bax KO/Bak KO and Bax/Bak double KO (DKO) mouse embryonic fibroblasts (MEFs), NIH 3T3 murine fibroblasts, and DU145 prostate cancer cells were cultured in 5% CO2 at 37°C. HeLa cells and the four MEF cell lines were grown in complete Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% heat-inactivated fetal bovine serum, 100 U/ml penicillin, and 100 μg/ml streptomycin. NIH 3T3 fibroblasts (ATCC) were grown in DMEM supplemented with 4 mM l-glutamine, 1 mM sodium pyruvate, 10% heat-inactivated calf bovine serum, 100 U/ml penicillin, and 100 μg/ml streptomycin. DU145 cells were grown in RPMI 1640 medium supplemented with 1 mM sodium pyruvate, 4 mM l-glutamine, 10% heat-inactivated fetal bovine serum, 100 U/ml penicillin, and 100 μg/ml streptomycin.
For mitochondrial analysis during MCMV infection, 4 × 105 cells were seeded in confocal chambers and infected with MCMV-green fluorescent protein (GFP), a recombinant MCMV expressing enhanced GFP (a gift from D. Margulies) at a multiplicity of infection (MOI) of 3. Infectious medium was removed 1 h postinfection (hpi), and fresh medium was added to each well. Cells were fixed at 48 hpi and imaged by confocal microscopy.
Mito-yellow fluorescent protein (YFP) (Clontech) was used to image the mitochondrial matrix. pcDNA3.1 (Invitrogen) was used as a control vector in transient transfections. m38.5-myc was amplified from the LNCX-m38.5 construct (30) using the primers 5′-CGATTCTCGAGGAATGTCTGAGCAAAAGCTCATTTC-3′ and 5′-ATCGCGGGCCCATTAAGATCCTCCTCGGATATTA-3′ and was cloned into the XhoI and ApaI sites of pcDNA3.1 to generate m38.5-myc. Bases 1 through 105 of m38.5 were amplified from the m38.5-myc construct using the primers 5′-CCGGACGAATTCATGGAGAGTGTGCGCCGACC-3′ and 5′-GGGAATGGATCCAAATTAGAGAGAATCCAACCGGCT-3′ and were cloned into the EcoRI and BamHI sites of YFP-N1 (BD Biosciences) to generate 1-35 m38.5-YFP. Bases 106 through 588 of m38.5 were amplified from the m38.5-myc construct using the primers 5′-CCGGACGAATTCATGTGGTTAACCAGGCGGCGGG-3′ and 5′-GGGAATGGATCCAAGAATGTGTAATCTCCATCTTCTG-3′ and were cloned into the EcoRI and BamHI sites of YFP-N1 (BD Biosciences) to generate m38.5Δ2-35-YFP. Human GFP-tagged Bax and BaxΔC, YFP-Bcl-xL, and cyan fluorescent protein (CFP)-tagged Bax, as well as human Bax and BaxΔC in the pcDNA3 vector, have been previously described (22, 34, 49).
Primary Abs used in this study were Bak rabbit polyclonal Ab (PAb) (NT; Upstate), Bax rabbit PAb (NT; Upstate), mouse 6A7 mAb (Sigma), c-myc mouse monoclonal Ab (9E10; Roche), GFP rabbit PAb (BD Biosciences), and Tim23 mouse monoclonal Ab (BD Biosciences). Anti-mouse and anti-rabbit immunoglobulin G horseradish peroxidase-conjugated secondary Abs (Amersham) were used for immunoblotting. For immunostaining, cytochrome c (PharMingen) was used as a primary Ab, with Alexa 594-conjugated goat anti-mouse as the secondary Ab (Molecular Probes).
Subcellular fractionation and carbonate extraction.
Cells were transfected with Effectene (Qiagen) according to the manufacturer's protocols, using 4 μg per 15-cm2 plate. Cells were collected with 0.1 mM EDTA in phosphate-buffered saline (PBS), washed, and then resuspended in sucrose lysis buffer (250 mM sucrose, 20 mM HEPES-KOH, pH 7.5, 10 mM KCl, 1.5 mM MgCl2, 1 mM EDTA, 1 mM EGTA, 1 mM dithiothreitol [DTT], and 0.1 mM phenylmethylsulfonyl fluoride). Cells were broken by needle passage (six passages through 25-gauge needles), followed by serial centrifugation at 4°C; a 2,000 × g spin yielded postnuclear supernatant (PNS), which then was repassaged and centrifuged at 20,000 × g for 10 min, yielding another pellet consisting of the heavy-membrane (HM) fraction and a supernatant. The HM samples were resuspended in membrane buffer (0.22 M mannitol, 0.07 M sucrose, 10 mM HEPES-KOH, pH 7.5, 1 mM MgCl2, 1 mM DTT, 1 mM EDTA). The equivalent amount of protein from the supernatant fraction was used. The pellet and supernatant fractions were heated at 98°C for 10 min in Tris-glycine sample buffer (Invitrogen) containing 10% β-mercaptoethanol.
For carbonate extraction, 100 μg protein from HM samples was suspended in a mixture of HEPES buffer (10 mM HEPES-KOH, pH 7.5) and 0.1 M sodium carbonate solution and then incubated on ice for 30 min. Samples then were centrifuged at 60,000 rpm for 30 min at 4°C in a Beckman ultracentrifuge. After centrifugation, the carbonate extraction pellet and supernatant fractions were heated at 98°C for 10 min in Tris-glycine sample buffer (Invitrogen) containing 10% β-mercaptoethanol.
Transient transfection, confocal microscopy, and imaging.
Cells were transiently transfected with Fugene6 (Roche) according to the manufacturer's protocol and using 1 μg DNA per chamber slide. Images were captured with a microscope (model LSM 510; Carl Zeiss MicroImaging, Inc.) using a ×63 magnification/1.4-numerical aperture or a ×100 magnification/1.45-numerical aperture Apochrome objective. The excitation lengths were 405 nm for CFP, 488 nm for GFP, 514 nm for YFP, or 543 nm for red immunostaining (with Alexa 594-conjugated secondary Abs). Postacquisition processing was performed using an image viewer (LSM 510; Carl Zeiss MicroImaging, Inc.).
Immunofluorescence.
For immunofluorescence, cells were seeded in chamber slides (5 × 105/well) followed by transient transfection or infection. Cells then were fixed in 4% paraformaldehyde and immunostained with cytochrome c Abs (Pharmingen) as previously described (27).
Immunoblotting.
For immunoblotting, proteins were separated on 4 to 12% gradient NUPAGE Bis-Tris gels (Invitrogen) and transferred onto nitrocellulose membranes (Invitrogen) for blotting. ECL plus (Amersham) was used as a detection reagent.
IP.
For immunoprecipitation (IP), cells were transfected with Effectene (Qiagen) according to the manufacturer's protocols, using 4 μg of DNA per 15-cm2 plate. Whole-cell lysates (WCL) were collected in CHAPS lysis buffer {25 mmol HEPES-KOH, 300 mmol NaCl, 2% 3-[(3-cholamidopropyl)-dimethylammonio]-1-propanesulfonate, complete protease inhibitor cocktail (Roche)}. WCL (1 to 2 mg) was incubated with 2 μg Bax 6A7 Ab (Sigma) or c-myc Ab (Roche) and then with 25 μl Protein A/G agarose beads (Pierce). IP and WCL samples were analyzed by immunoblotting.
FACS analysis.
