Abstract
Patients with von Hippel-Lindau (VHL) disease develop tumors in a range of tissues, but existing mouse models of Vhlh mutation have failed to reproduce these lesions. Epididymal cystadenomas arise frequently in VHL patients, but VHL mutation alone is believed to be insufficient for tumor formation, implying a requirement for cooperating mutations in epididymal pathogenesis. Here we show that epididymal cystadenomas from VHL patients frequently also lack expression of the PTEN tumor suppressor and display activation of phosphatidylinositol 3-kinase (PI3K) pathway signaling. Strikingly, while conditional inactivation of either Vhlh or Pten in epithelia of the mouse genital tract fails to produce a tumor phenotype, their combined deletion causes benign genital tract tumors with regions of squamous metaplasia and cystadenoma. The latter are histologically identical to lesions found in VHL patients. Importantly, these lesions are characterized by expansion of basal stem cells, high levels of expression and activity of HIF1α and HIF2α, and dysregulation of PI3K signaling. Our studies suggest a model for cooperative tumor suppression in which inactivation of PTEN facilitates epididymal cystadenoma genesis initiated by loss of VHL.
Inheritance of a mutant allele of the von Hippel-Lindau (VHL) tumor suppressor gene results in the VHL familial cancer syndrome, in which patients are predisposed to develop a variety of tumors, including central nervous system and retinal hemangioblastomas, endolymphatic sac tumors, phaeochromocytomas, pancreatic microcystic adenomas, neuroendocrine tumors, clear cell renal cell carcinomas, epididymal cystadenomas in males, and broad ligament cystadenomas (also known as APMO [adnexal papillary cystadenoma of probable mesonephric origin]) in females (15, 21). The last two tumor types occur in tissues that derive from the embryonic mesonephric duct (also called the Wolffian duct), suggesting that similar genetic events may underlie tumorigenesis in these two tissues.
While epididymal cystadenomas are benign lesions that occur very rarely in the general population, macroscopic lesions arise in approximately 50% of male VHL patients (4), and it is believed that microscopic lesions may be present in almost all male VHL patients (9). These lesions are characterized by deletions or mutations of the remaining wild-type VHL allele (7, 9, 38). Consistent with the known role of pVHL as a component of a ubiquitin ligase complex that targets the hypoxia-inducible factor α (HIFα) transcription factors for normoxic proteolytic degradation (26), epididymal cystadenomas display accumulation of HIF1α and HIF2α, as well as high levels of expression of the HIFα-inducible proteins CA9, GLUT-1, and vascular endothelial growth factor (VEGF) (9, 20). Similar to conclusions drawn from analysis of other tumor-prone tissues in VHL patients (8, 25, 47, 48), mutational inactivation of VHL alone appears to be insufficient to cause the formation of an epididymal cystadenoma. Rather, while small VHL mutant lesions are detected frequently within the efferent ductules of VHL patients, frank cystadenomas occur relatively rarely (9). One interpretation of this result is that additional mutations may be required for tumor progression.
The phosphatase and tensin homologue (PTEN) tumor suppressor gene is mutated at high frequency in many different tumor types, and patients that inherit a mutant allele of PTEN (Cowden's disease) are predisposed to develop multiple hamartomatous and mucocutaneous lesions and are at increased risk of developing diverse neoplasms (reviewed in reference 2). Among other functions, PTEN acts as a lipid phosphatase to dephosphorylate phosphatidylinositol 3,4,5-triphosphate that is formed as a result of the activity of phosphatidylinositol 3-kinase (PI3K) enzymes (24). PTEN thereby opposes signal transduction by numerous cell surface receptors. Inactivation of PTEN results in constitutive activation of multiple signaling cascades that are activated by PI3K, AKT, mTOR, S6K, and c-Jun N-terminal kinases (2, 46) and leads to alteration of transcriptional programs that are controlled by FOXO family and Jun family transcription factors (1, 46). In general, loss of PTEN function and constitutive signaling via the PI3K signaling pathway result in cellular proliferation.
Here we demonstrate that loss of PTEN expression and activation of PI3K signaling occur frequently in epididymal cystadenomas from human VHL patients. While the individual deletion of Vhlh or Pten in genital epithelia of mice causes a relatively mild phenotype, Vhlh Pten double mutant mice develop tumors that contain regions of clear cell cystadenoma that mimic the epididymal clear cell cystadenomas that develop in human VHL patients. These studies highlight a novel cooperative genetic interaction with respect to tumor suppression by Vhlh and Pten.
MATERIALS AND METHODS
VHL patient epididymal cystadenomas.
Two paraffin-embedded epididymal cystadenoma biopsies from VHL patients were previously described (9), and one sample was obtained from the pathology collection of the French VHL Group.
Mouse genetics.
C57BL/6J Ksp1.3-Cre mice (37) were obtained from Peter Igarashi (UT Southwestern), BALB/c;129S mixed-background Vhlhfl/fl mice (12) were obtained from The Jackson Laboratory, and BALB/c Ptenfl/fl mice (18) were obtained from Andreas Trumpp (ISREC, Switzerland). Ksp1.3-Cre/+ mice were intercrossed with Vhlhfl/fl; Ptenfl/fl mice to generate Ksp1.3-Cre/+; Vhlhfl/+; Ptenfl/+ and +/+; Vhlhfl/+; Ptenfl/+ mice. These mice were subsequently intercrossed to provide sets of parents (that contained genetic contributions from all of the different strain backgrounds) from which Ksp1.3-Cre/+; Vhlhfl/fl; Pten+/+ (VhlhΔ/Δ), Ksp1.3-Cre/+; Vhlh+/+; Ptenfl/fl (PtenΔ/Δ), and Ksp1.3-Cre/+; Vhlhfl/fl; Ptenfl/fl (VhlhΔ/Δ PtenΔ/Δ) mice were generated. Littermate mice that lacked the Ksp1.3-Cre transgene were used as controls. B6.129S4-Gt(ROSA)26Sortm1Sor/J mice (ROSA26R mice) were obtained from The Jackson Laboratory. Mouse embryo fibroblasts were derived from embryonic day 13.5 wild-type (C57BL/6J background), Vhlhfl/fl, Ptenfl/fl, and Vhlhfl/fl; Ptenfl/fl mice by using standard techniques and were infected with high-titer adenovirus expressing Cre recombinase (17).
Immunohistochemistry and X-Gal staining.
Immunohistochemistry was performed as previously described (45), using the following antibodies: anti-CA9 (M75) (31), anti-involucrin (SY5; Sigma), anti-keratin 6 (PRB-169P; Covance), anti-keratin 14 (PRB-155P; Covance), anti-Notch4 (sc-5594; Santa Cruz Biotechnology), anti-p63 (clone 4A4; Lab Vision), anti-PTEN (9559; Cell Signaling Technology), anti-phospho-Ser240/244 S6 ribosomal protein (2215; Cell Signaling Technology), anti-phospho-Ser65 4E-BP1 (9451; Cell Signaling Technology), anti-von Willebrand factor (F3520; Sigma), and anti-mouse pVHLCT (45). Immunohistochemistry using anti-HIF1α (NB100-105; Novus Biologicals) and anti-HIF2α (PM8) (34) antibodies was performed using a Dako Cytomation catalyzed signal amplification system. X-Gal (5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside) staining was performed on 50-μm-thick cryosections of freshly frozen tissue as previously described (37).
Western blotting.
Whole epididymal protein extracts were prepared by powdering freshly frozen epididymis under liquid nitrogen, followed by homogenization in TNN buffer (25 mM Tris-HCl, pH 7.2, 150 mM sodium chloride, 10% glycerol, 1% NP-40, 10 mM sodium fluoride, 1 mM Na3VO4, 1 mM phenylmethylsulfonyl fluoride, 1 mM dithiothreitol, 10 μg/ml aprotinin). Thirty to 60 μg of boiled protein extract was run in 8 to 15% acrylamide gels, transferred to nitrocellulose membranes, and visualized by immunoblotting with the following antibodies: anti-phospho-Ser473-AKT (4058; Cell Signaling Technology), anti-AKT (sc-1619; Santa Cruz), anti-phospho-Thr202/Tyr204 ERK1/2 (9101; Cell Signaling Technology), anti-phospho-Ser9 GSK3β (2215; Cell Signaling Technology), anti-HIF2α (PM8) (34), anti-LDH-A (sc-27230; Santa Cruz), anti-PTEN (sc-7974; Santa Cruz), anti-phospho-Ser240/244 S6 ribosomal protein (2215; Cell Signaling Technology), anti-S6 ribosomal protein (2212; Cell Signaling Technology), and anti-mouse pVHLCT antibody (45). To detect GLUT-1 using anti-GLUT-1 antibody (ab14683; Abcam), protein lysates were not boiled prior to sodium dodecyl sulfate-polyacrylamide gel electrophoresis.
