Abstract
Cytotoxic T lymphocytes (CTL) and natural killer (NK) cells play key roles in limiting herpesvirus infections; consequently, many herpesviruses, including herpes simplex virus (HSV), have evolved diverse strategies to evade and/or disarm these killer lymphocytes. Previous studies have shown that CTL and NK cells are functionally inactivated following contact with HSV-infected fibroblasts. During studies of the mechanisms involved, we discovered that HSV-inactivated NK-92 NK cells and Jurkat T cells contain a strikingly prominent, novel, ca. 90-kDa tyrosine-phosphorylated protein that we identified as the HSV tegument protein VP11/12. Inasmuch as VP11/12 produced in fibroblasts and epithelial cells is not obviously tyrosine phosphorylated, these data suggested that VP11/12 serves as the substrate of a cell-type-specific protein tyrosine kinase. Consistent with this hypothesis, VP11/12 was also tyrosine phosphorylated in B lymphocytes, and this modification was severely reduced in Jurkat T cells lacking the lymphocyte-specific Src family kinase Lck. These findings demonstrate that HSV tegument proteins can be differentially modified depending on the cell type infected. Our data also raise the possibility that VP11/12 may modulate one or more lymphocyte-specific signaling pathways or serve another lymphocyte-specific function. However, HSV type 1 mutants lacking the UL46 gene retained the ability to block signaling through the T-cell receptor in Jurkat cells and remained competent to functionally inactivate the NK-92 NK cell line, indicating that VP11/12 is not essential for lymphocyte inactivation. Further studies are therefore required to determine the biological function of tyrosine-phosphorylated VP11/12.
Natural killer (NK) cells and cytotoxic T lymphocytes (CTL) contribute to host antiviral defense by secreting antiviral cytokines and triggering apoptosis of cells that display evidence of virus infection (2, 12, 24). These killer lymphocytes play crucial roles in limiting the severity of many viral diseases (18, 27, 35, 41), including those caused by herpes simplex virus (HSV) and other herpesviruses (11, 35, 46). HSV, in turn, has evolved at least three distinct strategies for evading and/or disarming CTL and NK cells. First, HSV, like many other viruses, suppresses host major histocompatibility complex class I antigen presentation pathways, thereby impairing CTL recognition of infected cells (17, 21, 49, 57; reviewed in reference 1). Second, also like other viruses, HSV produces several antiapoptotic gene products (3, 6, 15, 19, 26), which can protect infected cells from cytolysis by killer lymphocytes (4, 7, 20). Third, NK cells and CTL are functionally inactivated (or disarmed) following contact with HSV-infected fibroblasts or epithelial cells (8, 40). Such inactivation requires direct contact between the infected cell and the killer lymphocyte (8, 40, 44) and abrogates the ability of the lymphocyte to kill other target cells. The mechanism of HSV-induced lymphocyte inactivation is of great interest but remains to be fully defined.
The bulk of the available evidence indicates that killer lymphocytes are inactivated by one or more HSV virion components that are transferred from the infected fibroblast, likely via progeny virions and/or enveloped subviral particles (39, 42, 44, 58). Consistent with this hypothesis, NK cells and CTL can also be inactivated by direct infection with high multiplicities of cell-free HSV (38, 42, 58), and in the case of Jurkat CD4+ T cells, such inactivation requires viral penetration but not viral gene expression (42). Remarkably, transfer of viral DNA to the lymphocyte does not seem to be required, since infected fibroblasts retain their ability to inactivate CTL following treatment with acyclovir, an antiviral drug that blocks viral DNA replication and hence the production of infectious progeny virions (42, 44). Taken in combination, these data suggest that noninfectious enveloped subviral assemblies lacking capsids and viral DNA, such as L particles (48) or PREPs (10), are also competent, arguing that one or more tegument proteins are likely responsible for inactivation (42). One report suggested that the serine/threonine protein kinase encoded by HSV gene US3 (a tegument protein) is required for lymphocyte inactivation mediated by infected fibroblasts (44); however, it is not yet clear if the requirement for US3 is direct or indirect, and the virion component that triggers inactivation has yet to be identified. Indeed, more than one mechanism of lymphocyte inactivation may be operative, since CTL can also be inactivated by exposure to fibroblasts infected with HSV mutants incapable of cell-to-cell spread, but only if such exposure occurs very early (2 h) after infection of the fibroblasts (39).
Studies by Sloan and coworkers (42, 43, 44) have partially defined the molecular mechanism underlying HSV-induced inactivation of T lymphocytes by showing that HSV remodels signaling through the T-cell receptor (TCR). Thus, HSV-inactivated Jurkat cells fail to display the expected calcium flux or Th1 cytokine responses following TCR ligation; moreover, TCR ligation results in p38-dependent induction of interleukin-10, a Th2 cytokine that suppresses the T-cell response (43). The HSV-induced TCR signaling modification is downstream of activation of Zap70 and correlates with decreased tyrosine phosphorylation of the T-cell signaling adaptor molecule LAT (linker of activated T cells) (42). These findings indicate that inactivation stems from virus-induced alterations to the protein tyrosine kinase signaling cascade emanating from the TCR. Consistent with this view, inactivation was partially attenuated by inhibitors of protein tyrosine phosphatases (42).
Given the foregoing observations, we sought to broadly examine changes in protein tyrosine phosphorylation in HSV-inactivated lymphocytes in order to gain further insight into the mechanism of inactivation. We report here that HSV-inactivated lymphocytes contain a prominent, novel, ca. 90-kDa tyrosine-phosphorylated protein, which we identify as the HSV tegument protein VP11/12, encoded by gene UL46. Tyrosine phosphorylation of VP 11/12 was lymphocyte specific, and in Jurkat cells it required the Src family kinase Lck, a key player in the activation pathways of both T and NK cells. However, our data demonstrate that VP11/12 is not essential for the HSV-induced blockade of TCR signaling in Jurkat cells or for functional inactivation of the NK-92 NK cell line, indicating that any putative effect of VP11/12 on lymphocyte function is likely to be more subtle than complete abrogation of signaling pathways. Thus, further studies are required to define the role of VP11/12 in the biology of HSV-lymphocyte interactions.
MATERIALS AND METHODS
Cells.
NK-92 cells (a gift from D. Burshtyn, University of Alberta; originally obtained from the ATCC) were grown in α-minimal essential medium (αMEM; Gibco) supplemented with 12.5% horse serum (Sigma), 12.5% fetal bovine serum (FBS) (PAA Laboratories Inc.), 0.2 mM inositol (Sigma), 0.1 mM 2-mercaptoethanol (BDH), 0.02 mM folic acid (Sigma), 2 mM l-glutamine (Gibco), 20 μg/ml gentamicin (Gibco), and 100 U/ml interleukin-2 (Hoffmann-LaRoche Inc.). 721.221 B cells (23) (a gift from D. Burshtyn) were maintained in Iscove's modified Dulbecco's medium supplemented with 10% FBS, 4 mM l-glutamine, and 100 U/ml penicillin-streptomycin (Gibco). Jurkat 6.8 and JCaM 1.6 cells (14) (donated by H. Ostergaard, University of Alberta; originally obtained from the ATCC) were maintained in RPMI 1640 medium (Gibco) supplemented with 10% FBS, 200 mM l-glutamine, 100 mM sodium pyruvate (Gibco), and 100 U/ml penicillin-streptomycin. Human embryonic lung (HEL) fibroblasts (obtained from the ATCC) were maintained in Dulbecco's modified Eagle's medium (DMEM; Gibco) supplemented with 10% FBS, 100 mM sodium pyruvate, and 100 U/ml penicillin-streptomycin. Vero cells (obtained from the ATCC) were maintained in DMEM supplemented with 5% FBS and 100 U/ml penicillin-streptomycin.
Coculture of lymphocytes with HSV-infected fibroblasts.
HEL fibroblasts in 6-well culture dishes were infected with 10 PFU/cell of the indicated HSV strain, and the infection was allowed to proceed for 12 h. The infected cells were then washed with phosphate-buffered saline, and lymphocytes growing in the appropriate medium were added to the culture and incubated for 3 to 4 h. Lymphocytes were added to the fibroblasts at a 1:1 ratio unless otherwise stated. Where indicated, the Src family kinase inhibitor PP2 (Calbiochem) was added to a final concentration of 10 μM during the coculture period. The lymphocytes were then collected and either used directly for a lytic assay or processed for mass spectrometry or Western blot analysis.
Mass spectrometry.