For fluorescent-activated cell sorting (FACS) analysis, cells were transfected with Effectene (Qiagen) according to the manufacturer's protocols, using 4 μg per 15-cm2 plate. At 18 to 20 h posttransfection, apoptosis was induced using 1 μM staurosporine (STS; Sigma) for 5 h. An equivalent amount of dimethylsulfoxide was used as a control in untreated samples. Cells were harvested using trypsin with 0.5% EDTA and washed once in medium and twice in 1× PBS. Cell density was adjusted to 1 × 106 cells in 1 ml 1× PBS. Hoechst 33342 and propidium iodide were added as recommended by the manufacturer's protocols (Vybrant apoptosis assay kit no. 5; Molecular Probes/Invitrogen). Cells were incubated for 20 to 30 min at room temperature and then measured by flow cytometry using UV/488 dual excitation and measuring fluorescence emission at approximately 460 and >575 nm, as described previously (19).
RESULTS
HCMV-encoded vMIA protects cells against a variety of apoptotic stimuli through interaction with the proapoptotic Bcl-2 family member Bax (5, 39). Although vMIA functionally inhibits apoptosis upstream of Bax, as does the antiapoptotic Bcl-2 protein, it does not share any sequence or known structural homology with Bcl-2 family members (16). However, a recent model of vMIA suggests it adopts a Bcl-2-like fold (37), and the structural analysis of other antiapoptotic viral proteins, such as myxoma virus M11L and the vaccinia N1L proteins, has shown that, despite a lack of sequence similarity, these proteins adopt a structure that closely resembles that of Bcl-xL (4, 14, 28). The unusual glutamic acid (E)-rich sequence in vMIA (Fig. 1A) is a unique feature absent from known Bcl-2 family members.
FIG. 1.
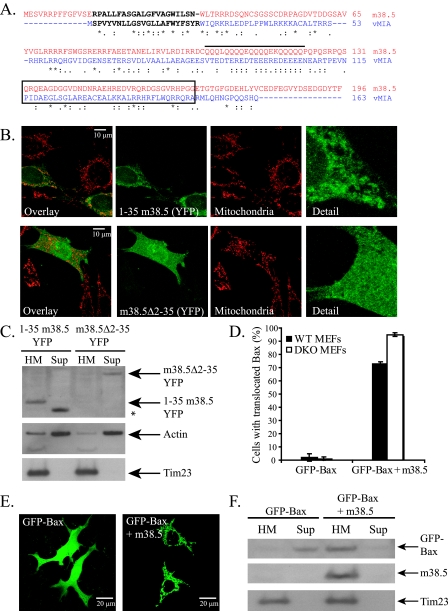
m38.5, like vMIA, causes the mitochondrial translocation of Bax. (A) ClustalW analysis of the m38.5 (red) and vMIA (blue) amino acid sequence alignment. Asterisks represent identical residues, colons represent conserved substitutions and a single dot represents a semiconserved substitution. Black letters in each sequence represent the vMIA MLS and the putative m38.5 MLS. The vMIA Bax-binding domain and aligned m38.5 amino acids are outlined with a black box. The black line delineates the E/Q-rich region. (B) Confocal images of cytochrome c staining of mitochondria (red) and YFP-tagged m38.5 amino acids 1-35 (1-35 m38.5-YFP; green) (top) or Δ2-35 m38.5-YFP (bottom) and overlay images of Bax/Bak DKO MEFs. (C) Western blot of HM and supernatant (Sup) fractions of Bax/Bak DKO MEFs transiently expressing either 1-35 m38.5-YFP or Δ2-35 m38.5-YFP. Immunoblots for YFP-tagged proteins is shown, as well as those for actin and Tim23 as cytosol and mitochondrial loading controls, respectively. The low-molecular-weight-band denoted by an asterisk is likely cleaved GFP. (D) Quantification of GFP-Bax translocation in wild type (WT; black bars) and Bax/Bak DKO (white bars) MEFs transiently transfected with GFP-Bax and either empty vector (pcDNA3.1) or m38.5. Data represent the percentage ± standard errors of 100 cells counted per condition per cell type in two independent experiments (n = 2). (E) Confocal microscope images of Bax/Bak DKO MEFs expressing GFP-tagged Bax with empty vector (left) or m38.5 (right). (F) Western blot of HM and Sup fractions of Bax/Bak DKO MEFs transiently expressing GFP-Bax with empty vector or m38.5. Immunoblots for GFP-Bax (anti-GFP), m38.5 (anti-myc), and a mitochondrial marker (anti-Tim23) are shown.
m38.5 in MCMV localizes to mitochondria (6, 25, 30) but displays little sequence similarity to vMIA (Fig. 1A) and also lacks detectable sequence homology to Bcl-2. There are two regions of the m38.5 sequence that show some common features with vMIA. There is a glutamine (Q)-rich region in m38.5 in which vMIA is rich in glutamic acid, and both proteins have a predicted N-terminal transmembrane domain (Fig. 1A), which were analyzed according to the transmembrane helix prediction software TMHMM (42). To ascertain if the N terminus of m38.5 targets membranes, amino acids 1 to 35 of m38.5 were fused to the N terminus of YFP (1-35 m38.5-YFP) and found to localize to mitochondria (Fig. 1B, top), the endoplasmic reticulum (data not shown), and the membrane fraction of cells (Fig. 1C), indicating that this region of m38.5 comprises a membrane-targeting function like that of the N terminus of vMIA. Consistently with the N terminus targeting m38.5 to mitochondria, m38.5 lacking the N terminus (m38.5Δ2-35) does not localize to mitochondria in Bax/Bak DKO MEFs (Fig. 1B and C; also see Fig. 3A).
FIG. 3.
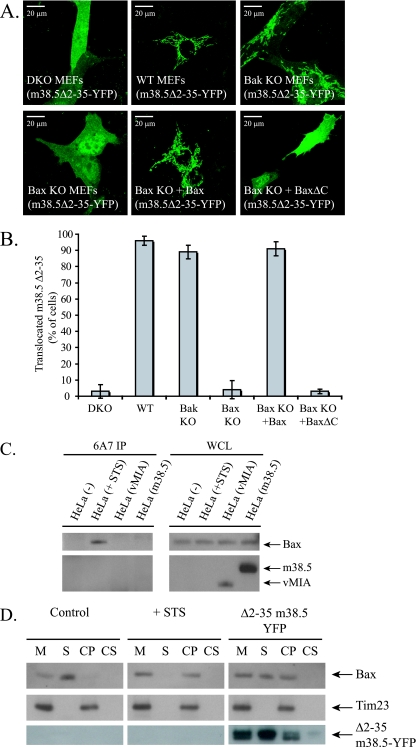
m38.5 induces Bax conformational change. (A) Confocal microscope images of Δ2-35 m38.5-YFP expression in DKO, wild-type (WT), Bak KO, and Bax KO MEFs. Bax or BaxΔC was transiently coexpressed with Δ2-35 m38.5-YFP in Bax KO MEFs. (B) Quantification of cells with Δ2-35 m38.5-YFP on the mitochondria of the cells shown in panel A. Data represent the percentage ± standard errors of 100 cells counted per condition per cell type in two independent experiments (n = 2). (C) IP using the 6A7 anti-Bax Ab of WCL from control (untreated) HeLa cells, STS-treated HeLa cells, and HeLa cells transiently transfected with myc-tagged vMIA or myc-tagged m38.5. The Western blot shows IP and WCL samples immunoblotted with anti-Bax (NT), anti-myc for vMIA, and anti-myc for m38.5. (D) Western blot of subcellular fractionation of control (untreated) HeLa cells, STS-treated HeLa cells, or HeLa cells transiently transfected with Δ2-35 m38.5-YFP. A portion of the membrane (M) pellets was further subjected to carbonate extraction (see Materials and Methods). S, supernatant; CP, pellet from carbonate extraction; and CS, supernatant from carbonate extraction. Tim23 was used as a carbonate-nonextractable and HM control.
m38.5 expression recruits Bax to mitochondria.