Real-time PCR analysis.
Total RNA was prepared from powdered tissue by use of an RNeasy minikit (Qiagen), and cDNA was prepared using random hexanucleotide primers and Ready-to-Go You-Prime first-strand beads (GE Healthcare). Genomic DNA was isolated by digesting powdered tissue overnight at 55°C in buffer containing 500 mg/ml proteinase K, 1% sodium dodecyl sulfate, 50 mM EDTA, 150 mM NaCl, and 10 mM Tris-HCl, pH 7.5, followed by phenol-chloroform treatment and precipitation with isopropanol. Real-time PCR analysis of cDNA and genomic DNA was conducted using Absolute Sybr green (Abgene) and the following primer pairs: for TAp63, 5′GTGGATGAACCTTCCGAAAA3′ and 5′GAGGAGCCGTTCTGAATCTG3′; for ΔNp63, 5′CAAAACCCTGGAAGCAGAAA3′ and 5′ GAGGAGCCGTTCTGAATCTG3′; for Glut-1, 5′GCTTATGGGCTTCTCCAAACT3′ and 5′GGTGACACCTCTCCCACATAC3′; for Vegf-a, 5′CTTGTTCAGAGCGGAGAAAGC3′ and 5′ACATCTGCAAGTACGTTCGTT3′; for Delta-1, 5′CAGGACCTTCTTTCGCGTATG3′ and 5′AAGGGGAATCGGATGGGGTT3′; for Jag-2, 5′GCGACCAGTACGGCAACAA3′ and 5′GGGACACACTCGTCACAGAA3′; for Hes-1, 5′CCAGCCAGTGTCAACACGA3′ and 5′AATGCCGGGAGCTATCTTTCT3′; for Hey-1, 5′GTCTCCCATCTCAACAACTACG3′ and 5′GTGTGGGTGATGTCCGAAGG3′; for Hey-2, 5′CAATTCACCGACAACTACCTCTC3′ and 5′GCCTTTTCTAACTTGGCAGATCC3′; for 18S rRNA, 5′TGGCCGACCATAAACGATGCC3′ and 5′TGGTGGTGCCCTTCCGTCAAT3′; for the Vhlh locus, 5′ACAGTAGGACCACATGAAGATAGG3′ and 5′TCGAGCTTTACCCACCTCTCTAG3′; and for the p63 locus, 5′GAGGAGCCGTTCTGAATCTG3′ and 5′TAATGCCAATTTTATTGAGC3′.
RESULTS
Loss of PTEN expression in epididymal cystadenomas in VHL patients.
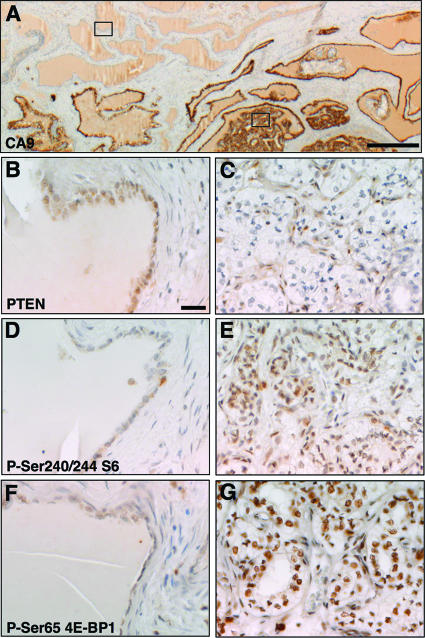
To investigate pathways that may contribute to the development of human epididymal cystadenoma, we identified 15 microscopic VHL mutant clear cell papillary cystadenomas in epididymal biopsies from three patients with VHL disease. The lesions stained positively for the HIFα target gene CA9 (Fig. 1A) (9). While the tumor suppressor protein PTEN was detected by immunostaining in normal epididymal tubules that lacked expression of CA9 (Fig. 1B), its expression was reduced or absent in tumor cells of 11 of 15 cystadenomas (Fig. 1C) but maintained in the stromal tissue of these tumors, likely due to the absence of mutations in the stroma. Since PTEN negatively regulates signaling by the PI3K-AKT-mTOR signaling pathway, we examined the expression of downstream markers of activation of this pathway in VHL patient epididymal cystadenomas. In comparison to normal tubules, tumors exhibited increased staining for phospho-Ser240/244 S6 ribosomal protein (Fig. 1D and E) and for phospho-Ser65 4E-BP1 (Fig. 1F and G), consistent with the idea that the PI3K signaling pathway is hyperactivated in these lesions. Thus, mechanisms that lead to loss or reduction of the PTEN protein and/or activation of PI3K pathway signaling may be involved in the formation of cystadenomas in VHL patients.
FIG. 1.
Activation of PI3K pathway signaling in VHL patient epididymal cystadenomas. Immunohistochemical staining of an epididymal section from a VHL patient is shown. (A) VHL-negative cells were identified by staining with an antibody against CA9. (B, D, and F) Staining of normal epididymal tubules from the region shown by the upper left box in panel A. (C, E, and G) Staining of a region of VHL-negative epididymal cystadenoma from the region shown by the lower right box in panel A. Antibodies against PTEN (B and C), phospho-Ser240/244 ribosomal S6 protein (D and E), or phospho-Ser65 4E-BP1 (F and G) were used for staining. Panels B to G are at the same magnification. Bars, 100 μm.
Cystadenoma and squamous metaplasia following Vhlh and Pten deletion.
To test this hypothesis and to model the genetic changes that occur in human cystadenoma, mice were generated in which Vhlh and Pten were deleted singly and in combination in epithelial cells of the genital tract by crossing Vhlhfl/fl and Ptenfl/fl mice to Ksp1.3-Cre transgenic mice (37). In addition to expression in the kidney, this transgene directs Cre recombinase expression to the Wolffian and Müllerian ducts of the mesonephros during embryonic development (37). The Wolffian duct gives rise to the epididymis, vas deferens, and vesicular glands in males, and the Müllerian duct gives rise to the oviducts, uterus, and cervix in females (51). X-Gal staining of mice carrying the Ksp1.3-Cre transgene and a ROSA26 Cre reporter transgene (ROSA26R) (40), in which transcription of the lacZ gene occurs only after Cre-mediated excision of a loxP-flanked transcriptional termination sequence, confirmed that the Ksp-1.3 transgene directs Cre activity to the epithelia of genital tissues (Fig. 2). Western blotting of epididymal tissue demonstrated that Ksp1.3-Cre efficiently reduced the expression of pVHL and PTEN proteins (see Fig. 10L). These mice displayed a range of striking phenotypes in tissues of the male and female genital tracts.
FIG. 2.

Ksp1.3-Cre directs Cre-mediated recombination to epithelia of the male and female genital tracts. The images show X-Gal staining of epididymal tubules (A and B), vas deferens (C and D), vesicular gland (E and F), endometrial lumen (G and H), and endometrial glands (I and J) from transgenic mice containing the ROSA26R Cre reporter transgene (A, C, E, G, and I) or containing Ksp1.3-Cre and ROSA26R transgenes (B, D, F, H, and J). All panels are the same magnification. Bar, 50 μm.