Approximately 4 × 107 Jurkat or NK-92 cells were exposed to HEL fibroblasts infected with HSV type 2 (HSV-2) strain HG52 as described above, recovered, and then lysed by incubation in lysis buffer (1% Nonidet, 0.25% sodium deoxycholate, 150 mM NaCl, 1 mM EGTA, 1 mM NaF, 1 mM Na3VO4, 50 mM Tris-HCl [pH 7.4]) supplemented with a phosphatase inhibitor cocktail (Sigma) and Complete protease inhibitor cocktail (Roche). Nuclei were pelleted; the supernatants were incubated overnight at 4°C with an antiphosphotyrosine monoclonal antibody (4G10; dilution, 1:20,000; Upstate); and immune complexes were then recovered by incubation with 50% protein G agarose (Roche) for 1 h at 4°C. Bound proteins were isolated by centrifugation and then separated by electrophoresis on a 10% sodium dodecyl sulfate (SDS)-polyacrylamide gel along with a Precision Plus molecular weight standard (Bio-Rad). The proteins were silver stained (SilverQuest; Invitrogen) according to the manufacturer's protocol. Immunoprecipitated proteins were then analyzed by mass spectrometry at the Institute for Biomolecular Design, University of Alberta.
Western blotting.
Samples separated by electrophoresis through an SDS-10% polyacrylamide gel were transferred to a nitrocellulose membrane (Hybond ECL; Amersham Pharmacia). In most experiments, specific proteins were detected using horseradish peroxidase-conjugated secondary antibodies (Promega) and an ECL Plus detection system (Amersham Biosciences). In some cases, membranes were stripped by incubation in 62.5 mM Tris (pH 6.7)-2% SDS-0.7% β-mercaptoethanol prior to reprobing with another antibody. In the experiments for which results are shown in Fig. 7 and 9, signals were detected and quantified with fluorochome-conjugated secondary antibodies by using an Odyssey infrared imager (LI-COR Biosciences). Signals were detected with the Odyssey infrared imager (LI-COR Biosciences) and quantified using Odyssey application software, version 1.2.
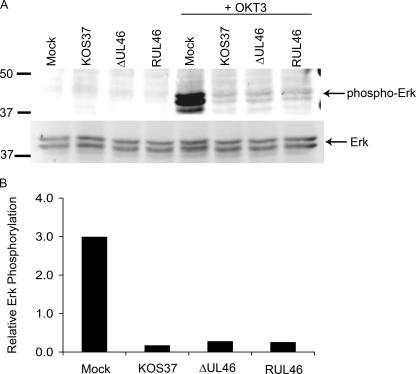
FIG. 7.
UL46 is not required for HSV-induced inhibition of TCR signaling. (A) Jurkat T cells were either mock infected or infected with 20 PFU/cell of the indicated virus for 8 h. Each culture was then divided in half and incubated on ice for 15 min with or without 10 μg/ml of an anti-CD3 antibody (OKT3). The anti-CD3 antibody was then cross-linked by incubation on ice for an additional 15 min with goat anti-mouse IgG2a (15 μg/ml). Samples were then incubated for 10 min at 37°C, followed by Western blot analysis using antibodies that detect total and phosphorylated ERK1/2. (B) The levels of phosphorylated ERK present in OKT3-treated cells (see panel A) were quantified using an Odyssey infrared imager and normalized to total ERK levels. Values are expressed in arbitrary units.
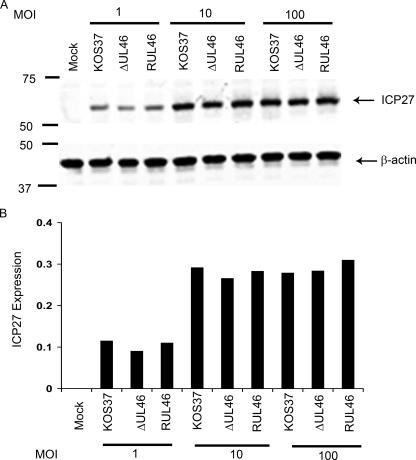
FIG. 9.
Effect of UL46 on the expression of the IE ICP27 gene in lymphocytes. Jurkat T cells were either mock infected or infected with the indicated viruses at a multiplicity of infection (MOI) of 1, 10, or 100 PFU/cell. At 4 h postinfection, the accumulation of the IE protein ICP27 was assessed by Western blotting. (A) ICP27 and host β-actin were simultaneously detected with fluorescently labeled secondary antibodies using an Odyssey infared imager. (B) ICP27 signal intensities normalized to β-actin levels are displayed.
Antibodies.
Antibodies used for Western blotting included mouse monoclonal antibodies directed against phosphotyrosine (4G10; dilution, 1:20,000; Upstate), actin (AC-15; 1:3,000; Sigma), phospho-p44/42 mitogen-activated protein kinase (extracellular signal-regulated kinases 1 and 2 [ERK1/2]) (Thr202/Tyr204) (E10; 1/1,000; Cell Signaling), HSV-1 VP16 (LP1; 1/32,000; provided by Tony Minson), and HSV-1 ICP27 (catalog no. 1113; 1/2,500; Goodwin Institute); rabbit polyclonal antisera raised against β-actin (1/2,000; Sigma) and HSV-2 UL46 (1:30,000; provided by Yukihiro Nishiyama); and IR800-conjugated donkey anti-mouse (1:15,000; Rockland), Alexa Fluor 680-conjugated goat anti-rabbit (1:15,000; Invitrogen), and IRDye800-conjugated goat anti-rabbit (1/15,000; Rockland) immunoglobulin G (IgG). Monoclonal antibody OKT3, directed against CD3 (eBioscience), was used to initiate signaling through the TCR following cross-linking with goat anti-mouse IgG2a (Sigma), as described in the legend to Fig. 7.
Construction of UL46 mutants.
KOS-37 (13), an infectious bacterial artificial chromosome (BAC) bearing the entire HSV-1 strain KOS genome, was used as the substrate for generating HSV-1 isolates bearing null mutations at the UL46 locus. Mutagenesis was performed using bacteriophage λ Red-mediated homologous recombination in Escherichia coli (recombineering [reviewed in reference 9]) using the E. coli galK gene as the selectable marker (52) (see also the protocol at http://recombineering.ncifcrf.gov/Protocol.asp). Mutagenesis was accomplished in two steps using sequential rounds of positive and negative selection for galK in the galK-deficient E. coli strain SW102, as described by Warming et al. (52). First, the HSV-1 UL46 open reading frame (ORF) was precisely deleted from KOS-37 and replaced with a galK expression cassette, yielding KOS-37 ΔUL46galK. Second, the galK cassette was either precisely removed from KOS-37 ΔUL46galK to form a seamless deletion of the UL46 ORF (KOS-37 ΔUL46) or replaced by the UL46 ORF to yield a UL46 repair construct (KOS-37 RUL46).
For the construction of KOS-37 ΔUL46galK, a linear galK expression cassette was amplified by PCR from pgalK (52) using a pair of galK primers that bear 5′ sequences corresponding to the 50 nucleotides (nt) immediately upstream of the UL46 start codon and the 54 nt immediately downstream of the UL46 stop codon, respectively: GACAAACAGGGGGAAAGGGGCGTGGTCTAGCGACGGCAGCACGGGCGGAGGCGTcctgttgacaattaatcatcggca-3′ and 5′-GGACGCGGCATAACTCCGACCGGCGGGTCCCGACCGAACGGGCGTCACCt cagcactgtcctgctcctt-3′. (HSV-1 sequences are capitalized, and galK sequences are lowercased.)
The construct bearing a seamless UL46 deletion, KOS-37 ΔUL46, was produced using a double-stranded linear oligonucleotide homologous to the regions flanking the UL46 ORF. Specifically, the oligonucleotide was composed of sequences corresponding to 49 nt immediately upstream of the UL46 start codon directly fused to 54 nt located immediately downstream of the UL46 stop codon: 5′-GACAAACAGGGGGAAAGGGGCGTGGTCTAGCGACGGCAGCACG GGCGGAGGCGT/GGTGACGCCCGTTCGGTCGGGACCCGCCGGTCGGAGTTATGCCGCGTCC-3′.
For construction of KOS-37 RUL46, a linear cassette of the UL46 ORF extending from 52 nt upstream of the UL46 start codon to 57 nt downstream of the UL46 stop codon was amplified from KOS-37 by using primers 5′-GTCGACAAACAGGGGGAAAG-3′ and 5′-CTGGACGCGGCATAACTC-3′.
Appropriately modified BAC clones were initially identified by PCR using primers internal to the galK ORF (CCATTGTCGCACATGAAAAC and TCCAGTGAAGCGGAAGAACT) and the HSV-1 UL46 ORF (GGACTCAGCCGGTGACATAC and AAGTACCTGCAGACGGTGGT). The presence of the desired modification of the UL46 locus was then confirmed by PCR with primers flanking the UL46 ORF (GACAAACAGGGGGAAAGG and GGACGCGGCATAACTCC) and Southern blot analysis. Following verification, BAC DNA was isolated using the large-construct purification kit from Qiagen and was converted to infectious HSV-1 by transfection into Cre-Vero cells (13) using Lipofectamine 2000 (Invitrogen).
Plasmids.
A plasmid containing the HSV-1 KOS UL46 ORF (pUL46) was constructed by inserting a PCR product extending from 10 nt upstream of the UL46 start codon to 30 nt downstream of the stop codon between the EcoRI and XhoI restriction sites of pcDNA3.1 (Invitrogen) by using the primer pair ttgaattcGGGCGTCACCATGCAG-tttctcgagGTCTAGCGACGGCAGCAC.