The inhibition of cell death through Bax inactivation has been suggested to be the mechanism of action of vMIA (5, 39). Previous work (5) suggested that vMIA inactivated Bax selectively, as a 37-amino-acid-long peptide of vMIA was shown to bind Bax but not Bak. However, recently it has been shown that full-length vMIA is able to coimmunoprecipitate with Bak and block Bak-mediated apoptosis (27). To compare the mechanism of action of m38.5 to that of vMIA, GFP-tagged Bax was expressed in Bax/Bak DKO MEFs with either empty vector or m38.5. Interestingly, Bax was recruited to mitochondria in cells expressing m38.5, in contrast to its normal cytosolic distribution in cells lacking m38.5 expression (Fig. 1D and E). To confirm that m38.5 alters Bax localization, supernatant and HM fractions were separated by the differential centrifugation of extracts of cells transfected with GFP-Bax and empty vector or m38.5. As shown in Fig. 1F, GFP-Bax is mainly in the supernatant in the absence of m38.5, whereas it is primarily localized in the membrane fraction in cells expressing m38.5. Thus, both m38.5 and vMIA recruit Bax to mitochondria.
m38.5 and vMIA alter Bax localization independently of the Bax transmembrane domain.
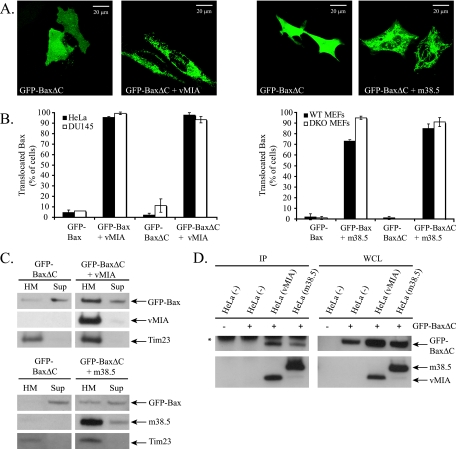
Bax translocates to mitochondria during apoptosis following a conformational change that allows the C terminus to disengage from a hydrophobic pocket and mediate mitochondrial targeting and membrane insertion (50). A Bax mutant lacking the 20 C-terminal amino acids (BaxΔC) has a cytosolic localization in healthy cells and fails to translocate to mitochondria after apoptotic stimulation (49). To determine whether Bax translocation to mitochondria in the presence of vMIA or m38.5 requires the Bax C-terminal membrane insertion sequence, human (DU145) or mouse (Bax/Bak DKO MEFs) cells lacking endogenous Bax were transiently transfected with GFP-tagged wild-type Bax or BaxΔC and either empty vector, vMIA (DU145), or m38.5 (Bax/Bak DKO MEFs). Transfected cells were imaged (Fig. 2A) and quantified (Fig. 2B). Whereas GFP-BaxΔC is localized to the cytosol in control cells, vMIA or m38.5 expression resulted in GFP-BaxΔC localization to mitochondria. Western blotting with anti-Bax Abs of HM and supernatant fractions from cells confirmed that GFP-BaxΔC is recruited to mitochondria by vMIA or m38.5 in DU145 cells or Bax/Bak DKO MEFs, respectively (Fig. 2C). vMIA and m38.5 also were found to interact with GFP-BaxΔC by coimmunoprecipitation (Fig. 2D). Therefore, both viral proteins recruit Bax to mitochondria independently of the Bax C-terminal membrane anchor. As shown in Fig. 1B, the mutant m38.5 lacking a mitochondrial localization signal (m38.5Δ2-35-YFP) fails to bind mitochondria in cells lacking Bax (Fig. 3A and B). Interestingly, if endogenous or ectopic Bax is present, m38.5Δ2-35 localizes predominantly to mitochondria (Fig. 3A and B). vMIA lacking the mitochondrial localization sequence (MLS) mutant (vMIAΔ2-23-YFP) also localizes to mitochondria when ectopic Bax is expressed (data not shown). This suggests that the mitochondrial targeting of the Bax-m38.5 complex can occur through either the Bax C-terminal mitochondrial binding domain or the m38.5 N-terminal mitochondrial binding domain. Consistently with this model, m38.5Δ2-35 remains in the cytosol when coexpressed in Bax/Bak DKO cells with BaxΔC (Fig. 3A and B). Taken together, these data indicate that m38.5 can bind to Bax in the cytosol and induce a conformational change in Bax that results in Bax and m38.5 translocation to the mitochondria if either Bax or m38.5 retains the mitochondria targeting domain.
FIG. 2.
m38.5 and vMIA recruit a Bax C-terminal deletion mutant to the mitochondria. (A) Confocal microscope images of DU145 cells (left two images) or Bax/Bak DKO MEFs (right two images) expressing GFP-BaxΔC alone or with vMIA (left) or with m38.5 (right). (B) Quantification of GFP-Bax or GFP-BaxΔC localization with and without vMIA in HeLa and DU145 cells or with m38.5 in WT and Bax/Bak DKO MEFs. Data represent the percentage ± standard errors of 100 cells counted per condition per cell type in two independent experiments (n = 2). (C) Western blot of the subcellular fractionation of DU145 cells expressing GFP-BaxΔC alone or with vMIA or Bax/Bak DKO MEFs expressing GFP-BaxΔC alone or with m38.5. Immunoblots for GFP-BaxΔC (anti-GFP), myc-tagged vMIA (anti-myc), myc-tagged m38.5 (α-myc), and a mitochondrion-specific marker (anti-Tim23) are shown. Sup, supernatant. (D) myc IP of vMIA or m38.5 from untransfected HeLa cells or HeLa cells expressing GFP-BaxΔC alone or with the coexpression of vMIA or m38.5. Immunoblots of GFP-BaxΔC (anti-Bax NT) and myc-tagged vMIA (anti-myc) or m38.5 (anti-myc) in IP and WCL samples are shown.
To examine if Bax changes conformation upon binding m38.5 as it does during apoptosis-associated translocation to mitochondria, we immunoprecipitated Bax using the conformation-specific monoclonal Ab 6A7. This Ab recognizes an N-terminal epitope of Bax that is buried in the cytosolic conformation and revealed in the mitochondrial membrane-inserted state, which is normally associated with apoptosis (21). Control lanes show that Bax is bound by 6A7 in cells treated with STS, a broad-specificity kinase inhibitor that promotes apoptosis, but not in healthy cells (Fig. 3C). However, neither vMIA expression, as previously revealed by immunofluorescence (5), nor m38.5 expression promotes Bax IP by 6A7. Either vMIA and m38.5 induce Bax activation and simultaneously block Ab recognition of the Bax N terminus, or they recruit Bax to mitochondria in a conformation that sequesters the N-terminal 6A7 epitope, as is found in cytosolic Bax in healthy cells.
When Bax undergoes the conformational change associated with mitochondrial translocation during apoptosis, it becomes integrally bound in the mitochondrial outer membrane (50). Carbonate extraction of membrane pellets of control, apoptotic (STS-treated) or transiently transfected cells expressing m38.5Δ2-35-YFP was performed (Fig. 3D). Bax is mainly in the cytosolic fraction and is not tightly associated with mitochondria in control cells, in contrast to the presence of Bax in the carbonate pellet upon apoptotic stimulation (Fig. 3D). In cells expressing m38.5Δ2-35-YFP, Bax can be found in the carbonate extraction pellet, indicating that a conformational change in Bax has occurred that results in Bax insertion into the mitochondrial membrane. The cell population transiently transfected with m38.5Δ2-35-YFP contained a percentage of untransfected cells that likely accounts for the residual pool of cytosolic Bax shown in Fig. 3D.