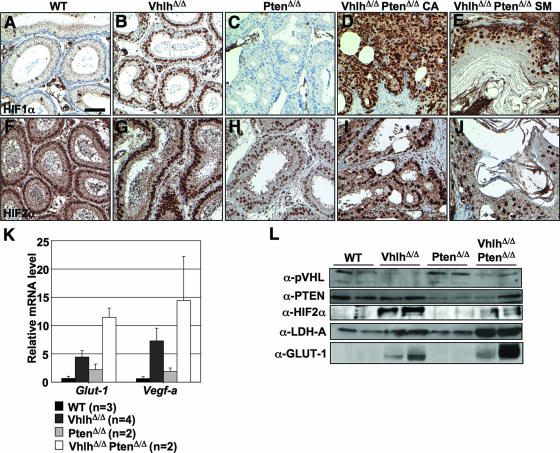
FIG. 10.
Activation of HIFα activity in epididymal tumors in VhlhΔ/Δ PtenΔ/Δ mice. (A to J) Immunohistochemical staining for HIF1α (A to E) and HIF2α (F to J) in epididymal tubules of 8-week-old wild-type (A and F), VhlhΔ/Δ (B and G), PtenΔ/Δ (C and H), and VhlhΔ/Δ PtenΔ/Δ (D, E, I, and J) mice. Panels D and I display regions of cystadenoma (CA), and panels E and J display regions of squamous metaplasia (SM). Panels A to J are all at the same magnification. Bar, 50 μm. (K) Real-time quantitative PCR analysis of mRNA expression in epididymides from 8- to 14-week-old wild-type, VhlhΔ/Δ, PtenΔ/Δ, and VhlhΔ/Δ PtenΔ/Δ mice, using specific primers for Glut-1 and Vegf-a. Expression changes were normalized against the expression level of the 18S rRNA. Data represent means ± standard deviations (n > 2) or means ± ranges (n = 2). Each sample was analyzed in triplicate. (L) Western blotting analysis of protein lysates of epididymides from two independent mice of each of the genotypes shown in the figure. Blots were probed with antibodies against pVHL, PTEN, HIF2α, LDH-A, GLUT-1, phospho-Ser473-AKT, AKT1, phospho-Ser240/244 S6 ribosomal protein, and S6 ribosomal protein. Note that the apparent lack of decrease in PTEN expression in the second VhlhΔ/Δ PtenΔ/Δ mouse (last lane) likely reflects the high stromal component of this particular tumor (data not shown). Stromal tissue, which is not subject to Cre-mediated gene deletion, exhibits high PTEN expression (data not shown).
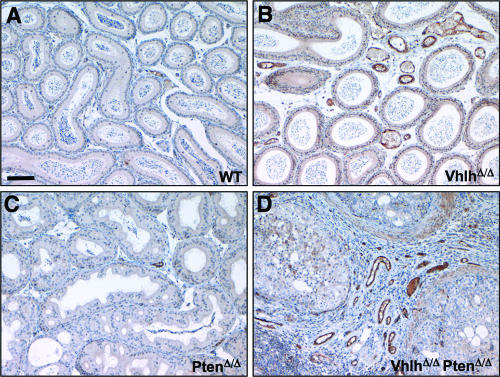
In Ksp1.3-Cre; Vhlhfl/fl mice (hereafter referred to as VhlhΔ/Δ mice), the male and female genital tracts appeared morphologically and histologically normal (Fig. 3D and H; 4B, F, and J; and 5D), with the exception of increased vascularization, as shown by the aberrant presence of dilated blood capillaries surrounding epididymal tubules (Fig. 3H and 4B, arrowheads). Staining using an antibody against the endothelial cell marker von Willebrand factor confirmed that these structures represent blood vessels (Fig. 6B). This finding is consistent with the known role of VHL inactivation in promoting angiogenesis and with the elevated levels of Vegf-a mRNA in this tissue of VhlhΔ/Δ mice (see Fig. 10K). Moreover, these findings are also consistent with the results of analysis of human VHL epididymal tissue that argue that loss of VHL alone causes neoangiogenesis but is not sufficient to cause formation of cystadenoma (9).
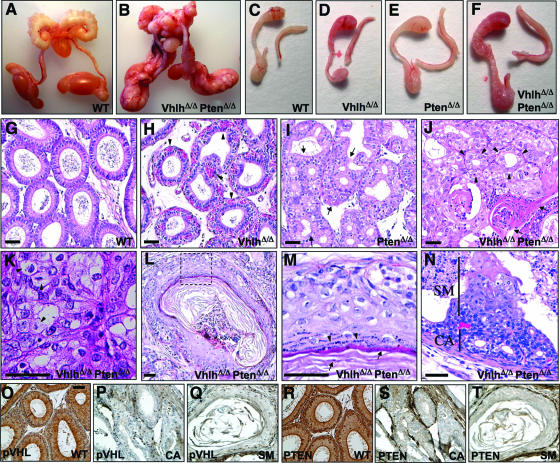
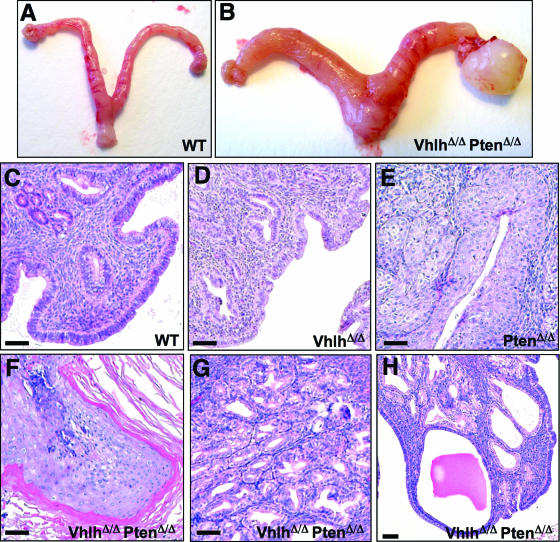
FIG. 3.
Phenotypic analysis of Vhlh and Pten deletion in the male genital tract. (A and B) Genital tracts of 14-week-old male wild-type (A) and VhlhΔ/Δ PtenΔ/Δ (B) mice. (C to F) Epididymides and vasa deferentia of 8-week-old wild-type (C), VhlhΔ/Δ (D), PtenΔ/Δ (E), and VhlhΔ/Δ PtenΔ/Δ (F) mice. (G to J) Hematoxylin- and eosin-stained sections of epididymides of 8-week-old wild-type (G), VhlhΔ/Δ (H), PtenΔ/Δ (I), and VhlhΔ/Δ PtenΔ/Δ (J) mice. Arrowheads in panel H point to blood vessels, regions between arrows in panel I highlight regions of hyperplasia, arrowheads in panel J point to adenoid structures in a region of cystadenoma, and arrows in panel J point to a region of squamous differentiation. (K) Region of cystadenoma in the epididymis of a 14-week-old VhlhΔ/Δ PtenΔ/Δ mouse with examples of clear cells shown by arrowheads. (L) Epididymal tubule showing squamous metaplasia in a 14-week-old VhlhΔ/Δ PtenΔ/Δ mouse. (M) Zoom of the dotted boxed region in panel L. (N) Early lesion in an epididymal tubule of an 8-week-old VhlhΔ/Δ PtenΔ/Δ mouse. A region that appears to be a precursor of cystadenoma is labeled CA, and a region of cells undergoing squamous metaplasia is labeled SM. (O to Q) Immunohistochemical staining using an antibody against pVHL in epididymal tubules from a wild-type (O) mouse or from regions of cystadenoma (P) and squamous metaplasia (Q) from a VhlhΔ/Δ PtenΔ/Δ mouse. (R to T) Immunohistochemical staining using an antibody against Pten in epididymal tubules from a wild-type (R) mouse or from regions of cystadenoma (S) and squamous metaplasia (T) from a VhlhΔ/Δ PtenΔ/Δ mouse. Panels O to T are at the same magnification. Bars, 50 μm.
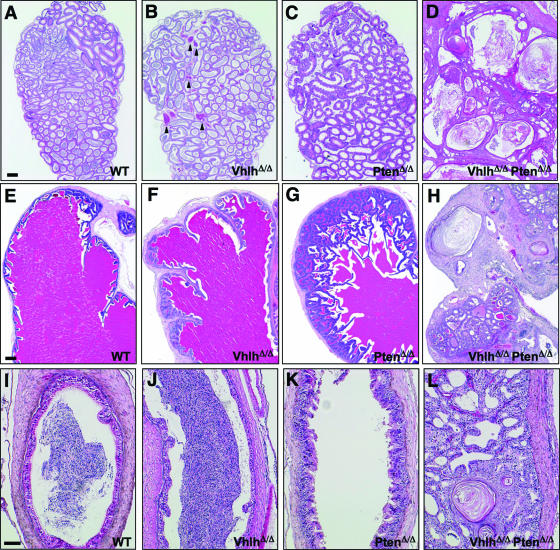
FIG. 4.