A plasmid containing the HSV-1 DNA sequences that flank the HSV-1 KOS UL46 ORF (pL1R1) was made in two steps. First, HSV-1 genomic sequences extending from 18 to 472 nt downstream of the UL46 stop codon (amplified using primers tctctagaGTCCAGTCGGCAAGATCCTC and ttttgcggccgCACGCCTCCGCCCGTGCTGC) were inserted between the XbaI and NotI sites of pBluescript SK(+) (Fermentas), producing pL1. Second, a PCR product extending from 1 to 350 nt upstream of the UL46 start codon was inserted between the NotI and SacI sites of pL1 by using primers gtttgcggccgcGGTGACGCCCGTTCGGT and ttgagctcCGGAGCACGTGGATCTGC.
Southern blotting.
BAC or viral DNA was digested with the indicated restriction endonucleases and analyzed by Southern blotting using 32P-labeled probes as previously described (31). A probe corresponding to the UL46 ORF was generated from pUL46 by digestion with EcoRI and XhoI; the galK probe was isolated from pgalK following digestion with EcoRI and NotI; and the sequences that flank the HSV-1 UL46 were isolated from pL1R1 following digestion with XbaI, NotI, and SacI.
Chromium release assay for NK cell-mediated lysis of target cells.
NK cell lytic activity was measured by a chromium release assay (54). Target 721.221 B cells were labeled with 100 μCi/ml Na251CrO4 (Perkin-Elmer) for 1.5 to 3 h at 37°C; then they were washed and resuspended in NK-92 cell medium. NK-92 and 721.221 cells were coincubated at various effector-to-target ratios for 4 to 5 h at 37°C. Radioactivity released from the target cells was then measured using a 1450 Wallac Microbeta TriLux liquid scintillation counter. The percentage of lysis was calculated as (radioactivity released from the sample − radioactivity released from the spontaneous target lysis control)/(radioactivity released from the maximum target lysis control − radioactivity released from the spontaneous target lysis control). (Radioactivity was measured in counts per minute.) The radioactivity released via spontaneous target lysis was determined by using 721.221 cells incubated in the absence of NK-92 effectors. Maximal target lysis was measured by determining the radioactivity released from 721.221 cells in the absence of NK-92 effectors and in the presence of 10% Igepal CA-630 (Sigma).
RESULTS
Lymphocytes exposed to HSV-infected fibroblasts display a prominent, ca. 90-kDa protein detected with an antiphosphotyrosine antibody.
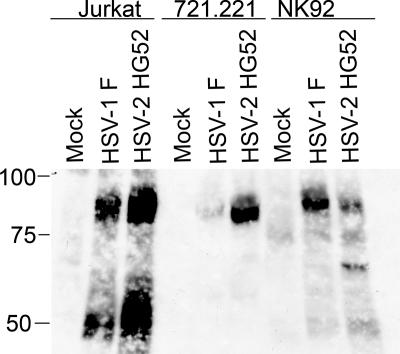
As reviewed in the introduction, T lymphocytes and NK cells are functionally inactivated following exposure to HSV-infected fibroblasts (8, 40). Recent studies suggest that inactivation of Jurkat T lymphocytes is accomplished by blocking signaling through the TCR at one or more steps prior to activation of LAT (42). Moreover, this functional inactivation can be attenuated by inhibitors of protein tyrosine phosphatases, implying that it involves modulation of tyrosine phosphorylation (42). We therefore sought to broadly examine changes in protein tyrosine phosphorylation in lymphocytes following exposure to HSV-infected fibroblasts. To this end, HEL fibroblasts were either mock infected or infected with HSV-1 strain F or HSV-2 strain HG52 for 12 h. Jurkat, 721.221, and NK-92 cells (CD4+ T cells, B cells, and NK cells, respectively) were then exposed to the infected fibroblasts for 4 h. The lymphocytes were removed and lysed, and extracts were analyzed by Western blotting with monoclonal antibody 4G10, directed against phosphotyrosine (Fig. 1). Strikingly, all three lymphocyte cell lines displayed a novel, prominent 4G10-reactive protein of ca. 90 kDa after exposure to HSV-1- or HSV-2-infected fibroblasts (although the signal induced by HSV-1 in 721.221 cells was weaker than those in the other cell types). Similar results were obtained with a CD8+ CTL clone (SA) and an additional Epstein-Barr virus-immortalized B-cell line (data not shown). In contrast, no such prominent tyrosine-phosphorylated species could be discerned in lymphocytes exposed to mock-infected fibroblasts (Fig. 1) or in the HSV-infected fibroblasts themselves (see Fig. 5 below). In many experiments, the exposed lymphocytes also displayed a prominent tyrosine-phosphorylated band (or bands) of ca. 55 to 60 kDa (for example, see the Jurkat cell samples in Fig. 1), but this was less consistently observed. Exposed Jurkat cells also displayed a much weaker, ca. 37-kDa tyrosine-phosphorylated band (for example, see Fig. 4B). These results indicated that cells representing three distinct lymphocyte lineages contain a prominent, novel, ca. 90-kDa tyrosine-phosphorylated protein after exposure to HSV-infected fibroblasts.
FIG. 1.
Analysis of tyrosine-phosphorylated proteins in lymphocytes after exposure to HSV-infected fibroblasts. The indicated lymphocytes were incubated for 4 h with HEL fibroblasts that had been either mock infected or infected with HSV-1 F or HSV-2 HG52 12 h previously. The lymphocytes were then removed, and extracts were analyzed by Western blotting with an antiphosphotyrosine antibody. The mobilities of protein markers are indicated on the left.
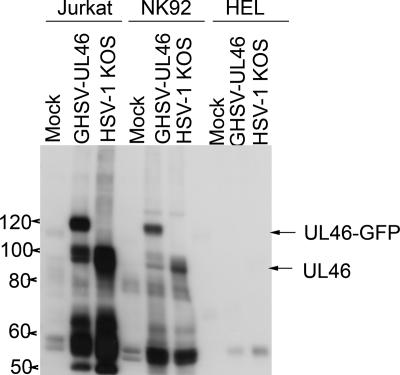
FIG. 5.
Lymphocyte-specific tyrosine phosphorylation of VP11/12. HEL fibroblasts were infected with 10 PFU/cell of the indicated viruses. Twelve hours later, Jurkat T cells or NK-92 cells were added to some of the cultures. Four hours later, the lymphocytes were removed, and extracts were analyzed for tyrosine-phosphorylated proteins as before. Extracts prepared at the same time from HEL cells that had not been exposed to lymphocytes were analyzed in parallel. The mobilities of the prominent ca. 90-kDa and 120-kDa tyrosine-phosphorylated proteins produced by HSV-1 KOS and GHSV-UL46 are indicated (UL46 and UL46-GFP, respectively).
FIG. 4.
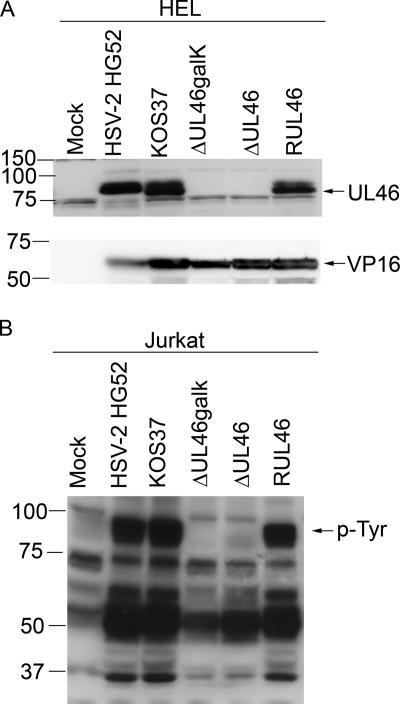
Analysis of UL46 and tyrosine-phosphorylated proteins in cells infected with UL46 mutants. (A). HEL fibroblasts were either mock infected or infected with wild-type HSV (HSV-2 HG52, HSV-1 KOS-37), the UL46-null viruses (ΔUL46galk, ΔUL46), or the UL46 repair virus (RUL46). Cell extracts prepared 16 h postinfection were then analyzed by Western blotting using a rabbit polyclonal antiserum directed against HSV-2 strain 186 UL46 (top panel) and monoclonal antibody LP1, directed against VP16 (bottom panel). (B) Jurkat cells were exposed as before to HEL fibroblasts that had been either mock infected or infected by wild-type HSV (HSV-2 HG52, HSV-1 KOS-37), a UL46-null virus (ΔUL46galk, ΔUL46), or the UL46 repair virus (RUL46). The Jurkat cells were then removed, and extracts were analyzed by Western blotting with an antiphosphotyrosine antibody. The mobility of the prominent 90-kDa tyrosine-phosphorylated protein is indicated (p-Tyr).
The 90-kDa tyrosine-phosphorylated protein is HSV VP11/12, encoded by gene UL46.