The involvement of the Bax N terminus in the subcellular localization of the protein remains a matter of debate (8, 47). To investigate whether the Bax N terminus is required for membrane translocation occurring with vMIA or m38.5 expression, GFP-tagged BaxΔN and vMIA or m38.5 were expressed in DU145 or Bax/Bak DKO MEFs, respectively, and analyzed by confocal microscopy. BaxΔN was localized to the cytosol of cells expressing empty vector control but was recruited to mitochondria to a degree similar to that of wild-type Bax and BaxΔC when either m38.5 or vMIA was expressed (data not shown). This finding is consistent with findings a previous study that demonstrated that in vitro-translated BaxΔN interacts with vMIA (39).
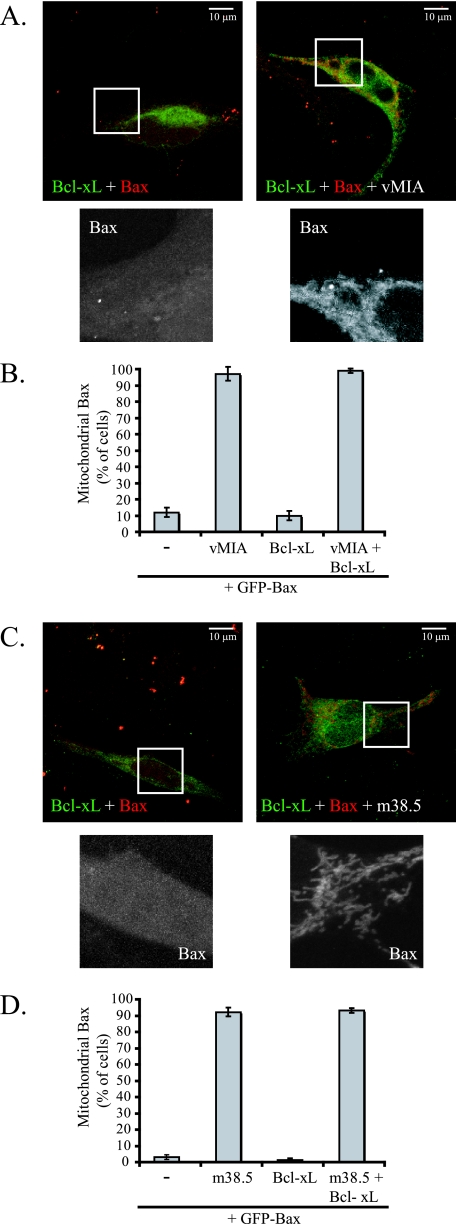
Bcl-xL overexpression blocks Bax translocation to mitochondria during apoptosis induced with STS (unpublished data), as does Bcl-2 overexpression (33). However, upon the expression of vMIA (Fig. 4A and B) or m38.5 (Fig. 4C and D), CFP-tagged Bax localizes to mitochondria even with the overexpression of YFP-Bcl-xL. Thus, Bcl-xL is unable to block Bax translocation in the presence of vMIA or m38.5. The mechanism of Bax inhibition by vMIA and m38.5 with the induction of Bax localization to mitochondria appears distinct from that of antiapoptotic Bcl-2 family members that inhibit Bax translocation.
FIG. 4.
Bcl-xL does not block Bax translocation with vMIA or m38.5 expression. (A) Confocal microscope images of CFP-Bax (red; white in zoom image) with YFP-Bcl-xL (green) in the absence (left) or presence (right) of vMIA in DU145 cells. (B) Quantification of the effect of YFP-Bcl-xL overexpression on the mitochondrial localization of CFP-Bax in the absence or presence of vMIA in DU145 cells. Data represent the percentage ± standard errors of 100 cells counted per condition in two independent experiments (n = 2). (C) Confocal microscope images of CFP-Bax with YFP-Bcl-xL in the absence (left) or presence (right) of m38.5 in Bax/Bak DKO MEFs. (D) Quantification of the effect of YFP-Bcl-xL overexpression on the mitochondrial localization of CFP-Bax in the absence or presence of m38.5 in Bax/Bak DKO MEFs. Data represent the percentage ± standard errors of 100 cells counted per condition in two independent experiments (n = 2).
Cellular effects of m38.5.
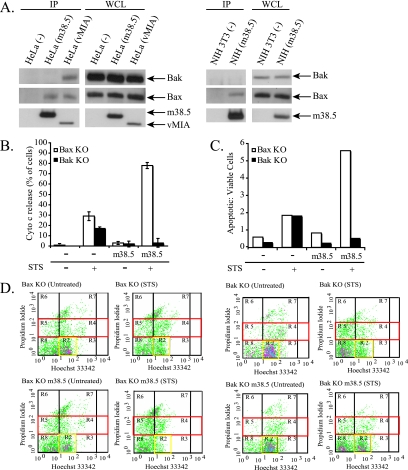
We investigated the respective abilities of vMIA and m38.5 to interact with Bak, another proapoptotic Bcl-2 family protein that is similar to Bax and redundant with Bax, for several apoptotic pathways (27, 48). In contrast to vMIA, m38.5 does not interact with Bak in human or mouse cells (Fig. 5A) and selectively binds to Bax. Due to the lack of interaction between m38.5 and Bak, we hypothesized that m38.5 also would lack protection from apoptosis in cells with endogenous Bak but lacking Bax. We transiently transfected Bax KO and Bak KO MEFs with mito-YFP and empty vector or m38.5 and then treated the cells with STS to induce apoptosis. Cells were immunostained for cytochrome c, and the number of apoptotic cells was quantified (Fig. 5B). While m38.5 protected cells expressing endogenous Bax (Fig. 5B) from cytochrome c release following STS treatment, m38.5 did not inhibit cytochrome c release in Bax KO cells (Fig. 5B). Unexpectedly, Bax KO cells expressing m38.5 displayed even greater apoptosis than control cells treated with STS.
FIG. 5.
m38.5 does not protect against apoptosis in cells lacking Bax. (A) The images on the left show the IP of myc-tagged m38.5 or vMIA transiently expressed in HeLa cells. The Western blot shows Bax and Bak in IP and WCL samples. The myc blotting shows m38.5 and vMIA pulldown and expression in WCL. The images on the right show the IP of myc-tagged m38.5 in transiently transfected mouse NIH 3T3 cells. The Western blot shows Bax and Bak in IP and WCL samples. The myc blotting shows m38.5 pulldown and expression in WCL. (B) Quantification of Bax KO (white) or Bak KO (black) cells expressing mito-YFP and empty vector or m38.5, with or without treatment with STS (1 μM) for 6 h. Data represent the percentage ± standard errors of 100 cells counted per condition per cell type in two independent experiments (n = 2). (C) Control or STS-treated Bax KO (white bars) and Bak KO (black bars) MEFs transiently expressing mito-YFP and empty vector or m38.5 were sorted using FACS, and the viability of YFP-positive cells was analyzed based on Hoechst DNA dye fluorescence and propidium iodide permeability as previously described (19). Five thousand cells were counted per cell type and condition, and the data were plotted as a ratio of apoptotic (red box in panel D) to viable (yellow box in panel D) cells. (D) FACS analysis of YFP-positive cells was performed, and the distribution of cells based upon Hoechst and PI staining is plotted. Red boxes indicate apoptotic cells, and yellow boxes represent viable cells.