Histological analysis of Vhlh and/or Pten deletion in the male genital tract. (A to D) Sections of caput epididymides from 14-week-old wild-type (A), VhlhΔ/Δ (B), PtenΔ/Δ (C), and VhlhΔ/Δ PtenΔ/Δ (D) mice. Bar, 200 μm. (E to H) Sections of vesicular glands from 8-week-old wild-type (E), VhlhΔ/Δ (F), PtenΔ/Δ (G), and VhlhΔ/Δ PtenΔ/Δ (H) mice. Bar, 200 μm. (I to L) Sections of vasa deferentia from 11-week-old wild-type (I), VhlhΔ/Δ (J), PtenΔ/Δ (K), and VhlhΔ/Δ PtenΔ/Δ (L) mice. Bar, 20 μm.
FIG. 5.
Phenotypic analysis of Vhlh and Pten deletion in the female genital tract. (A and B) Genital tracts of 20-week-old female wild-type (A) and VhlhΔ/Δ PtenΔ/Δ (B) mice. Note the thickened uterus and presence of an ovarian duct tumor in panel B. (C to H) Hematoxylin- and eosin-stained sections of uteri of 20-week-old wild-type (C), VhlhΔ/Δ (D), PtenΔ/Δ (E), and VhlhΔ/Δ PtenΔ/Δ (F to H) mice. (F) Region of squamous metaplasia of the endometrial lumen; (G) region of hyperplasia of endometrial glands; (H) endometrial hyperplasia with cyst formation. Bars, 50 μm.
FIG. 6.
Increased vascularization in epididymides of VhlhΔ/Δ and VhlhΔ/Δ PtenΔ/Δ mice. (A to D) Immunohistochemical staining for the endothelial cell marker von Willebrand factor in epididymides of littermate 11-week-old wild-type (A), VhlhΔ/Δ (B), PtenΔ/Δ (C), and VhlhΔ/Δ PtenΔ/Δ (D) mice. All panels are the same magnification. Bar, 100 μm.
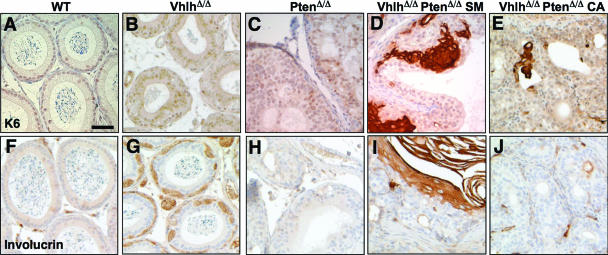
Genital tissues of Ksp1.3-Cre; Ptenfl/fl mice (hereafter referred to as PtenΔ/Δ mice) were enlarged (Fig. 3E and data not shown). Epithelial hyperplasia was observed in the epididymis (Fig. 3I and 4C), vesicular glands (Fig. 4G), and vas deferens (Fig. 4K) in male mice. Mice aged up to 1 year did not show any tumor formation, demonstrating that the loss of Pten alone is not sufficient to cause transition from epithelial hyperplasia to a tumorigenic state. Female PtenΔ/Δ mice displayed hyperplasia of the endometrial glands and lumen (Fig. 5E), similar to the phenotypes that arise in this tissue in Pten+/− mice (33, 41). Endometrial hyperplasia was accompanied by partial squamous differentiation, as demonstrated by the expression of keratin 14 (K14) (Fig. 7C), which normally is expressed in basal layer keratinocytes that maintain proliferative capacity, and of keratin 6 (K6) (Fig. 7G), which is expressed by proliferating keratinocytes during wound healing and in culture (6). These lesions lacked expression of involucrin (Fig. 7K), a component of cornified epithelia and a marker of terminally differentiated keratinocytes (5).
FIG. 7.
Squamous differentiation in endometrial lumen of PtenΔ/Δ and VhlhΔ/Δ PtenΔ/Δ mice. Shown is immunohistochemical staining for K14 (A to D), K6 (E to H), and involucrin (I to L) in endometrial lumina of wild-type (A, E, and I), VhlhΔ/Δ (B, F, and J), PtenΔ/Δ (C, G, and K), and VhlhΔ/Δ PtenΔ/Δ (D, H, and L) mice. All panels are the same magnification. Bar, 50 μm.
In contrast to the relatively mild phenotypes of VhlhΔ/Δ and PtenΔ/Δ single mutant mice, all Ksp1.3-Cre; Vhlhfl/fl; Ptenfl/fl (hereafter referred to as VhlhΔ/Δ PtenΔ/Δ mice) double mutant mice developed benign mixed adenosquamous genital tract tumors. In 8-week-old male mice, genital tissues were noticeably enlarged (Fig. 3F and data not shown), and by 14 weeks of age, the entire genital tract was grossly deformed (Fig. 3B). Histological analysis of tumors in the epididymis (Fig. 3J and 4D), vesicular glands (Fig. 4H), and vas deferens (Fig. 4L) revealed gross disorganization of tissue morphology. Strikingly, tumors contained regions of cystadenoma characterized by adenoid structures (Fig. 3J) that were histologically identical to the cystadenoma lesions that arise in the epididymides of VHL patients. Consistent with the role of pVHL in suppressing angiogenesis, epididymal cystadenomas exhibited a highly vascularized stromal component (Fig. 6D). Epididymal cystadenomas and renal cell carcinomas in VHL patients are characterized by clear cell morphology, which results from the accumulation of lipids and glycogen, which prevent staining by the cytoplasmic dye eosin. Similarly, regions of cystadenoma in VhlhΔ/Δ PtenΔ/Δ mice displayed clear cell morphology (Fig. 3K).
Intermingled with regions of cystadenoma in VhlhΔ/Δ PtenΔ/Δ mice were regions of squamous differentiation (Fig. 3J and L) that recapitulate epidermal differentiation. Evidence for this conclusion includes hematoxylin and eosin staining that revealed the presence of a multilayered epidermis-like structure, blue-staining keratin droplets in terminally differentiated cells (Fig. 3M, arrowheads), and pink-staining secreted keratin fibers (Fig. 3M, arrows). Immunohistochemical staining for the expression of the squamous differentiation markers K14, K6, and involucrin (Fig. 8J and 9D and I) confirmed that lesions in VhlhΔ/Δ PtenΔ/Δ mice express markers of proliferating and terminally differentiated keratinocytes. In contrast, regions of cystadenoma did not display markers of squamous differentiation (Fig. 9E and J).
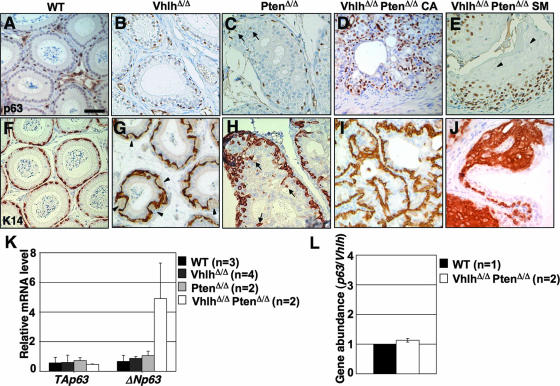
FIG. 8.