We sought to identify the 90-kDa tyrosine-phosphorylated protein as a prelude to studies of its possible role(s) in the biology of HSV-lymphocyte interactions. As the first step, we used mass spectrometry to suggest potential candidates for its identity. To this end, Jurkat and NK-92 cells were exposed to HEL fibroblasts infected with HSV-2 strain HG52 as described for Fig. 1. The cells were then removed and lysed, and tyrosine-phosphorylated proteins were isolated by immunoprecipitation with the antiphosphotyrosine antibody, followed by SDS-polyacrylamide gel electrophoresis. Gel slices containing proteins migrating at ca. 90 and 60 kDa were separately excised and analyzed by mass spectrometry. Many host proteins were present in the ca. 90- and 60-kDa gel slices; however, in one of two experiments, we also identified peptides derived from the HSV-2 strain HG52 tegument protein VP11/12, encoded by gene UL46. Specifically, one UL46 peptide was detected in the 90-kDa sample from Jurkat cells, and two additional UL46 peptides were found in the 60-kDa sample from NK-92 cells (data not shown). No other viral proteins were identified. Previous studies have shown that HSV-2 VP11/12 produced in Vero cells migrates in SDS-polyacrylamide gels with an apparent molecular mass of 82 to 86 kDa (22), so it seems likely that the UL46 peptides found in the 60-kDa sample arose from partially degraded or otherwise truncated VP11/12. Although these mass spectrometry data were by no means definitive, the results raised the possibility that the prominent, ca. 90-kDa phosphoprotein might correspond to a tyrosine-phosphorylated form of HSV VP11/12.
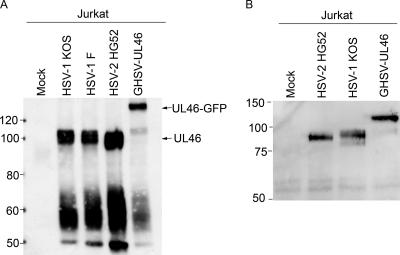
We therefore asked if the major tyrosine-phosphorylated protein was altered in Jurkat cells exposed to fibroblasts infected with HSV-1 stain KOS mutant GHSV-UL46, which encodes a UL46-green fluorescent protein (GFP) fusion protein of ca. 120 kDa (53). As observed previously, wild-type HSV-1 or HSV-2 triggered the appearance of a prominent, ca. 90-kDa tyrosine-phosphorylated band detected by the antiphosphotyrosine antibody, as well as a broader band or bands in the 55- to 60-kDa range. By contrast, the ca. 90-kDa signal was greatly reduced and replaced by a prominent, ca. 120-kDa species in Jurkat cells exposed to fibroblasts infected with GHSV-UL46 (Fig. 2A). This result indicated that most or all of the 90-kDa tyrosine-phosphorylated material corresponds to VP11/12. Also consistent with tyrosine phosphorylation of VP11/12, immunoprecipitation/Western blot analysis using a polyclonal antibody raised against HSV-2 VP11/12 (22) documented that both wild-type VP11/12 and the UL46-GFP fusion protein were immunoprecipitated by the antiphosphotyrosine antibody 4G10 (Fig. 2B). Unexpectedly, GHSV-UL46 produced small amounts of a ca. 90-kDa tyrosine-phosphorylated protein (Fig. 2A) in addition to the predicted 120-kDa UL46-GFP fusion protein. This residual 90-kDa band, which differed in relative abundance between experiments (for example, compare Fig. 2A to Fig. 5), likely corresponds to a degradation product of the UL46-GFP fusion protein, since it reacts with the anti-VP11/12 antibody (Fig. 2B). GHSV-UL46 continued to induce the appearance of tyrosine-phosphorylated bands at ca. 55 to 60 kDa, albeit at somewhat reduced levels compared to those with wild-type HSV-1 KOS. The identity of the protein or proteins that give rise to this 55- to 60-kDa signal has yet to be determined, but we note that these bands do not obviously react with the anti-VP11/12 antibody (Fig. 2A; also data not shown). Taken in combination, these data establish that the prominent 90-kDa tyrosine-phosphorylated protein detected in inactivated lymphocytes is the HSV tegument protein VP11/12.
FIG. 2.
Tyrosine phosphorylation of the HSV tegument protein VP11/12, encoded by gene UL46. (A). VP11/12 is tyrosine phosphorylated. HEL fibroblasts were either mock infected or infected with the indicated viruses for 12 h. Jurkat T cells were then coincubated with the fibroblasts for 4 h, and extracts of the lymphocytes were prepared. Cell lysates were analyzed by Western blotting with an antiphosphotyrosine antibody as for Fig. 1. The prominent ca. 90- and 120-kDa bands, presumed to correspond to wild-type VP11/12 (UL46) and the VP11/12-GFP fusion protein encoded by GHSV-UL46 (UL46-GFP), respectively, are indicated by arrows. The mobilities of protein markers are indicated on the left. (B) VP11/12 is immunoprecipitated by an antiphosphotyrosine antibody. Jurkat cells were exposed to HEL fibroblasts as described for panel A and were then harvested 9 h later. Extracts were immunoprecipitated with the antiphosphotyrosine antibody, and precipitated UL46-related proteins were detected by Western blotting with a polyclonal antibody directed against HSV-2 VP11/12. The mobilities of protein markers are indicated on the left.
Construction of UL46-null viruses.
We wished to examine the phenotypes of HSV UL46-null mutants in order to further confirm the identity of the major ca. 90-kDa tyrosine-phosphorylated protein and investigate the possible role of VP11/12 in the biology of HSV-lymphocyte interactions. McKnight and coworkers have previously derived viable HSV-1 strain F mutants bearing null mutations at the UL46 locus (59, 60); however, the mutants obtained in these studies were no longer available to us. We therefore constructed additional HSV-1 UL46-null mutants by using bacteriophage λ Red-mediated targeted homologous recombination (recombineering) (9) of an infectious BAC clone of the HSV-1 KOS genome (KOS-37). We first generated a UL46-null mutant (ΔUL46galK) in which the UL46 ORF is precisely deleted and replaced with an E. coli galK expression cassette (Fig. 3A) (Materials and Methods). The ΔUL46galK BAC was then used as the template for a second round of recombineering employing galK counterselection to produce a seamless UL46-null deletion (ΔUL46) and a UL46 repair construct (RUL46) (Fig. 3A).
FIG. 3.
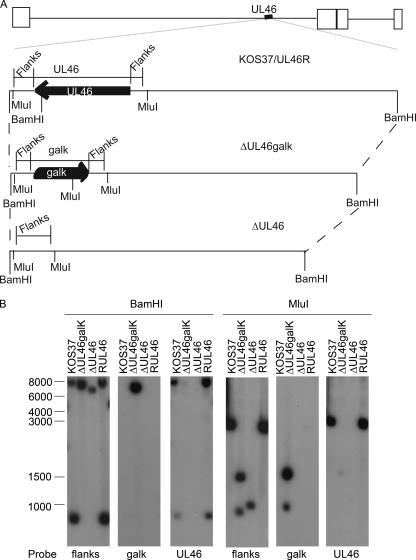
Verification of the UL46 mutations produced by recombineering of the KOS-37 BAC. (A) Genomic structures of wild-type and UL46 mutant viruses. Below the schematic diagram of the HSV-1 KOS-37 genome is an expanded view of the UL46 locus. The location and orientation of the UL46 ORF are indicated, along with the locations of cleavage sites for BamHI and MluI. “Flanks” indicates the probe that detects the UL46 5′ and 3′ flanking sequences. ΔUL46galK bears a deletion/substitution mutation that replaces the UL46 ORF with the E. coli galK gene; ΔUL46 bears a precise deletion of the UL46 ORF; and RUL46 is a UL46 repair virus derived from ΔUL46galK. (B) Southern blot analysis of viral DNA. Total cellular DNA extracted from Vero cells infected with the indicated viruses was cleaved with BamHI or MluI and analyzed by Southern blot hybridization using the indicated probes. Mobilities of molecular weight markers (in nucleotides) are indicated.
We used Southern blot analysis to confirm that the virus stocks derived from the modified BACs displayed the appropriate structure at the UL46 locus (Fig. 3; also data not shown). Total DNA extracted from infected Vero cells was digested with BamHI or MluI, which cut within the UL46 ORF or the galK expression cassette, respectively (diagrammed in Fig. 3A). The resulting fragments were then detected using probes corresponding to the sequences that flank the UL46 ORF (Flanks), the galK gene, or the UL46 ORF. As predicted, the wild-type UL46 locus present in KOS-37 and RUL46 DNA gave rise to 8,055-bp and 717-bp BamHI fragments that were detected by both the UL46 and Flanks probes (Fig. 3A and B). These wild-type DNAs also displayed the single expected 3,027-bp MluI fragment that hybridized to the UL46 and Flanks probes, and neither viral genome reacted with the galK probe (Fig. 3B). Consistent with replacement of the UL46 ORF by the galK gene, ΔUL46galK DNA displayed a single 7,933-bp BamHI fragment that bound to the Flanks and galK probes but not to the UL46 probe. Also consistent with acquisition of the galK cassette and loss of UL46, ΔUL46galK DNA generated the predicted 1,317-bp and 784-bp fragments upon digestion with MluI, both of which hybridized to the galK and Flanks probes but not to the UL46 probe. Finally, as expected, ΔUL46 DNA gave rise to a single fragment that hybridized only to the Flanks probe following digestion with BamHI or MluI; moreover, the size of the ΔUL46 MluI fragment was consistent with the predicted seamless deletion of the 2,157-bp UL46 ORF.