To confirm the difference in protection of Bax KO and Bak KO MEFs by m38.5, we performed a FACS analysis of control or STS-treated Bax KO and Bak KO cells transiently expressing mito-YFP and empty vector or m38.5 (Fig. 5C and D). The viability of cells was determined using propidium iodide and Hoechst dyes as described previously (19). Figure 5C shows the ratio of apoptotic cells to viable cells for 5,000 cells counted for each cell type and condition. In Bak KO MEFs, which contain endogenous Bax, cells expressing m38.5 treated with STS were protected from apoptosis (apoptotic-to-viable ratio of 0.51) compared to cells expressing empty vector (apoptotic-to-viable ratio of 1.81). Bax KO MEFs, on the other hand, were not protected from STS-induced apoptosis and displayed greater apoptosis sensitivity upon m38.5 expression (an untreated apoptotic-to-viable ratio of 0.826 and a treated apoptotic-to-viable ratio of 5.596) than empty vector-transfected cells (untreated apoptotic-to-viable ratio of 0.586; treated apoptotic-to-viable ratio of 1.86). These data support the hypothesis that the failure of m38.5 to protect cells from Bak-mediated apoptosis is due to the lack of interaction of m38.5 with Bak. m38.5 was previously shown to act analogously to vMIA by protecting HeLa cells from apoptosis induced by the inhibition of the proteasome with MG132 treatment (30). Recent work has shown that MG132-induced apoptosis is Bax dependent (13), which is consistent with the conclusion that m38.5 selectively interacts with and inactivates Bax.
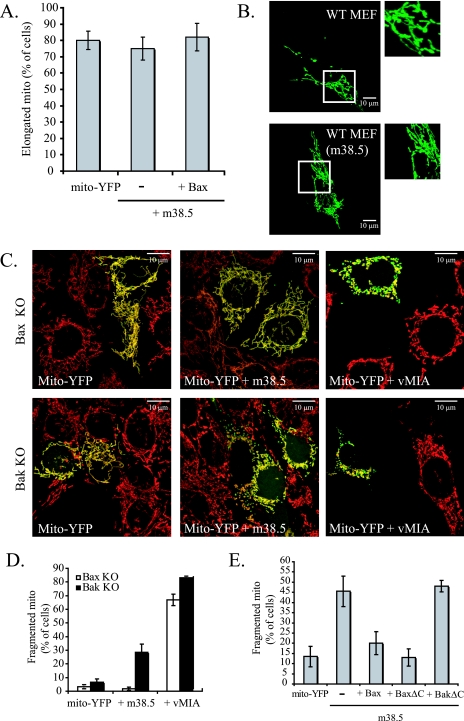
vMIA expression disrupts the mitochondrial network, leading to smaller and more numerous mitochondria (32). This has been proposed to be due either to the PiC (36) or to the inactivation of Bax and Bak, as these two proteins are required for mitochondrial fusion in healthy cells, and the vMIA-induced fragmentation of mitochondria is reversed by ectopic Bax expression (27). In contrast to that of vMIA, m38.5 expression does not cause the fragmentation of mitochondria in WT MEFs (Fig. 6A and B). We hypothesized that the difference between vMIA and m38.5 in causing mitochondrial fragmentation is due to the failure of m38.5 to bind or inactivate Bak (Fig. 5). We expressed empty vector control or m38.5 in Bax KO or Bak KO MEFs and analyzed mitochondrial morphology. Figure 6D shows that only about 5% of Bax KO or Bak KO cells have a fragmented mitochondrial morphology, and this is unaltered by m38.5 expression in cells lacking Bax (Bax KO). However, in cells lacking endogenous Bak (Bak KO), m38.5 expression results in a sixfold increase in the number of cells with a fragmented mitochondrial phenotype (Fig. 6C and D). However, even in Bak−/− cells, m38.5 is less effective than vMIA in fragmenting mitochondria.
FIG. 6.
m38.5 and vMIA affect mitochondrial morphology. (A) Quantification of mitochondrial morphology in wild type (WT) MEFs expressing mito-YFP and empty vector, m38.5, and m38.5 with wild-type Bax. Data represent the percentage ± standard errors of 100 cells counted per condition in two independent experiments (n = 2). (B) Confocal microscope images of WT MEFs expressing mito-YFP with empty vector (top) or m38.5 (bottom). Images to the right are 5× magnifications. (C) Confocal images of Bax KO or Bak KO MEFs expressing mito-YFP (yellow) and empty vector, m38.5, or vMIA. Cytochrome c staining shows mitochondria in red. (D) Quantification of cells with fragmented mitochondrial phenotype in Bax KO (white) or Bak KO (black) cells expressing mito-YFP and empty vector, m38.5, or vMIA. Data represent the percentage ± standard errors of 100 cells counted per condition per cell type in two independent experiments (n = 2). (E) Quantification of mitochondrial fragmentation in Bak KO cells expressing mito-YFP alone or with m38.5, m38.5 and Bax, m38.5 and BaxΔC, or m38.5 and BakΔC. Data represent the percentage ± standard errors of 100 cells counted per condition per cell type in two independent experiments (n = 2).
We also found that, as in vMIA-expressing cells (27), ectopic expression of Bax reverses the fragmentation of mitochondria induced by m38.5 (Fig. 6E). Interestingly, BaxΔC but not BakΔC was at least as potent as full-length Bax in reversing the mitochondrial fragmentation induced by m38.5, which is consistent with the results showing an interaction of m38.5 with Bax but not with Bak.
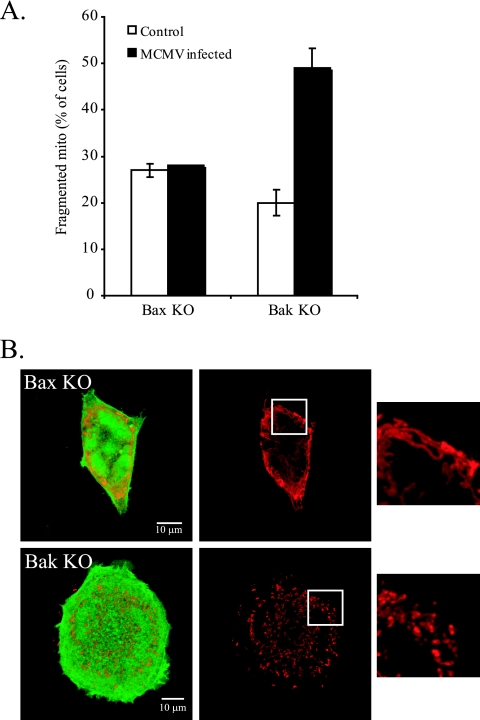
We additionally sought to determine whether mitochondrial morphology differs between Bak KO and Bax KO MEFs following MCMV infection. We used MCMV-GFP, an MCMV encoding enhanced GFP at an innocuous site (7), to infect Bax KO and Bak KO MEFs for 48 h, and then we determined the mitochondrial morphology following the immunostaining of mitochondria. As seen in Fig. 7A, the number of Bax KO cells with fragmented mitochondria was similar with or without MCMV infection. Bak KO cells, on the other hand, had a 2.5-fold increase in the number of cells with a fragmented mitochondrial morphology at 48 hpi (Fig. 7A). Figure 7B shows representative images of mitochondria in MCMV-GFP infected Bax KO or Bak KO MEFs showing mitochondrial fragmentation in the Bak KO cells.
FIG. 7.
MCMV infection alters mitochondrial morphology. (A) Quantification of fragmented mitochondria in control (uninfected) or MCMV-GFP-infected Bax or Bak KO MEFs. Cells were fixed at 48 hpi, and the mitochondrial were immunostained with anti-cytochrome c. Data represent the percentage ± standard errors of 100 cells counted per condition per cell type in two independent experiments (n = 2). (B) Representative images of mitochondria (red) in MCMV-GFP (green)-infected Bax KO (top) or Bak KO (bottom) MEFs at 48 hpi.