Expansion of basal cells in epididymal tumors in VhlhΔ/Δ PtenΔ/Δ mice. (A to J) Immunohistochemical staining for p63 (A to E) and K14 (F to J) in epididymal tubules of 8-week-old wild-type (A and F), VhlhΔ/Δ (B and G), PtenΔ/Δ (C and H), and VhlhΔ/Δ PtenΔ/Δ (D, E, I, and J) mice. Panels D and I display regions of cystadenoma (CA), and panels E and J display regions of squamous metaplasia (SM). Arrows in panel C highlight p63-positive cells that are not localized to the basal layer, and arrowheads in panel E show examples of terminally differentiated cells that do not express p63. Arrowheads in panel G highlight small blood vessels, and arrows in panel H highlight K14-positive cells that are not localized to the basal layer. Panels A to J are all at the same magnification. Bar, 50 μm. (K) Real-time quantitative PCR analysis of mRNA expression in epididymides from 8- to 14-week-old wild-type, VhlhΔ/Δ, PtenΔ/Δ, and VhlhΔ/Δ PtenΔ/Δ mice, using isoform-specific primers for TAp63 and ΔNp63. Expression changes were normalized against the expression level of the 18S rRNA. Data represent means ± standard deviations (n > 2) or means ± ranges (n = 2). Each sample was analyzed in triplicate. (L) Real-time quantitative PCR analysis of epididymal genomic DNAs from wild-type (n = 1) and VhlhΔ/Δ PtenΔ/Δ (n = 2) mice, using primers that detect intronic regions of the p63 and Vhlh genes (the latter region lies outside the loxP-flanked sequences and is not deleted by Cre). The ratio of p63 to Vhlh gene abundance is presented as the mean ± range. Each sample was analyzed in triplicate.
FIG. 9.
Squamous differentiation in the epididymis of VhlhΔ/Δ PtenΔ/Δ mice. Immunohistochemical staining was done for K6 (A to E) and involucrin (F to J) in epididymides of wild-type (A and F), VhlhΔ/Δ (B and G), PtenΔ/Δ (C and H), and VhlhΔ/Δ PtenΔ/Δ (D, E, I, and J) mice. Panels D and I display regions of squamous metaplasia (SM), and panels E and J display regions of cystadenoma (CA). All panels are the same magnification. Bar, 50 μm.
Immunohistochemical staining using antibodies against pVHL (Fig. 3O to Q) and PTEN (Fig. 3R to T) confirmed the deletion of both genes in regions of cystadenoma and squamous metaplasia. In the epididymides of 8-week-old mice, we could identify discrete sites that presumably represent the earliest stage of development of these lesions. Regions of cystadenoma and squamous differentiation appear to coarise within otherwise morphologically normal epididymal tubules. Regions that are apparently precursors of cystadenoma occur at the basal surface of the tubule (Fig. 3N, area labeled CA), and layers of cells that are undergoing squamous differentiation overlie these cystadenoma precursors and project toward the center of the epididymal tubule (Fig. 3N, area labeled SM). Thus, regions of cystadenoma and squamous differentiation appear to arise at the same site.
Tumor phenotypes were also observed in the genital tracts of female VhlhΔ/Δ PtenΔ/Δ mice (Fig. 5B), including hyperplasia and full squamous differentiation of the uterine endometrial lumen (Fig. 5F and 7D, H, and L), endometrial hyperplasia (Fig. 5G), and endometrial hyperplasia with cyst formation (Fig. 5H). The latter histologically resembles the cystadenoma and cystic lesions seen in the broad ligament in human female VHL patients. Cysts and squamous epithelial hyperplasia also occurred in the cervixes and oviducts of VhlhΔ/Δ PtenΔ/Δ mice (data not shown).
Genital tracts of VhlhΔ/+ PtenΔ/+ mice were indistinguishable from those of wild-type mice. In contrast, the genital tracts of VhlhΔ/Δ PtenΔ/+ and VhlhΔ/+ PtenΔ/Δ mice were initially indistinguishable from those of VhlhΔ/Δ and PtenΔ/Δ mice, respectively, but by 6 months of age these mice developed histologically identical lesions to those seen in VhlhΔ/Δ PtenΔ/Δ mice (data not shown). However, whereas VhlhΔ/Δ PtenΔ/Δ mice developed tumors simultaneously in all genital tract epithelia, tumors arose only at discrete sites in VhlhΔ/Δ PtenΔ/+ and VhlhΔ/+ PtenΔ/Δ mice. Therefore, it is likely that these tumors arose due to somatic mutation of the remaining wild-type Vhlh or Pten allele.
In summary, genital tract lesions seen in VhlhΔ/Δ PtenΔ/Δ mice recapitulate the clear cell cystadenoma lesions that are found in male and female human VHL patients. Squamous metaplasia, however, is not a clinical feature of human VHL disease.
Expansion of basal cells in tumors in VhlhΔ/Δ PtenΔ/Δ mice.
Pseudostratified epithelia of the genitourinary tract, including the epididymal tubules, vesicular glands, vas deferens, prostate, and urothelium, are lined by a population of p63-expressing basal cells (13, 36). While the functions of basal cells are incompletely characterized for most tissues, detailed studies of the prostate have shown that basal cells act as progenitor cells that are necessary for prostatic development (10). Based on these findings and the findings that p63-expressing basal cells are important for the formation of other epithelia, including the epidermis and salivary, lachrymal, and mammary glands (27, 52), it is believed that basal cells may function in adult tissues as progenitor or stem cell-like cells that have the capacity to self-renew as well as to differentiate into different lineages and which thereby act to maintain epithelia (reviewed in reference 10). This idea, however, remains to be proven formally.
To gain insight into the molecular changes that cause normal genital tract epithelia to be reprogrammed to form cystadenomas and epidermis-like structures in VhlhΔ/Δ PtenΔ/Δ mice, we focused further analysis on the epididymis. Since deletion of Pten within the prostatic epithelium caused expansion of basal cells (49), we analyzed expression of the basal cell markers p63 and K14 (49, 50) in the epididymis. This analysis revealed that epididymal tubules in VhlhΔ/Δ mice (Fig. 8B and G) and PtenΔ/Δ mice (Fig. 8C and H) contained normal and slightly increased numbers of basal cells, respectively. In VhlhΔ/Δ mice, the pattern of staining of basal cells appears distorted by the presence of capillaries associated with the tubules (Fig. 8G, arrowheads). In PtenΔ/Δ mice, basal cells were not restricted to the basal layer of tubules but were present within regions of hyperproliferation (Fig. 8C and H, arrows). This finding is consistent with the role of PTEN as a regulator of cell migration (19). Mislocalization of basal cells may contribute to the altered architecture of epithelia in PtenΔ/Δ mice. In contrast to the relatively mild alteration in basal cell number and localization in VhlhΔ/Δ and PtenΔ/Δ mice, dramatically increased numbers of cells that stained positively for p63 and K14 were observed throughout regions of cystadenoma (Fig. 8D and I) and squamous metaplasia (Fig. 8E and J) in the epididymides of VhlhΔ/Δ PtenΔ/Δ mice.
p63 exists as two isoforms, ΔNp63 and TAp63, that are generated by alternate promoters. ΔNp63 is the isoform that is expressed in basal cells in genital tract epithelia (13, 36) and has been shown to be necessary for the development of epithelial tissues (42). Consistent with these findings, real-time PCR analysis demonstrated that ΔNp63, not TAp63, is the isoform that is expressed by the expanded pool of basal cells in the epididymides of VhlhΔ/Δ PtenΔ/Δ mice (Fig. 8K). Real-time quantitative PCR analysis of genomic DNA demonstrated that the increased expression of ΔNp63 mRNA does not result from amplification of the p63 gene (Fig. 8L).
Collectively, these findings suggest that an expansion of basal cells may underlie tumor formation in genital epithelia in VhlhΔ/Δ PtenΔ/Δ mice. Cystadenomas appear to result from basal cell proliferation without differentiation, whereas regions of squamous metaplasia contain abundant basal cells in basal layers but also cells that express markers of epidermal differentiation in overlying layers of cells. Thus, combined loss of Vhlh and Pten must alter molecular signaling cascades that influence the proliferation and differentiation of basal cells.
Elevated HIFα activity in tumors in VhlhΔ/Δ PtenΔ/Δ mice.