Taken in combination, these and other data document that the viral mutants derived by recombineering of the KOS-37 BAC displayed the intended genome configurations at the UL46 locus.
The UL46 gene product VP11/12 is required for the appearance of the 90-kDa tyrosine-phosphorylated protein.
As documented above, the UL46-null viruses ΔUL46galK and ΔUL46 lack the UL46 ORF and hence cannot express the UL46 gene product VP11/12. We therefore used these mutant isolates to determine if production of VP11/12 is required for the appearance of the 90-kDa tyrosine-phosphorylated protein in lymphocytes exposed to infected fibroblasts.
We first confirmed that the UL46 null mutations indeed abrogate the production of VP11/12. HEL fibroblasts were infected with HSV-2 HG52, HSV-1 KOS-37, or the KOS-37 derived mutants, ΔUL46galk, ΔUL46, and RUL46. Extracts prepared 16 h postinfection were then analyzed by Western blotting using the rabbit polyclonal antibody directed against HSV-2 VP11/12 (Fig. 4A). As a control, the samples were also probed with a monoclonal antibody directed against another abundant tegument protein, VP16, the product of the UL48 gene. All of the wild-type viruses gave rise to a strong VP11/12 signal; in contrast, no VP11/12 could be detected in cells infected with the UL46-null mutants ΔUL46galK and ΔUL46, although these samples displayed levels of VP16 comparable to those of the other virus isolates. These data confirmed that ΔUL46galK and ΔUL46 fail to produce VP11/12 and that this defect is rescued in the UL46-repaired virus RUL46. Comparable results were obtained with infected Vero cells (data not shown).
We then asked if inactivation of the UL46 gene abrogates the appearance of the ca. 90-kDa tyrosine-phosphorylated protein in lymphocytes exposed to HSV-infected fibroblasts. Jurkat cells were exposed to infected HEL fibroblasts, and tyrosine-phosphorylated proteins were examined as described above (Fig. 4B). HSV-2 HG52 and wild-type HSV-1 KOS-37 triggered the appearance of the prominent ca. 90-kDa tyrosine-phosphorylated protein as before; in marked contrast, the 90-kDa band was entirely absent from Jurkat cells exposed to fibroblasts infected with either of the UL46 null mutants. The 90-kDa band was, however, restored when the UL46 repair virus RUL46 was used. Similar data were obtained when NK-92 cells were exposed to HEL cells infected with the various HSV mutants (data not shown). Thus, VP11/12 is required for the appearance of the 90-kDa tyrosine-phosphorylated protein in lymphocytes exposed to HSV-infected fibroblasts. Of note, the UL46 null mutations also reduced the levels of the ca. 50- to 60-kDa and ca. 37-kDa tyrosine-phosphorylated species in the exposed Jurkat cells (Fig. 4B).
Tyrosine phosphorylation of VP11/12 is cell type specific.
A previous study has documented that HSV-1 and HSV-2 induce the production of multiple tyrosine-phosphorylated proteins during infection of Vero cells, including the immediate-early (IE) viral protein ICP22 (5); however, tyrosine phosphorylation of VP11/12 has not been reported previously. To determine if tyrosine phosphorylation of VP11/12 is lymphocyte specific, we first infected HEL fibroblasts with HSV-1 KOS and GHSV-UL46 and then compared extracts of these cells prepared 16 h postinfection to those of Jurkat and NK-92 cells exposed to parallel cultures of infected fibroblasts from 12 to 16 h after infection with the same viruses (Fig. 5). The ca. 90- and 120-kDa tyrosine-phosphorylated bands corresponding to UL46 and UL46-GFP were readily detected only in the lymphocytes, despite the fact that the HEL cells contained easily detectable levels of total VP11/12 (Fig. 4; also data not shown). However, upon heavy overexposure of the blots, we occasionally observed an extremely weak, ca. 90-kDa tyrosine-phosphorylated band in the infected fibroblasts (data not shown). These observations document that efficient tyrosine phosphorylation of VP11/12 is cell type specific, since it is readily detected in exposed lymphocytes but not in infected fibroblasts. The data obtained to date are consistent with the hypothesis that tyrosine phosphorylation of VP11/12 is lymphocyte specific, although we cannot exclude the possibility that this modification also occurs in additional cell types that have yet to be identified.
Phosphorylation of VP11/12 requires members of the Src kinase family, including the lymphocyte-specific Src kinase Lck.
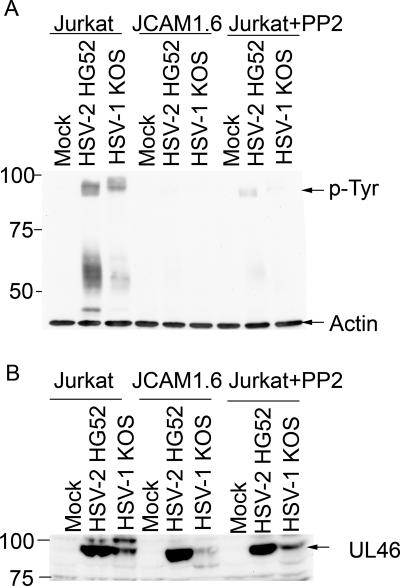
The cell-type-specific nature of VP11/12 tyrosine phosphorylation suggested that lymphocyte-specific protein tyrosine kinases might be involved. As one approach to examining this possibility, we asked if the Src family kinase Lck (lymphocyte-specific protein tyrosine kinase) plays any role. Lck is expressed in a tissue-specific fashion, being largely restricted to lymphocytes and neurons (34, 36, 37, 50). In T cells, Lck plays a key role in signaling through the TCR, where it phosphorylates the CD3 and TCR ζ chains of the TCR complex in response to TCR ligation (reviewed in reference 36). To determine if Lck is required for tyrosine phosphorylation of VP11/12 in Jurkat T cells, we exposed wild-type and Lck-deficient (JCaM 1.6) (14, 47) Jurkat cells to HEL fibroblasts infected with HSV-1 or HSV-2 as before; in addition, the Src family kinase inhibitor PP2 was added to some of the Jurkat cell cultures during the coincubation period. The lymphocytes were then removed, lysed, and analyzed for tyrosine-phosphorylated (Fig. 6A) and total (Fig. 6B) VP11/12 by Western blotting. Strikingly, the Lck-deficient JCaM 1.6 cells displayed severely reduced levels of 90-kDa tyrosine-phosphorylated VP11/12 compared to the parental Jurkat cell line (Fig. 6A); in contrast, the levels of total VP11/12 appeared to be roughly comparable in the two cell lines, at least in the samples derived from cells exposed to fibroblasts infected with HSV-2. Along the same lines, PP2 reduced the ca. 90-kDa phosphotyrosine signal in wild-type Jurkat cells (Fig. 6A) without greatly altering the overall levels of VP11/12 (Fig. 6B). These data suggest that Src family kinases, including Lck, are required for efficient tyrosine phosphorylation of VP11/12 in Jurkat T cells. However, we note that in some experiments, the total levels of VP11/12 were also somewhat reduced in the JCaM 1.6 cells and in the Jurkat cells treated with PP2 (data not shown), raising the possibility that Lck and/or other Src family kinases may additionally play a role in controlling the overall abundance of VP11/12 in these cells.
FIG. 6.
Src family kinase activity is required for efficient tyrosine phosphorylation of VP11/12 in Jurkat T cells. (A) HEL fibroblasts were either mock infected or infected with HSV-2 HG52 or HSV-1 KOS for 12 h in the absence of PP2. Jurkat cells or cells of the Lck-deficient Jurkat derivative JCaM 1.6 were then added to the cultures, and extracts prepared 4 h later were analyzed for tyrosine-phosphorylated proteins and β-actin by Western blotting as before. Where indicated, PP2 was added at the same time as the Jurkat cells. The mobility of the 90-kDa tyrosine-phosphorylated protein is indicated. (B) The Western blot described for panel A was stripped and reprobed with antisera raised against the HSV-2 186 UL46 protein.
UL46 gene products are not required for the HSV-induced blockade of TCR signaling or functional inactivation of NK-92 cells.