DISCUSSION
Apoptosis is an important host defense mechanism against viral infection, and many viruses, including CMV, express proteins that counteract this cellular response. Some viruses, such as Kaposi's sarcoma-associated herpesvirus and Epstein Barr virus, encode antiapoptotic proteins that share functional as well as sequence and structural homology with Bcl-2 (9, 10, 23, 24, 45). Other viruses, such as myxoma virus and vaccinia virus, encode proteins that block apoptosis and are structurally similar to Bcl-2 without having any clear conservation in sequence (4, 14, 28).
HCMV-encoded vMIA inhibits apoptosis and localizes to mitochondria, like certain Bcl-2 family members (16, 29). However, vMIA appears distinct in sequence from Bcl-2 and appears to function by a unique mechanism. vMIA expression results in Bax movement to mitochondria, in contrast to Bcl-2 (33), which not only fails to recruit Bax to mitochondria but also actually inhibits Bax translocation that occurs during apoptosis.
Recent work indicates that MCMV encodes a functional homolog of vMIA, m38.5, that is able to protect against apoptosis induced by proteasome inhibition (30).
We compared the cellular effects of m38.5 to those of vMIA in order to better characterize the proposed MCMV homolog as well as the mechanism of apoptosis inhibition of these two viral proteins, because vMIA and m38.5 share little sequence homology. m38.5 expression, like that of vMIA, causes Bax translocation to mitochondria in mouse cells, and, in contrast to Bax translocation during apoptosis, this m38.5- or vMIA-induced translocation cannot be inhibited by Bcl-xL overexpression. Interestingly, m38.5 that lacked its mitochondrial targeting sequence was able to translocate to mitochondria with Bax, indicating that the binding of m38.5 to Bax in the cytosol induces a conformational change in Bax, allowing mitochondrial translocation via the Bax C terminus and deep insertion into the mitochondrial membrane resisting carbonate extraction, a conformation distinct from that of Bax in healthy cells (17, 22).
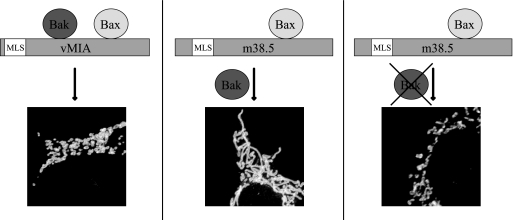
vMIA inactivates Bax and Bak seemingly to protect against their proapoptotic function and disrupts the normally interconnected mitochondrial network. As Bax and Bak play a role in maintaining normal mitochondrial morphology in healthy cells, vMIA may disrupt normal mitochondrial morphology through the inactivation of their mitochondrial morphogenesis activities. In contrast to vMIA, m38.5 did not detectably change the mitochondrial morphology. We found that this discrepancy likely was due to the inability of m38.5 to bind human or murine Bak. In mouse cells lacking endogenous Bak, m38.5 induced the fragmentation of the mitochondrial network (Fig. 8).
FIG. 8.
Model of vMIA and m38.5 effects on mitochondrial morphology. (Left) vMIA interacts with Bax and Bak, inactivating the fusion activity of both proteins and resulting in mitochondrial fragmentation, as shown by confocal image. (Middle) m38.5 selectively interacts with Bax, allowing Bak to regulate mitochondrial fusion and leading to a normal mitochondrial network. (Right) m38.5 inactivates Bax fusion activity, and, in cells lacking Bak, this results in mitochondrial fragmentation similar to that of vMIA.
The hypothesis that differences between m38.5 and vMIA arise from m38.5 specificity for Bax was corroborated with experiments measuring the m38.5 inhibition of apoptosis. m38.5 inhibited cell death induced by STS, a broad-specificity kinase inhibitor that works through either Bax or Bak, only in cells lacking Bak. Thus, m38.5 appears to selectively inhibit Bax-dependent apoptosis. MCMV infection induces the oligomerization of both Bax and Bak while also inhibiting apoptosis (1). In contrast, the transient transfection of m38.5 into HeLa cells does not induce detectable Bak oligomerization upon cross-linking with bismaleimidohexane (data not shown). This indicates that MCMV encodes multiple proteins to perform the functions of vMIA in HCMV infection, namely inactivating both Bax and Bak at the mitochondria upstream of cytochrome c release. It also is possible that the induction of apoptosis due to viral infection is primarily a Bax-dependent pathway in murine cells. This could be one explanation for the species specificity of MCMV, which is unable to replicate in human cells unless apoptosis is blocked by Bcl-2 or vMIA (25).
In experiments addressing the differences in the bioactivity of m38.5 in murine cells lacking endogenous Bax (Bax KO MEFs) or Bak (Bak KO MEFs), we found that m38.5 not only lacks a protective function in Bax KO MEFs but also seems to further sensitize mitochondria to release cytochrome c (Fig. 5). This increase in cell death in Bax KO MEFs expressing m38.5 could be due to the activation of an apoptotic pathway responding to the presence of this viral protein.
Interestingly, the GFP-tagged wild type and BaxΔC localize along the entire outer mitochondrial membrane upon m38.5 expression rather than in the foci that Bax forms upon translocation to mitochondria during apoptosis (35). Even GFP-Bax recruited to mitochondria through its own C-terminal domain by m38.5Δ2-35 fails to form foci, suggesting that m38.5 binding inhibits a second step in the Bax conformational change and therefore that Bax focus formation is key for the release of cytochrome c and apoptosis. We propose that vMIA or m38.5 recruitment of Bax to mitochondria is not lethal, because these viral proteins block apoptotic focus formation.
During apoptosis, Bax foci form and often become sites of mitochondrial division, leading to the fragmentation of the mitochondrial network (26). These foci include the mitochondrial fusion protein, Mfn2, as well as the fission protein Drp1. vMIA has been shown to recruit Bax to mitochondria, but we found that vMIA expression has no effect on Drp1 localization. Another cytosolic protein, Endophilin B1, interacts with Bax (11, 38) most prominently during apoptosis, when it may aid in Bax translocation and oligomerization (44). We found that vMIA does not recruit Endophilin B1 to mitochondria. vMIA also had no effect on the localization of the proapoptotic Bcl-2 family member Bid, the antiapoptotic Bcl-2 family member Bcl-xL, or the cytosolic transmembrane mutant of Bcl-xL (Bcl-xLΔC), demonstrating that vMIA selectively influences Bax translocation to the mitochondria.
The CMV proteins vMIA and m38.5 share a number of biological activities, including the ability to inhibit apoptosis, bind and recruit Bax to mitochondria, and induce mitochondrial fragmentation. However, they differ in their primary sequences and in their abilities to neutralize Bak. Our results indicate that they share a common mechanism of apoptosis inhibition through Bax sequestration that differs substantially in mechanism from that of antiapoptotic Bcl-2 family proteins.
Acknowledgments
We thank V. Goldmacher for vMIA-myc, E. Mocarski for the LNCX-m38.5 construct, S. Korsmeyer for the wild-type, Bax KO, Bak KO, and Bax/Bak DKO MEFs, and D. Margulies (with permission from J. Stipan and U. Koszinowski) for the GFP-MCMV (7). We also are grateful to C. Smith (LIF, NINDS) for the use of the confocal microscopes, S. Smith for assistance with tissue culture, D. Maric for assistance in the FACS analysis, and the NINDS DNA sequencing facility. We also thank C. Williamson, A. Neutzner, C. Wang, and M. Cleland for their critical reading of the manuscript.
Footnotes
Published ahead of print on 16 April 2008.