To investigate such potential molecular changes, we first analyzed the status of activation of the HIFα pathway. Consistent with the known role of pVHL in degrading HIFα transcription factors, immunohistochemical staining revealed strong nuclear accumulation of HIF1α in epididymal tubules in VhlhΔ/Δ mice (Fig. 10B) and in regions of cystadenoma (Fig. 10D) and squamous metaplasia (Fig. 10E) in VhlhΔ/Δ PtenΔ/Δ mice but not in tubules from wild-type or PtenΔ/Δ mice (Fig. 10A and C). While nuclear staining for HIF2α was observed even in wild-type and PtenΔ/Δ mice (Fig. 10F and H), VhlhΔ/Δ (Fig. 10G) and VhlhΔ/Δ PtenΔ/Δ (Fig. 10I and J) mice displayed more intense staining. Upregulation of HIF2α in epididymal tissues of VhlhΔ/Δ and VhlhΔ/Δ PtenΔ/Δ mice was confirmed by Western blotting (Fig. 10L). Surprisingly, the extent of upregulation of HIF2α in VhlhΔ/Δ PtenΔ/Δ mice was less than that in VhlhΔ/Δ mice, suggesting that a loss of PTEN activity may alter the balance of expression of HIFα family members in response to loss of pVHL. HIF1α was not detectable by Western blotting using three commercially available antibodies. Real-time quantitative PCR analysis of the HIFα-inducible genes Glut-1 and Vegf-a (Fig. 10K) and Western blotting for the HIFα-inducible proteins GLUT-1 and LDH-A (Fig. 10L) confirmed that HIFα activity is induced by Vhlh and by Vhlh Pten gene deletion. The trend toward increased HIFα target gene abundance in the epididymides of VhlhΔ/Δ PtenΔ/Δ mice in comparison to those of VhlhΔ/Δ mice likely reflects the expansion of Vhlh-negative cells in VhlhΔ/Δ PtenΔ/Δ tumors. In summary, since VhlhΔ/Δ mouse epididymides display abundant HIFα activity but do not develop tumors, we conclude that tumor formation in VhlhΔ/Δ PtenΔ/Δ mice must involve pathways in addition to HIFα.
Vhlh influences downstream signaling following Pten deletion.
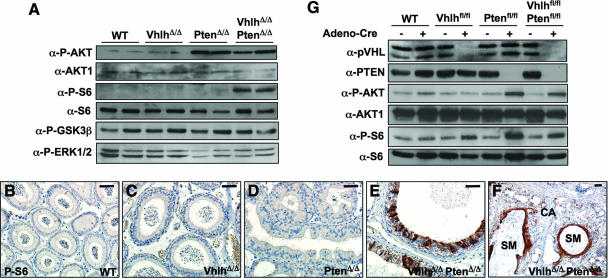
Since the PI3K signaling pathway promotes the proliferation and prevents the differentiation of stem cells and progenitor cells (reviewed in reference 44) and since tumors of VhlhΔ/Δ PtenΔ/Δ mice contain an expansion of basal cells, we investigated the activation status of the PI3K signaling cascade in epididymal tissue following Vhlh and/or Pten deletion. As expected, deletion of Pten alone or Pten and Vhlh together, but not of Vhlh alone, led to accumulation of phospho-Ser473-AKT, indicative of activation of PI3K (Fig. 11A). However, phospho-Ser240/244-S6 ribosomal protein, a downstream marker of mTOR and p70S6K activation, accumulated only in VhlhΔ/Δ PtenΔ/Δ mice, not in PtenΔ/Δ mice (Fig. 11A). This effect appears to be specific to S6 ribosomal protein, as Ser9 phosphorylation of glycogen synthase kinase 3β (GSK3β; a site targeted by AKT) and Thr202/Tyr204 phosphorylation of ERK1/2 were not altered by Vhlh and Pten deletion (Fig. 11A). Immunohistochemical staining confirmed that phospho-Ser240/244-S6 ribosomal protein expression is dysregulated in preneoplastic epididymal epithelium of VhlhΔ/Δ PtenΔ/Δ (Fig. 11E) mice but not in VhlhΔ/Δ (Fig. 11C) or PtenΔ/Δ (Fig. 11D) mice. Phospho-Ser240/244-S6 ribosomal protein expression is also dysregulated in regions of cystadenoma and is highly upregulated in regions of squamous metaplasia (Fig. 11F). The mechanism that underlies the cooperative effects of Vhlh and Pten mutation on S6 phosphorylation appears to be tissue specific, as Cre-mediated deletion of Pten alone in mouse embryo fibroblasts (Fig. 11G) or in the kidney (data not shown) was sufficient to induce phosphorylation of AKT and of S6 ribosomal protein in the absence of serum and this was not further modified by combined deletion of Vhlh.
FIG. 11.
Dysregulation of PI3K signaling in epididymal tumors in VhlhΔ/Δ PtenΔ/Δ mice. (A) Western blotting analysis of protein lysates from Fig. 10L, using antibodies against phospho-Ser473-AKT, AKT1, phospho-Ser240/244 S6 ribosomal protein, S6 ribosomal protein, phospho-Ser9 GSK3β, and phospho-Thr202/Tyr204 ERK1/2. (B to F) Immunohistochemical staining for phospho-Ser240/244 S6 ribosomal protein in epididymal tubules of 8-week-old wild-type (B), VhlhΔ/Δ (C), PtenΔ/Δ (D), and VhlhΔ/Δ PtenΔ/Δ (E and F) mice. Regions of cystadenoma (CA) and squamous metaplasia (SM) are labeled. Bar, 50 μm. (G) Western blotting analysis of protein lysates from wild-type, Vhlhfl/fl, Ptenfl/fl, and Vhlhfl/flPtenfl/fl mouse embryonic fibroblasts, with or without infection with adenovirus expressing Cre and growth for 3 days in medium containing no serum. Blots were probed with antibodies against pVHL, PTEN, phospho-Ser473-AKT, AKT1, phospho-Ser240/244 S6 ribosomal protein, and S6 ribosomal protein.
These findings suggest that pVHL normally functions in the epididymis to suppress the activation of downstream components of the PI3K signaling pathway. It is possible that pVHL may therefore limit basal cell proliferation in response to Pten mutation and PI3K and AKT activation. Combined mutation of Vhlh and Pten may allow mTOR and p70S6K activation, which may drive basal cell proliferation.
Elevated Notch signaling in tumors in VhlhΔ/Δ PtenΔ/Δ mice.
A striking feature of tumors in all genital tract tissues in VhlhΔ/Δ PtenΔ/Δ mice is the presence of epidermis-like tissue that arises from the normal simple epithelia that line these tissues. Epidermal differentiation is normally regulated by cross talk between the p63 transcription factor and the Notch signaling pathway that ensures the correct balance between keratinocyte self-renewal and differentiation (29). Notch downregulates expression of p63 to allow keratinocyte terminal differentiation, and p63 negatively regulates transcription by Notch to inhibit its ability to induce keratinocyte differentiation (29). Given that combined Vhlh and Pten deletion causes expansion of p63-expressing basal cells and also leads to HIF1α activation, it is noteworthy that HIF1α has been shown to promote Notch signaling by binding to the cleaved Notch intracellular domain at the promoters of Notch target genes, inducing their expression (11). HIF1α has also been shown to induce the expression of the Notch ligand Jag-2 (3). Finally, expression of the active intracellular domains of either Notch1 or Notch4 in transgenic mice induces epididymal hyperplasia (14, 23).
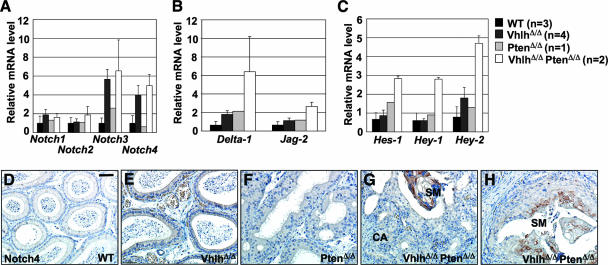
Given these connections between Notch, HIFα, hyperproliferation, and squamous differentiation, we investigated the status of Notch signaling pathways in VhlhΔ/Δ PtenΔ/Δ mice. Real-time quantitative PCR analysis revealed upregulation of mRNA expression of Notch3 and Notch4, but not Notch1 and Notch2, in epididymides of VhlhΔ/Δ and VhlhΔ/Δ PtenΔ/Δ mice (Fig. 12A). Interestingly, epididymides of VhlhΔ/Δ PtenΔ/Δ mice, but not those of the other genotypes, displayed increased mRNA levels of the Notch ligands Delta-1 and Jag-2 (Fig. 12B) as well as increased levels of the Notch target genes Hes-1, Hey-1, and Hey-2 (Fig. 12C). These findings suggest that a combination of upregulation of Notch receptors and ligands leads to activation of Notch signaling in VhlhΔ/Δ PtenΔ/Δ mice. While an antibody against the active intracellular domain of Notch1 revealed staining in spermatocytes, as previously demonstrated (28), no staining was observed within the tumor tissue (data not shown), suggesting that Notch1 is not activated in epididymides of VhlhΔ/Δ PtenΔ/Δ mice. However, consistent with mRNA analysis, immunohistochemical staining revealed that Notch4 protein expression was upregulated and restricted to regions of terminally differentiated squamous metaplasia in VhlhΔ/Δ PtenΔ/Δ mice (Fig. 12H). Interestingly, regions of squamous differentiation in VhlhΔ/Δ PtenΔ/Δ mice display high levels of p63 in basal layers of cells and transient amplifying keratinocyte-like cells, but p63 expression is absent in terminally differentiated keratinocyte-like cells (Fig. 8E, arrowheads). In the epidermis, similar downregulation of p63 expression is mediated by Notch (16), supporting the notion that aberrant Notch4 signaling may underlie the squamous differentiation in VhlhΔ/Δ PtenΔ/Δ mice.