As outlined in the introduction, CTL and NK cells are functionally inactivated by HSV. As a first step in examining the role of VP11/12 in the biology of HSV-lymphocyte interactions, we asked if UL46 gene products are required for the HSV-induced blockade to TCR signaling in Jurkat T cells (42). Cultures of Jurkat cells were directly infected with 20 PFU/cell of wild-type KOS-37, the UL46-null mutant ΔUL46, and the UL46-repaired virus RUL46. Eight hours postinfection, each of the cultures was divided in half, and TCR signaling was initiated in one of the samples by cross-linking of the TCR using antibody OKT3, directed against CD3. Phosphorylation of the downstream ERK1/2 mitogen-activated protein kinase(s) was assessed by Western blotting using antibodies that detect phosphorylated and total ERK1/2 (Fig. 7A). As expected (42), ERK phosphorylation in response to TCR ligation was severely impaired in cells infected with KOS-37 and RUL46, confirming the block to TCR signaling imposed by wild-type HSV-1. We found that ΔUL46 was as effective as KOS-37 and RUL46 in blocking ERK phosphorylation (Fig. 7A and B), indicating that UL46 is not required for the HSV-induced signaling blockade in Jurkat T cells.
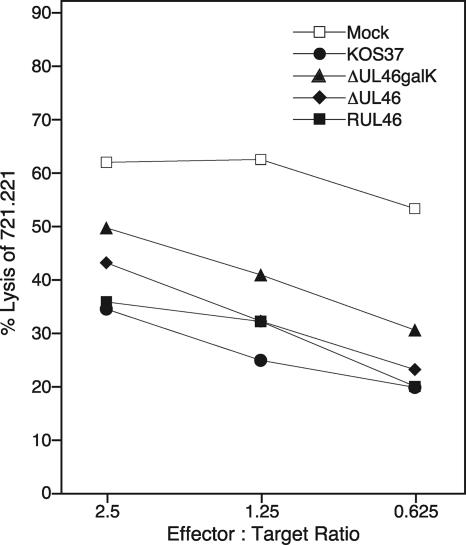
We also asked if UL46 is required for the ability of HSV-infected fibroblasts to attenuate the lytic activity of NK cells. Preliminary experiments indicated that the lytic activity of NK-92 cells toward the major histocompatibility complex class I-low B-cell line 721.221 is severely reduced following exposure of the NK-92 cells to HEL fibroblasts infected with wild-type HSV-1 or HSV-2 (data not shown). To examine the contribution of VP11/12 to this inhibition, NK-92 cells were exposed to HEL cells infected with wild-type KOS-37, the UL46-null mutants ΔUL46galK and ΔUL46, or the UL46-repaired virus RUL46 from 12 to 16 h postinfection. The NK-92 cells were then removed and assayed for their ability to lyse 51Cr-labeled 721.211 target cells in a 51Cr release assay (Fig. 8). We found that the UL46 mutants could not be distinguished from wild-type KOS-37 or the UL46 repair virus RUL46 in this assay: with all these viruses, infected HEL cells reduced the lytic activity of NK-92 cells to similar degrees relative to that for mock-infected controls. Thus, UL46 is not required for HSV-induced inactivation of the lytic activity of the NK-92 cell line under the conditions of our assay.
FIG. 8.
UL46 is not required for HSV-induced inhibition of NK-92 cells. HEL fibroblasts were either mock infected or infected with the indicated virus for 12 h. NK-92 cells were exposed to the fibroblasts from 12 to 16 h postinfection; then they were removed and analyzed for lytic activity against 721.221 target cells in a chromium release assay at various effector-to-target cell ratios. Data points are means from quadruplicate samples within a single experiment.
Deletion of VP11/12 does not alter viral IE gene expression in lymphocytes.
Previous studies have suggested that VP11/12 modulates viral IE gene expression in nonlymphoid cells. VP11/12 enhances the ability of HSV VP16 to transactivate viral IE promoters in transient transfection assays (22, 30), and one report indicates that VP11/12 also blocks IE promoter activity in the absence of VP16 in such assays (22). Consistent with these observations, VP11/12 physically interacts with VP16 (22, 51). Although such effects of VP11/12 on IE gene expression have not been convincingly demonstrated in the context of HSV-infected cells (59, 60), it seemed possible that tyrosine phosphorylation of VP11/12 might alter the ability of VP11/12 to stimulate HSV IE gene expression. To test this possibility, we infected Jurkat cells with varied multiplicities of cell-free KOS-37, ΔUL46, and RUL46 and compared the amounts of the IE protein ICP27 (normalized to β-actin) at 4 h postinfection (Fig. 9). We were unable to detect any difference in the ICP27 levels between cells infected with the UL46-null mutant ΔUL46 and those infected with the parental or repaired viral isolate KOS-37 or RUL46, respectively. Thus, UL46 has little if any effect on the expression of this IE gene under the conditions of our assay.
DISCUSSION
The data presented in this communication document that cells representing three distinct lymphocyte lineages (NK, T, and B cells) display dramatically increased levels of tyrosine-phosphorylated proteins following exposure to HSV-infected fibroblasts. Our results further demonstrate that a substantial fraction of this increase is due to tyrosine phosphorylation of the ca. 90-kDa HSV tegument protein VP11/12, encoded by gene UL46. In many experiments, we also detected novel tyrosine-phosphorylated proteins of ca. 55 to 60 kDa and 37 kDa, which have yet to be identified. One possibility is that one or more of these proteins corresponds to a truncated form of VP11/12, a possibility that is consistent with the detection of UL46-derived peptides in the ca. 60-kDa fraction in one experiment. However, we note that the 55- to 60-kDa species do not react with a polyclonal antibody directed against VP11/12 (Fig. 2B), and deletion of the UL46 gene did not completely abrogate production of at least some of these species (Fig. 4B). Hence, further studies are required to identify the origin of these bands.
Tyrosine phosphorylation of VP11/12 appears to be cell type specific, since it is not readily detected in the fibroblasts or epithelial cells that we have examined to date. A previous report showed that the HSV IE protein ICP22 is tyrosine phosphorylated in several nonlymphoid cell lines (5); however, as far as we are aware, our data provide the first example of tyrosine phosphorylation of an HSV tegument protein. VP11/12 and many other HSV tegument proteins have long been known as phosphoproteins (25, 33, 45, 59), but until now it has been generally assumed that they are phosphorylated solely on serine and/or threonine residues by viral (US3 and UL13) and/or cellular serine-threonine kinases (29, 33). Our data now indicate that HSV VP11/12 also serves as a substrate of one or more tyrosine or dual-specificity kinases, in a cell-type-specific fashion.
The marked cell type specificity of VP11/12 tyrosine phosphorylation raises the possibility that cell-type-specific protein kinases are responsible. Consistent with this hypothesis, our evidence suggests that the Src family tyrosine kinase Lck, which is largely restricted to lymphocytes and some neurons (34, 36, 37, 50), is required for the modification of VP11/12 in Jurkat T cells. Thus, tyrosine phosphorylation of VP11/12 was severely suppressed by the Src family kinase inhibitor PP2 and in the Lck-deficient Jurkat derivative JCaM 1.6. Although these data do not necessarily imply that Lck directly phosphorylates VP11/12, they do suggest that Lck activity is required for this event. Lck plays a critical role in signaling through the TCR by phosphorylating the immunoreceptor tyrosine-based activation motifs (ITAM) in the CD3 and TCR ζ chains of the TCR complex following TCR ligation (36), leading to recruitment and activation of Zap70 and downstream signaling events. Intriguingly, Lck plays a similar role in the activation of NK cells through a subset of NK activating receptors including Nkp46, which signal through the ITAM of CD3 ζ (reviewed in reference 32). Moreover, related Src kinases, including Lyn, play analogous roles in signaling through the B-cell receptor (BCR) in B lymphocytes, by phosphorylating the ITAM of the Igα and Igβ signaling subunits of the BCR (reviewed in reference 56). However, unlike receptor signaling in T and NK cells, HSV infection of B cells does not appear to alter BCR signaling (16). It will therefore be interesting to determine if Src family kinases are also required for tyrosine phosphorylation of VP11/12 in NK and B cells.
As noted above, Lck is normally activated in T cells in response to external signals such as TCR ligation. However, extremely robust Lck-dependent tyrosine phosphorylation of VP11/12 occurs in the absence of TCR engagement. These data raise the possibility that HSV infection may activate Lck (and perhaps other lymphocyte-specific Src family kinases) in the absence of receptor engagement. In this context, it is noteworthy that HSV infection has previously been shown to activate several Src family members in nonlymphoid cells in a process that requires the viral IE protein ICP0 and is modulated by the viral US3 and UL13 kinases (28); however, Lck and other lymphocyte-specific family members were not tested in that study. Moreover, exposure of T cells to HSV-infected fibroblasts has been reported to induce the phosphorylation of p38 and Jun N-terminal protein kinase in the absence of TCR stimulation (43), illustrating that HSV infection can activate certain signaling pathways in lymphocytes. We currently seek to determine if HSV infection activates Lck and/or other lymphocyte-specific Src family kinases in T and NK cells, and if so, which viral proteins are required.