REFERENCES
- 1.Andoniou, C. E., D. M. Andrews, M. Manzur, P. Ricciardi-Castagnoli, and M. A. Degli-Esposti. 2004. A novel checkpoint in the Bcl-2-regulated apoptotic pathway revealed by murine cytomegalovirus infection of dendritic cells. J. Cell Biol. 166827-837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andoniou, C. E., and M. A. Degli-Esposti. 2006. Insights into the mechanisms of CMV-mediated interference with cellular apoptosis. Immunol. Cell Biol. 8499-106. [DOI] [PubMed] [Google Scholar]
- 3.Antonsson, B., S. Montessuit, S. Lauper, R. Eskes, and J. C. Martinou. 2000. Bax oligomerization is required for channel-forming activity in liposomes and to trigger cytochrome c release from mitochondria. Biochem. J. 345271-278. [PMC free article] [PubMed] [Google Scholar]
- 4.Aoyagi, M., D. Zhai, C. Jin, A. E. Aleshin, B. Stec, J. C. Reed, and R. C. Liddington. 2007. Vaccinia virus N1L protein resembles a B cell lymphoma-2 (Bcl-2) family protein. Protein Sci. 16118-124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arnoult, D., L. M. Bartle, A. Skaletskaya, D. Poncet, N. Zamzami, P. U. Park, J. Sharpe, R. J. Youle, and V. S. Goldmacher. 2004. Cytomegalovirus cell death suppressor vMIA blocks Bax- but not Bak-mediated apoptosis by binding and sequestering Bax at mitochondria. Proc. Natl. Acad. Sci. USA 1017988-7993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brocchieri, L., T. N. Kledal, S. Karlin, and E. S. Mocarski. 2005. Predicting coding potential from genome sequence: application to betaherpesviruses infecting rats and mice. J. Virol. 797570-7596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brune, W., C. Menard, J. Heesemann, and U. H. Koszinowski. 2001. A ribonucleotide reductase homolog of cytomegalovirus and endothelial cell tropism. Science 291303-305. [DOI] [PubMed] [Google Scholar]
- 8.Cartron, P. F., M. Priault, L. Oliver, K. Meflah, S. Manon, and F. M. Vallette. 2003. The N-terminal end of Bax contains a mitochondrial-targeting signal. J. Biol. Chem. 27811633-11641. [DOI] [PubMed] [Google Scholar]
- 9.Cheng, E. H., J. Nicholas, D. S. Bellows, G. S. Hayward, H. G. Guo, M. S. Reitz, and J. M. Hardwick. 1997. A Bcl-2 homolog encoded by Kaposi sarcoma-associated virus, human herpesvirus 8, inhibits apoptosis but does not heterodimerize with Bax or Bak. Proc. Natl. Acad. Sci. USA 94690-694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cuconati, A., and E. White. 2002. Viral homologs of BCL-2: role of apoptosis in the regulation of virus infection. Genes Dev. 162465-2478. [DOI] [PubMed] [Google Scholar]
- 11.Cuddeback, S. M., H. Yamaguchi, K. Komatsu, T. Miyashita, M. Yamada, C. Wu, S. Singh, and H. G. Wang. 2001. Molecular cloning and characterization of Bif-1. A novel Src homology 3 domain-containing protein that associates with Bax. J. Biol. Chem. 27620559-20565. [DOI] [PubMed] [Google Scholar]
- 12.Delivani, P., C. Adrain, R. C. Taylor, P. J. Duriez, and S. J. Martin. 2006. Role for CED-9 and Egl-1 as regulators of mitochondrial fission and fusion dynamics. Mol. Cell 21761-773. [DOI] [PubMed] [Google Scholar]
- 13.Ding, W. X., H. M. Ni, X. Chen, J. Yu, L. Zhang, and X. M. Yin. 2007. A coordinated action of Bax, PUMA, and p53 promotes MG132-induced mitochondria activation and apoptosis in colon cancer cells. Mol. Cancer Ther. 61062-1069. [DOI] [PubMed] [Google Scholar]
- 14.Douglas, A. E., K. D. Corbett, J. M. Berger, G. McFadden, and T. M. Handel. 2007. Structure of M11L: a myxoma virus structural homolog of the apoptosis inhibitor, Bcl-2. Protein Sci. 16695-703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goldmacher, V. S. 2005. Cell death suppression by cytomegaloviruses. Apoptosis 10251-265. [DOI] [PubMed] [Google Scholar]
- 16.Goldmacher, V. S., L. M. Bartle, A. Skaletskaya, C. A. Dionne, N. L. Kedersha, C. A. Vater, J. W. Han, R. J. Lutz, S. Watanabe, E. D. Cahir McFarland, E. D. Kieff, E. S. Mocarski, and T. Chittenden. 1999. A cytomegalovirus-encoded mitochondria-localized inhibitor of apoptosis structurally unrelated to Bcl-2. Proc. Natl. Acad. Sci. USA 9612536-12541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goping, I. S., A. Gross, J. N. Lavoie, M. Nguyen, R. Jemmerson, K. Roth, S. J. Korsmeyer, and G. C. Shore. 1998. Regulated targeting of BAX to mitochondria. J. Cell Biol. 143207-215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hayajneh, W. A., A. M. Colberg-Poley, A. Skaletskaya, L. M. Bartle, M. M. Lesperance, D. G. Contopoulos-Ioannidis, N. L. Kedersha, and V. S. Goldmacher. 2001. The sequence and antiapoptotic functional domains of the human cytomegalovirus UL37 exon 1 immediate early protein are conserved in multiple primary strains. Virology 279233-240. [DOI] [PubMed] [Google Scholar]
- 19.Hillion, J. A., K. Takahashi, D. Maric, C. Ruetzler, J. L. Barker, and J. M. Hallenbeck. 2005. Development of an ischemic tolerance model in a PC12 cell line. J. Cereb. Blood Flow Metab. 25154-162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hsu, Y. T., K. G. Wolter, and R. J. Youle. 1997. Cytosol-to-membrane redistribution of Bax and Bcl-X(L) during apoptosis. Proc. Natl. Acad. Sci. USA 943668-3672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hsu, Y. T., and R. J. Youle. 1998. Bax in murine thymus is a soluble monomeric protein that displays differential detergent-induced conformations. J. Biol. Chem. 27310777-10783. [DOI] [PubMed] [Google Scholar]
- 22.Hsu, Y. T., and R. J. Youle. 1997. Nonionic detergents induce dimerization among members of the Bcl-2 family. J. Biol. Chem. 27213829-13834. [DOI] [PubMed] [Google Scholar]
- 23.Huang, Q., A. M. Petros, H. W. Virgin, S. W. Fesik, and E. T. Olejniczak. 2002. Solution structure of a Bcl-2 homolog from Kaposi sarcoma virus. Proc. Natl. Acad. Sci. USA 993428-3433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huang, Q., A. M. Petros, H. W. Virgin, S. W. Fesik, and E. T. Olejniczak. 2003. Solution structure of the BHRF1 protein from Epstein-Barr virus, a homolog of human Bcl-2. J. Mol. Biol. 3321123-1130. [DOI] [PubMed] [Google Scholar]
- 25.Jurak, I., and W. Brune. 2006. Induction of apoptosis limits cytomegalovirus cross-species infection. EMBO J. 252634-2642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Karbowski, M., Y. J. Lee, B. Gaume, S. Y. Jeong, S. Frank, A. Nechushtan, A. Santel, M. Fuller, C. L. Smith, and R. J. Youle. 2002. Spatial and temporal association of Bax with mitochondrial fission sites, Drp1, and Mfn2 during apoptosis. J. Cell Biol. 159931-938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Karbowski, M., K. L. Norris, M. M. Cleland, S. Y. Jeong, and R. J. Youle. 2006. Role of Bax and Bak in mitochondrial morphogenesis. Nature 443658-662. [DOI] [PubMed] [Google Scholar]