FIG. 12.
Activation of Notch signaling in epididymal tumors in VhlhΔ/Δ PtenΔ/Δ mice. (A to C) Real-time quantitative PCR analysis of mRNA expression in epididymides from 8- to 14-week-old wild-type, VhlhΔ/Δ, PtenΔ/Δ, and VhlhΔ/Δ PtenΔ/Δ mice, using specific primers for Notch1, Notch2, Notch3, and Notch4 (A), for Delta-1 and Jag-2 (B), and for Hes-1, Hey-1, and Hey-2 (C). Expression changes were normalized against the expression level of the 18S rRNA. Data represent means ± standard deviations (n > 2) or means ± ranges (n = 2). Each sample was analyzed in triplicate. (D to H) Immunohistochemical staining for Notch4 in epididymal tubules of 8-week-old wild-type (D), VhlhΔ/Δ (E), PtenΔ/Δ (F), and VhlhΔ/Δ PtenΔ/Δ (G and H) mice. Regions of cystadenoma (CA) and squamous metaplasia (SM) are labeled. Bar, 50 μm.
DISCUSSION
Previous mouse models of Vhlh mutation surprisingly did not develop the characteristic tumors that arise in human VHL patients. However, subsequent careful analysis of VHL patient tissue revealed that kidney, central nervous system, endolymphatic duct, and epididymal tissues all contain many sites of VHL mutation but relatively few frank tumors, suggesting that mutations in addition to VHL mutation may be required for tumor formation (8, 25, 47, 48). In this study, we present proof of concept of this idea of cooperative tumor suppression in VHL disease. We describe a mouse model of VHL-associated epididymal clear cell cystadenoma that results from combined deletion of the Vhlh and Pten tumor suppressor genes.
Consistent with a previous study of VHL patients that demonstrated that loss of pVHL function and HIFα activation alone are insufficient to induce the formation of frank epididymal cystadenoma (9), we found that deletion of Vhlh in the epididymis and other genital tract epithelia in mice is sufficient to activate HIFα but does not induce epithelial cell proliferation. We show here that epididymal cystadenomas in human VHL patients frequently display loss of PTEN expression, which may contribute to tumor formation in these lesions. Indeed, while deletion of Pten in the mouse genital tract caused mild epithelial hyperplasia, combined deletion of Vhlh and Pten together caused tumorigenic transformation. Clear cell cystadenomas that histologically resemble the clear cell cystadenomas that form in VHL disease patients arose in the epididymal ducts, vas deferens, vesicular glands, endometrium, and oviducts of VhlhΔ/Δ PtenΔ/Δ mice. Another striking histological feature of tumors in VhlhΔ/Δ PtenΔ/Δ mice is the presence of squamous differentiation that fully recapitulates the structure of the epidermis. While lesions in VHL patients have not been reported to involve squamous differentiation and we did not observe markers of squamous differentiation in VHL patient epididymal cystadenomas (data not shown), loss of PTEN expression and activation of the PI3K signaling pathway have been reported to occur in a variety of human squamous cell carcinomas (32, 39, 43).
What causes genital tract epithelia to be reprogrammed to form cystadenomas and epidermis-like structures upon Vhlh and Pten deletion? Part of the answer to this question likely lies in the dramatically increased number of basal cells in tumors of VhlhΔ/Δ PtenΔ/Δ mice. One possible signaling cascade that may regulate basal cell proliferation is the PI3K pathway. Several studies (reviewed in reference 44) have demonstrated that PI3K, AKT, and mTOR activities are necessary for maintenance and proliferation of stem and progenitor cells and that loss of PTEN leads to enhanced stem cell proliferation and expansion of tissue progenitor cells, including basal cells of the prostate (49). In this respect, we uncovered a cooperative role of Vhlh and Pten mutation in inducing the phosphorylation of ribosomal protein S6, a downstream effector of the PI3K-mTOR-S6K signaling pathway. The presence of Vhlh may thereby limit basal cell expansion in Pten mutant mice. However, Pten deletion in the absence of Vhlh leads to high levels of mTOR and S6K signaling, which may then drive the observed uncontrolled basal cell proliferation. The molecular nature of this genetic cooperation remains unclear and appears to be tissue specific. One possible mechanism that may apply to the epididymis may be that loss of pVHL leads to HIFα-mediated upregulation of growth factors, such as platelet-derived growth factor and transforming growth factor alpha, that could act in an autocrine or paracrine fashion on Pten-deficient cells to hyperactivate signal transduction through the PI3K-AKT-mTOR-S6K signaling cascade.
Another signaling pathway that may be relevant to tumor formation in the mouse model is the Notch pathway. Tumors of VhlhΔ/Δ PtenΔ/Δ mice displayed upregulation of mRNAs encoding the Notch3 and Notch4 receptors and the Notch ligands Delta-1 and Jag-2 and of several Notch-inducible target genes, suggesting that combined Vhlh and Pten deletion leads to aberrant Notch activity in genital epithelia. Since Notch signaling in keratinocytes promotes epidermal differentiation (22, 30, 35), we reasoned that aberrant Notch activation might contribute to the squamous differentiation phenotype of VhlhΔ/Δ PtenΔ/Δ mice. Indeed, we observed specific expression of Notch4 in regions of squamous differentiation. Thus, the epididymides of VhlhΔ/Δ PtenΔ/Δ mice reproduce at least two key elements that normally underlie the differentiation of the epidermis: the expanded population of p63-expressing basal cells may function analogously to the p63-expressing basal cells in the epidermis and may be acted upon by aberrant Notch4 signaling, resulting in squamous differentiation.
As discussed by Gläsker et al. (9), the tissue type of origin of cystadenomas in VHL patients is controversial, with various studies proposing different mesonephric duct-derived tissue, including the epididymal ducts, efferent ductules, vas deferens, testicular appendix, epididymal appendix, and paradidymis. Our findings may reconcile these observations. Since deletion of Vhlh and Pten in the mesonephric duct of mice induces cystadenoma formation in diverse genital tract tissues, it is possible that cystadenomas in VHL patients could form in all of the above-mentioned tissues. Slightly different molecular and cellular events may underlie tumorigenesis at different sites. For example, while tumors derived in mice are characterized by an expansion of basal cells, analysis of three epididymal cystadenomas from human VHL patients did not reveal p63-positive cells (data not shown). This finding is not unexpected, as these particular tumors are likely to have arisen from efferent ductules (9), an epithelium that is not characterized by the presence of basal cells. It will be interesting in further studies to analyze the activation status of the PI3K signaling pathway and of pathways including p63 and Notch in a larger sample of tumors from different mesonephric duct-derived tissues in VHL patients. The awareness that lesions in VHL patients may potentially occur at sites in the genital tract in addition to the epididymis may translate into improved diagnosis for these patients.