The role that tyrosine phosphorylation of VP11/12 plays in the biology of HSV-lymphocyte interactions remains to be defined. Based on previously published data, it seemed possible that this modification might serve to modulate viral gene expression in lymphocytes. VP11/12 binds to the HSV virion transactivator VP16 (22, 51), a tegument protein that plays a central role in launching viral IE gene expression (55), and this interaction has been suggested to augment VP16-induced transactivation (22, 30). However, we were unable to detect any effect of VP11/12 deletion on the expression of the viral IE protein ICP27 in Jurkat cells, indicating that any such effect on gene expression from the incoming viral genome is likely to be minor. It will be interesting to determine if the VP11/12 molecules produced de novo within the infected lymphocytes are tyrosine phosphorylated, and if so, whether this modification alters their interactions with other tegument proteins and/or assembly into progeny virions.
Perhaps a more plausible possibility is that VP11/12 alters one or more signaling pathways within the lymphocyte for the benefit of the virus. For example, as reviewed in the introduction, HSV functionally inactivates CTL and NK cells, and current evidence implicates one or more tegument proteins as the inactivating agent(s). We have shown that HSV mutants lacking VP11/12 retain the ability to block TCR signaling in Jurkat T cells and inhibit the cytolytic activity of NK-92 cells against 720.221 B-cell targets (Fig. 7), documenting that VP11/12 is not essential for HSV-induced inactivation of these lymphocytes. However, this result does not necessarily exclude a role for VP11/12 in modulating lymphocyte function. It is possible that HSV encodes several proteins each of which is sufficient to functionally inactivate lymphocytes, a redundancy that could mask the effects of deleting UL46. Alternatively, UL46 may alter lymphocyte functions other than receptor-induced activation of cytolytic activity. Given the key role that phosphorylation by Src family kinases plays in regulating lymphocyte activity, experiments that focus on possible interactions between VP11/12 and receptor-proximal signaling molecules may prove informative.
Acknowledgments
We thank David Leib, Mark Willard, and Yukihiro Nishiyama for providing the KOS-37 BAC, the HSV-1 mutant GHSV-UL46, and the anti-UL46 antibody, respectively. Protein identification by mass spectrometry was performed at the Institute for Biomolecular Design, University of Alberta, Edmonton, Alberta, Canada.
This work was funded by operating grants from the Canadian Institutes of Health Research (J.R.S.), the University of Alberta Hospital Foundation (G.Z. and J.R.S.), the Provincial Laboratory for Public Health of Alberta (G.Z. and J.R.S.), and NIH (K.R.J.). M.J.W. is supported by a graduate scholarship from the Alberta Heritage Foundation for Medical Research, and G.Z. held postdoctoral fellowships from the Canadian Institutes for Health Research and the Alberta Heritage Foundation for Medical Research. J.R.S. holds a Canada Research Chair in Molecular Virology.
Footnotes
Published ahead of print on 16 April 2008.
REFERENCES
- 1.Ambagala, A. P., J. C. Solheim, and S. Srikumaran. 2005. Viral interference with MHC class I antigen presentation pathway: the battle continues. Vet. Immunol. Immunopathol. 1071-15. [DOI] [PubMed] [Google Scholar]
- 2.Arnon, T. I., G. Markel, and O. Mandelboim. 2006. Tumor and viral recognition by natural killer cells receptors. Semin. Cancer Biol. 16348-358. [DOI] [PubMed] [Google Scholar]
- 3.Aubert, M., E. M. Krantz, and K. R. Jerome. 2006. Herpes simplex virus genes Us3, Us5, and Us12 differentially regulate cytotoxic T lymphocyte-induced cytotoxicity. Viral Immunol. 19391-408. [DOI] [PubMed] [Google Scholar]
- 4.Benedict, C. A., P. S. Norris, and C. F. Ware. 2002. To kill or be killed: viral evasion of apoptosis. Nat. Immunol. 31013-1018. [DOI] [PubMed] [Google Scholar]
- 5.Blaho, J. A., C. S. Zong, and K. A. Mortimer. 1997. Tyrosine phosphorylation of the herpes simplex virus type 1 regulatory protein ICP22 and a cellular protein which shares antigenic determinants with ICP22. J. Virol. 719828-9832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Branco, F. J., and N. W. Fraser. 2005. Herpes simplex virus type 1 latency-associated transcript expression protects trigeminal ganglion neurons from apoptosis. J. Virol. 799019-9025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cartier, A., E. Broberg, T. Komai, M. Henriksson, and M. G. Masucci. 2003. The herpes simplex virus-1 Us3 protein kinase blocks CD8 T cell lysis by preventing the cleavage of Bid by granzyme B. Cell Death Differ. 101320-1328. [DOI] [PubMed] [Google Scholar]
- 8.Confer, D. L., G. M. Vercellotti, D. Kotasek, J. L. Goodman, A. Ochoa, and H. S. Jacob. 1990. Herpes simplex virus-infected cells disarm killer lymphocytes. Proc. Natl. Acad. Sci. USA 873609-3613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Court, D. L., J. A. Sawitzke, and L. C. Thomason. 2002. Genetic engineering using homologous recombination. Annu. Rev. Genet. 36361-388. [DOI] [PubMed] [Google Scholar]
- 10.Dargan, D. J., A. H. Patel, and J. H. Subak-Sharpe. 1995. PREPs: herpes simplex virus type 1-specific particles produced by infected cells when viral DNA replication is blocked. J. Virol. 694924-4932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Divito, S., T. L. Cherpes, and R. L. Hendricks. 2006. A triple entente: virus, neurons, and CD8+ T cells maintain HSV-1 latency. Immunol. Res. 36119-126. [DOI] [PubMed] [Google Scholar]
- 12.Ertl, H. C. J. 2003. Viral immunology, p. 1201-1228. In W. E. Paul (ed.), Fundamental immunology, 5th ed. Lippincott, Williams & Wilkins, New York, NY.
- 13.Gierasch, W. W., D. L. Zimmerman, S. L. Ward, T. K. Vanheyningen, J. D. Romine, and D. A. Leib. 2006. Construction and characterization of bacterial artificial chromosomes containing HSV-1 strains 17 and KOS. J. Virol. Methods 135197-206. [DOI] [PubMed] [Google Scholar]
- 14.Goldsmith, M. A., and A. Weiss. 1987. Isolation and characterization of a T-lymphocyte somatic mutant with altered signal transduction by the antigen receptor. Proc. Natl. Acad. Sci. USA 846879-6883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gupta, A., J. J. Gartner, P. Sethupathy, A. G. Hatzigeorgiou, and N. W. Fraser. 2006. Anti-apoptotic function of a microRNA encoded by the HSV-1 latency-associated transcript. Nature 44282-85. [DOI] [PubMed] [Google Scholar]
- 16.Han, J. Y., D. D. Sloan, M. Aubert, S. A. Miller, C. H. Dang, and K. R. Jerome. 2007. Apoptosis and antigen receptor function in T and B cells following exposure to herpes simplex virus. Virology 359253-263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hill, A., P. Jugovic, I. York, G. Russ, J. Bennink, J. Yewdell, H. Ploegh, and D. Johnson. 1995. Herpes simplex virus turns off the TAP to evade host immunity. Nature 375411-415. [DOI] [PubMed] [Google Scholar]
- 18.Hui, C. K., and G. K. Lau. 2005. Immune system and hepatitis B virus infection. J. Clin. Virol. 34(Suppl. 1)S44-S48. [DOI] [PubMed] [Google Scholar]
- 19.Jerome, K. R., R. Fox, Z. Chen, A. E. Sears, H. Lee, and L. Corey. 1999. Herpes simplex virus inhibits apoptosis through the action of two genes, Us5 and Us3. J. Virol. 738950-8957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jerome, K. R., J. F. Tait, D. M. Koelle, and L. Corey. 1998. Herpes simplex virus type 1 renders infected cells resistant to cytotoxic T-lymphocyte-induced apoptosis. J. Virol. 72436-441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jugovic, P., A. M. Hill, R. Tomazin, H. Ploegh, and D. C. Johnson. 1998. Inhibition of major histocompatibility complex class I antigen presentation in pig and primate cells by herpes simplex virus type 1 and 2 ICP47. J. Virol. 725076-5084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kato, K., T. Daikoku, F. Goshima, H. Kume, K. Yamaki, and Y. Nishiyama. 2000. Synthesis, subcellular localization and VP16 interaction of the herpes simplex virus type 2 UL46 gene product. Arch. Virol. 1452149-2162. [DOI] [PubMed] [Google Scholar]
- 23.Kavathas, P., F. H. Bach, and R. DeMars. 1980. Gamma ray-induced loss of expression of HLA and glyoxalase I alleles in lymphoblastoid cells. Proc. Natl. Acad. Sci. USA 774251-4255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Klenerman, P., and A. Hill. 2005. T cells and viral persistence: lessons from diverse infections. Nat. Immunol. 6873-879. [DOI] [PubMed] [Google Scholar]