- 28.Kvansakul, M., M. F. van Delft, E. F. Lee, J. M. Gulbis, W. D. Fairlie, D. C. Huang, and P. M. Colman. 2007. A structural viral mimic of prosurvival Bcl-2: a pivotal role for sequestering proapoptotic Bax and Bak. Mol. Cell 25933-942. [DOI] [PubMed] [Google Scholar]
- 29.Mavinakere, M. S., and A. M. Colberg-Poley. 2004. Dual targeting of the human cytomegalovirus UL37 exon 1 protein during permissive infection. J. Gen. Virol. 85323-329. [DOI] [PubMed] [Google Scholar]
- 30.McCormick, A. L., C. D. Meiering, G. B. Smith, and E. S. Mocarski. 2005. Mitochondrial cell death suppressors carried by human and murine cytomegalovirus confer resistance to proteasome inhibitor-induced apoptosis. J. Virol. 7912205-12217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McCormick, A. L., A. Skaletskaya, P. A. Barry, E. S. Mocarski, and V. S. Goldmacher. 2003. Differential function and expression of the viral inhibitor of caspase 8-induced apoptosis (vICA) and the viral mitochondria-localized inhibitor of apoptosis (vMIA) cell death suppressors conserved in primate and rodent cytomegaloviruses. Virology 316221-233. [DOI] [PubMed] [Google Scholar]
- 32.McCormick, A. L., V. L. Smith, D. Chow, and E. S. Mocarski. 2003. Disruption of mitochondrial networks by the human cytomegalovirus UL37 gene product viral mitochondrion-localized inhibitor of apoptosis. J. Virol. 77631-641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Murphy, K. M., U. N. Streips, and R. B. Lock. 2000. Bcl-2 inhibits a Fas-induced conformational change in the Bax N terminus and Bax mitochondrial translocation. J. Biol. Chem. 27517225-17228. [DOI] [PubMed] [Google Scholar]
- 34.Nechushtan, A., C. L. Smith, Y. T. Hsu, and R. J. Youle. 1999. Conformation of the Bax C-terminus regulates subcellular location and cell death. EMBO J. 182330-2341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nechushtan, A., C. L. Smith, I. Lamensdorf, S. H. Yoon, and R. J. Youle. 2001. Bax and Bak coalesce into novel mitochondria-associated clusters during apoptosis. J. Cell Biol. 1531265-1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pauleau, A. L., L. Galluzzi, S. R. Scholz, N. Larochette, O. Kepp, and G. Kroemer. 2008. Unexpected role of the phosphate carrier in mitochondrial fragmentation. Cell Death Differ. 15616-618. [DOI] [PubMed] [Google Scholar]
- 37.Pauleau, A. L., N. Larochette, F. Giordanetto, S. R. Scholz, D. Poncet, N. Zamzami, V. S. Goldmacher, and G. Kroemer. 2007. Structure-function analysis of the interaction between Bax and the cytomegalovirus-encoded protein vMIA. Oncogene 267067-7080. [DOI] [PubMed] [Google Scholar]
- 38.Pierrat, B., M. Simonen, M. Cueto, J. Mestan, P. Ferrigno, and J. Heim. 2001. SH3GLB, a new endophilin-related protein family featuring an SH3 domain. Genomics 71222-234. [DOI] [PubMed] [Google Scholar]
- 39.Poncet, D., N. Larochette, A. L. Pauleau, P. Boya, A. A. Jalil, P. F. Cartron, F. Vallette, C. Schnebelen, L. M. Bartle, A. Skaletskaya, D. Boutolleau, J. C. Martinou, V. S. Goldmacher, G. Kroemer, and N. Zamzami. 2004. An anti-apoptotic viral protein that recruits Bax to mitochondria. J. Biol. Chem. 27922605-22614. [DOI] [PubMed] [Google Scholar]
- 40.Poncet, D., A. L. Pauleau, G. Szabadkai, A. Vozza, S. R. Scholz, M. Le Bras, J. J. Briere, A. Jalil, R. Le Moigne, C. Brenner, G. Hahn, I. Wittig, H. Schagger, C. Lemaire, K. Bianchi, S. Souquere, G. Pierron, P. Rustin, V. S. Goldmacher, R. Rizzuto, F. Palmieri, and G. Kroemer. 2006. Cytopathic effects of the cytomegalovirus-encoded apoptosis inhibitory protein vMIA. J. Cell Biol. 174985-996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Smith, G. B., and E. S. Mocarski. 2005. Contribution of GADD45 family members to cell death suppression by cellular Bcl-xL and cytomegalovirus vMIA. J. Virol. 7914923-14932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sonnhammer, E. L., G. von Heijne, and A. Krogh. 1998. A hidden Markov model for predicting transmembrane helices in protein sequences. Proc. Int. Conf. Intell. Syst. Mol. Biol. 6175-182. [PubMed] [Google Scholar]
- 43.Su, J., G. Wang, J. W. Barrett, T. S. Irvine, X. Gao, and G. McFadden. 2006. Myxoma virus M11L blocks apoptosis through inhibition of conformational activation of Bax at the mitochondria. J. Virol. 801140-1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Takahashi, Y., M. Karbowski, H. Yamaguchi, A. Kazi, J. Wu, S. M. Sebti, R. J. Youle, and H. G. Wang. 2005. Loss of Bif-1 suppresses Bax/Bak conformational change and mitochondrial apoptosis. Mol. Cell. Biol. 259369-9382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Takayama, S., D. L. Cazals-Hatem, S. Kitada, S. Tanaka, T. Miyashita, L. R. Hovey III, D. Huen, A. Rickinson, P. Veerapandian, S. Krajewski, et al. 1994. Evolutionary conservation of function among mammalian, avian, and viral homologs of the Bcl-2 oncoprotein. DNA Cell Biol. 13679-692. [DOI] [PubMed] [Google Scholar]
- 46.Tan, Y. J., W. Beerheide, and A. E. Ting. 1999. Biophysical characterization of the oligomeric state of Bax and its complex formation with Bcl-XL. Biochem. Biophys. Res. Commun. 255334-339. [DOI] [PubMed] [Google Scholar]
- 47.Upton, J. P., A. J. Valentijn, L. Zhang, and A. P. Gilmore. 2007. The N-terminal conformation of Bax regulates cell commitment to apoptosis. Cell Death Differ. 14932-942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wei, M. C., W. X. Zong, E. H. Cheng, T. Lindsten, V. Panoutsakopoulou, A. J. Ross, K. A. Roth, G. R. MacGregor, C. B. Thompson, and S. J. Korsmeyer. 2001. Proapoptotic BAX and BAK: a requisite gateway to mitochondrial dysfunction and death. Science 292727-730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wolter, K. G., Y. T. Hsu, C. L. Smith, A. Nechushtan, X. G. Xi, and R. J. Youle. 1997. Movement of Bax from the cytosol to mitochondria during apoptosis. J. Cell Biol. 1391281-1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Youle, R. J., and A. Strasser. 2008. The BCL-2 protein family: opposing activities that mediate cell death. Nat. Rev. Mol. Cell Biol. 947-59. [DOI] [PubMed] [Google Scholar]
- 51.Yu, D., M. C. Silva, and T. Shenk. 2003. Functional map of human cytomegalovirus AD169 defined by global mutational analysis. Proc. Natl. Acad. Sci. USA 10012396-12401. [DOI] [PMC free article] [PubMed] [Google Scholar]