- 25.Lemaster, S., and B. Roizman. 1980. Herpes simplex virus phosphoproteins. II. Characterization of the virion protein kinase and of the polypeptides phosphorylated in the virion. J. Virol. 35798-811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Leopardi, R., C. Van Sant, and B. Roizman. 1997. The herpes simplex virus 1 protein kinase US3 is required for protection from apoptosis induced by the virus. Proc. Natl. Acad. Sci. USA 947891-7896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Levy, J. A. 2006. HIV pathogenesis: knowledge gained after two decades of research. Adv. Dent. Res. 1910-16. [DOI] [PubMed] [Google Scholar]
- 28.Liang, Y., and B. Roizman. 2006. State and role of SRC family kinases in replication of herpes simplex virus 1. J. Virol. 803349-3359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Matsuzaki, A., Y. Yamauchi, A. Kato, F. Goshima, Y. Kawaguchi, T. Yoshikawa, and Y. Nishiyama. 2005. US3 protein kinase of herpes simplex virus type 2 is required for the stability of the UL46-encoded tegument protein and its association with virus particles. J. Gen. Virol. 861979-1985. [DOI] [PubMed] [Google Scholar]
- 30.McKnight, J. L., P. E. Pellett, F. J. Jenkins, and B. Roizman. 1987. Characterization and nucleotide sequence of two herpes simplex virus 1 genes whose products modulate α-trans-inducing factor-dependent activation of α genes. J. Virol. 61992-1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Minaker, R. L., K. L. Mossman, and J. R. Smiley. 2005. Functional inaccessibility of quiescent herpes simplex virus genomes. Virol. J. 285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Moretta, A., C. Bottino, M. Vitale, D. Pende, C. Cantoni, M. C. Mingari, R. Biassoni, and L. Moretta. 2001. Activating receptors and coreceptors involved in human natural killer cell-mediated cytolysis. Annu. Rev. Immunol. 19197-223. [DOI] [PubMed] [Google Scholar]
- 33.Morrison, E. E., Y. F. Wang, and D. M. Meredith. 1998. Phosphorylation of structural components promotes dissociation of the herpes simplex virus type 1 tegument. J. Virol. 727108-7114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Omri, B., P. Crisanti, M. C. Marty, F. Alliot, R. Fagard, T. Molina, and B. Pessac. 1996. The Lck tyrosine kinase is expressed in brain neurons. J. Neurochem. 671360-1364. [DOI] [PubMed] [Google Scholar]
- 35.Orange, J. S. 2002. Human natural killer cell deficiencies and susceptibility to infection. Microbes Infect. 41545-1558. [DOI] [PubMed] [Google Scholar]
- 36.Palacios, E. H., and A. Weiss. 2004. Function of the Src-family kinases, Lck and Fyn, in T-cell development and activation. Oncogene 237990-8000. [DOI] [PubMed] [Google Scholar]
- 37.Perlmutter, R. M., J. D. Marth, D. B. Lewis, R. Peet, S. F. Ziegler, and C. B. Wilson. 1988. Structure and expression of lck transcripts in human lymphoid cells. J. Cell. Biochem. 38117-126. [DOI] [PubMed] [Google Scholar]
- 38.Posavad, C. M., J. J. Newton, and K. L. Rosenthal. 1994. Infection and inhibition of human cytotoxic T lymphocytes by herpes simplex virus. J. Virol. 684072-4074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Posavad, C. M., J. J. Newton, and K. L. Rosenthal. 1993. Inhibition of human CTL-mediated lysis by fibroblasts infected with herpes simplex virus. J. Immunol. 1514865-4873. [PubMed] [Google Scholar]
- 40.Posavad, C. M., and K. L. Rosenthal. 1992. Herpes simplex virus-infected human fibroblasts are resistant to and inhibit cytotoxic T-lymphocyte activity. J. Virol. 666264-6272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shiina, M., and B. Rehermann. 2006. Hepatitis C vaccines: inducing and challenging memory T cells. Hepatology 431395-1398. [DOI] [PubMed] [Google Scholar]
- 42.Sloan, D. D., J. Y. Han, T. K. Sandifer, M. Stewart, A. J. Hinz, M. Yoon, D. C. Johnson, P. G. Spear, and K. R. Jerome. 2006. Inhibition of TCR signaling by herpes simplex virus. J. Immunol. 1761825-1833. [DOI] [PubMed] [Google Scholar]
- 43.Sloan, D. D., and K. R. Jerome. 2007. Herpes simplex virus remodels T cell receptor signaling resulting in p38-dependent selective synthesis of interleukin-10. J. Virol. 8112504-12514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sloan, D. D., G. Zahariadis, C. M. Posavad, N. T. Pate, S. J. Kussick, and K. R. Jerome. 2003. CTL are inactivated by herpes simplex virus-infected cells expressing a viral protein kinase. J. Immunol. 1716733-6741. [DOI] [PubMed] [Google Scholar]
- 45.Stevely, W. S., M. Katan, V. Stirling, G. Smith, and D. P. Leader. 1985. Protein kinase activities associated with the virions of pseudorabies and herpes simplex virus. J. Gen. Virol. 66661-673. [DOI] [PubMed] [Google Scholar]
- 46.Stone, S. F., P. Price, and M. A. French. 2006. Cytomegalovirus (CMV)-specific CD8+ T cells in individuals with HIV infection: correlation with protection from CMV disease. J. Antimicrob. Chemother. 57585-588. [DOI] [PubMed] [Google Scholar]
- 47.Straus, D. B., and A. Weiss. 1992. Genetic evidence for the involvement of the lck tyrosine kinase in signal transduction through the T cell antigen receptor. Cell 70585-593. [DOI] [PubMed] [Google Scholar]
- 48.Szilágyi, J. F., and J. Berriman. 1994. Herpes simplex virus L particles contain spherical membrane-enclosed inclusion vesicles. J. Gen. Virol. 751749-1753. [DOI] [PubMed] [Google Scholar]
- 49.Tigges, M. A., S. Leng, D. C. Johnson, and R. L. Burke. 1996. Human herpes simplex virus (HSV)-specific CD8+ CTL clones recognize HSV-2-infected fibroblasts after treatment with IFN-γ or when virion host shutoff functions are disabled. J. Immunol. 1563901-3910. [PubMed] [Google Scholar]
- 50.Van Tan, H., G. Allee, C. Benes, J. V. Barnier, J. D. Vincent, and R. Fagard. 1996. Expression of a novel form of the p56lck protooncogene in rat cerebellar granular neurons. J. Neurochem. 672306-2315. [DOI] [PubMed] [Google Scholar]
- 51.Vittone, V., E. Diefenbach, D. Triffett, M. W. Douglas, A. L. Cunningham, and R. J. Diefenbach. 2005. Determination of interactions between tegument proteins of herpes simplex virus type 1. J. Virol. 799566-9571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Warming, S., N. Costantino, D. L. Court, N. A. Jenkins, and N. G. Copeland. 2005. Simple and highly efficient BAC recombineering using galK selection. Nucleic Acids Res. 33e36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Willard, M. 2002. Rapid directional translocations in virus replication. J. Virol. 765220-5232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wonderlich, J., G. Shearer, A. Livingstone, and A. Brooks. 2006. Induction and measurement of cytotoxic T lymphocyte activity, p. 3.11.1-3.11.23. In J. E. Coligan, B. E. Bierer, D. H. Margulies, E. M. Shevach, and W. Strober (ed.), Current protocols in immunology. John Wiley & Sons Inc., New York, NY. [DOI] [PubMed]
- 55.Wysocka, J., and W. Herr. 2003. The herpes simplex virus VP16-induced complex: the makings of a regulatory switch. Trends Biochem. Sci. 28294-304. [DOI] [PubMed] [Google Scholar]
- 56.Xu, Y., K. W. Harder, N. D. Huntington, M. L. Hibbs, and D. M. Tarlinton. 2005. Lyn tyrosine kinase: accentuating the positive and the negative. Immunity 229-18. [DOI] [PubMed] [Google Scholar]
- 57.York, I., C. Roop, D. W. Andrews, S. R. Riddel, F. L. Graham, and D. C. Johnson. 1994. A cytosolic herpes simplex virus protein inhibits antigen presentation to CD8− T lymphocytes. Cell 77525-535. [DOI] [PubMed] [Google Scholar]
- 58.York, I. A., and D. C. Johnson. 1993. Direct contact with herpes simplex virus-infected cells results in inhibition of lymphokine-activated killer cells because of cell-to-cell spread of virus. J. Infect. Dis. 1681127-1132. [DOI] [PubMed] [Google Scholar]
- 59.Zhang, Y., and J. L. McKnight. 1993. Herpes simplex virus type 1 UL46 and UL47 deletion mutants lack VP11 and VP12 or VP13 and VP14, respectively, and exhibit altered viral thymidine kinase expression. J. Virol. 671482-1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhang, Y., D. A. Sirko, and J. L. McKnight. 1991. Role of herpes simplex virus type 1 UL46 and UL47 in αTIF-mediated transcriptional induction: characterization of three viral deletion mutants. J. Virol. 65829-841. [DOI] [PMC free article] [PubMed] [Google Scholar]