In summary, our study provides evidence that additional mutations can cooperate with loss of VHL function in tumorigenesis and suggests that mutations that lead to activation of the PI3K signaling pathway may contribute to the pathology of genital tract tumors associated with VHL deficiency. Since inheritance of VHL mutations predisposes patients to the development of a variety of tumors, including renal cell carcinoma, it is tempting to speculate that mutations that activate the PI3K pathway may contribute to tumor progression more generally in VHL disease. If so, our study suggests that drugs that interfere with the active PI3K signaling pathway may potentially be of therapeutic benefit in genital tract lesions and other cancers that involve VHL mutation.
Acknowledgments
We thank members of our laboratory for discussions. We are grateful to Peter Igarashi, Andreas Trumpp, Sabine Werner, Jan Zevada, and Patrick Pollard for providing mice and reagents, to Vincent Molonié, Sophie Ferlicot, and Marie-Christine Rousselet for providing pathological specimens, to Matteo Montani for pathological diagnosis, and to Claudio Thoma for discussions and comments on the manuscript.
This work was supported by grants to W.K. from the Josef Steiner Foundation and the Swiss National Science Foundation.
Footnotes
Published ahead of print on 12 May 2008.
REFERENCES
- 1.Burgering, B. M., and R. H. Medema. 2003. Decisions on life and death: FOXO forkhead transcription factors are in command when PKB/Akt is off duty. J. Leukoc. Biol. 73689-701. [DOI] [PubMed] [Google Scholar]
- 2.Cantley, L. C., and B. G. Neel. 1999. New insights into tumor suppression: PTEN suppresses tumor formation by restraining the phosphoinositide 3-kinase/AKT pathway. Proc. Natl. Acad. Sci. USA 964240-4245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chi, J. T., Z. Wang, D. S. Nuyten, E. H. Rodriguez, M. E. Schaner, A. Salim, Y. Wang, G. B. Kristensen, A. Helland, A. L. Borresen-Dale, A. Giaccia, M. T. Longaker, T. Hastie, G. P. Yang, M. J. van de Vijver, and P. O. Brown. 2006. Gene expression programs in response to hypoxia: cell type specificity and prognostic significance in human cancers. PLoS Med. 3e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Choyke, P. L., G. M. Glenn, J. P. Wagner, I. A. Lubensky, K. Thakore, B. Zbar, W. M. Linehan, and M. M. Walther. 1997. Epididymal cystadenomas in von Hippel-Lindau disease. Urology 49926-931. [DOI] [PubMed] [Google Scholar]
- 5.Eckert, R. L., J. F. Crish, T. Efimova, S. R. Dashti, A. Deucher, F. Bone, G. Adhikary, G. Huang, R. Gopalakrishnan, and S. Balasubramanian. 2004. Regulation of involucrin gene expression. J. Investig. Dermatol. 12313-22. [DOI] [PubMed] [Google Scholar]
- 6.Fuchs, E. 1995. Keratins and the skin. Annu. Rev. Cell Dev. Biol. 11123-153. [DOI] [PubMed] [Google Scholar]
- 7.Gilcrease, M. Z., L. Schmidt, B. Zbar, L. Truong, M. Rutledge, and T. M. Wheeler. 1995. Somatic von Hippel-Lindau mutation in clear cell papillary cystadenoma of the epididymis. Hum. Pathol. 261341-1346. [DOI] [PubMed] [Google Scholar]
- 8.Gläsker, S., R. R. Lonser, M. G. Tran, B. Ikejiri, J. A. Butman, W. Zeng, P. H. Maxwell, Z. Zhuang, E. H. Oldfield, and A. O. Vortmeyer. 2005. Effects of VHL deficiency on endolymphatic duct and sac. Cancer Res. 6510847-10853. [DOI] [PubMed] [Google Scholar]
- 9.Gläsker, S., M. G. Tran, S. B. Shively, B. Ikejiri, R. R. Lonser, P. H. Maxwell, Z. Zhuang, E. H. Oldfield, and A. O. Vortmeyer. 2006. Epididymal cystadenomas and epithelial tumourlets: effects of VHL deficiency on the human epididymis. J. Pathol. 21032-41. [DOI] [PubMed] [Google Scholar]
- 10.Grisanzio, C., and S. Signoretti. 2007. p63 in prostate biology and pathology. J. Cell Biochem. 1031354-1368. [DOI] [PubMed] [Google Scholar]
- 11.Gustafsson, M. V., X. Zheng, T. Pereira, K. Gradin, S. Jin, J. Lundkvist, J. L. Ruas, L. Poellinger, U. Lendahl, and M. Bondesson. 2005. Hypoxia requires notch signaling to maintain the undifferentiated cell state. Dev. Cell 9617-628. [DOI] [PubMed] [Google Scholar]
- 12.Haase, V. H., J. N. Glickman, M. Socolovsky, and R. Jaenisch. 2001. Vascular tumors in livers with targeted inactivation of the von Hippel-Lindau tumor suppressor. Proc. Natl. Acad. Sci. USA 981583-1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hayashi, T., A. Yoshinaga, R. Ohno, N. Ishii, S. Kamata, and T. Yamada. 2004. Expression of the p63 and Notch signaling systems in rat testes during postnatal development: comparison with their expression levels in the epididymis and vas deferens. J. Androl. 25692-698. [DOI] [PubMed] [Google Scholar]
- 14.Jhappan, C., D. Gallahan, C. Stahle, E. Chu, G. H. Smith, G. Merlino, and R. Callahan. 1992. Expression of an activated Notch-related int-3 transgene interferes with cell differentiation and induces neoplastic transformation in mammary and salivary glands. Genes Dev. 6345-355. [DOI] [PubMed] [Google Scholar]
- 15.Kim, W. Y., and W. G. Kaelin. 2004. Role of VHL gene mutation in human cancer. J. Clin. Oncol. 224991-5004. [DOI] [PubMed] [Google Scholar]
- 16.Koster, M. I., S. Kim, A. A. Mills, F. J. DeMayo, and D. R. Roop. 2004. p63 is the molecular switch for initiation of an epithelial stratification program. Genes Dev. 18126-131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Leone, D. P., J. B. Relvas, L. S. Campos, S. Hemmi, C. Brakebusch, R. Fassler, C. Ffrench-Constant, and U. Suter. 2005. Regulation of neural progenitor proliferation and survival by beta1 integrins. J. Cell Sci. 1182589-2599. [DOI] [PubMed] [Google Scholar]
- 18.Lesche, R., M. Groszer, J. Gao, Y. Wang, A. Messing, H. Sun, X. Liu, and H. Wu. 2002. Cre/loxP-mediated inactivation of the murine Pten tumor suppressor gene. Genesis 32148-149. [DOI] [PubMed] [Google Scholar]
- 19.Leslie, N. R., X. Yang, C. P. Downes, and C. J. Weijer. 2005. The regulation of cell migration by PTEN. Biochem. Soc. Trans. 331507-1508. [DOI] [PubMed] [Google Scholar]
- 20.Leung, S. Y., A. S. Chan, M. P. Wong, S. T. Yuen, Y. W. Fan, and L. P. Chung. 1998. Expression of vascular endothelial growth factor in von Hippel-Lindau syndrome-associated papillary cystadenoma of the epididymis. Hum. Pathol. 291322-1324. [DOI] [PubMed] [Google Scholar]
- 21.Lonser, R. R., G. M. Glenn, M. Walther, E. Y. Chew, S. K. Libutti, W. M. Linehan, and E. H. Oldfield. 2003. von Hippel-Lindau disease. Lancet 3612059-2067. [DOI] [PubMed] [Google Scholar]
- 22.Lowell, S., P. Jones, I. Le Roux, J. Dunne, and F. M. Watt. 2000. Stimulation of human epidermal differentiation by delta-notch signalling at the boundaries of stem-cell clusters. Curr. Biol. 10491-500. [DOI] [PubMed] [Google Scholar]
- 23.Lupien, M., A. Dievart, C. R. Morales, L. Hermo, E. Calvo, D. G. Kay, C. Hu, and P. Jolicoeur. 2006. Expression of constitutively active Notch1 in male genital tracts results in ectopic growth and blockage of efferent ducts, epididymal hyperplasia and sterility. Dev. Biol. 300497-511. [DOI] [PubMed] [Google Scholar]
- 24.Maehama, T., and J. E. Dixon. 1998. The tumor suppressor, PTEN/MMAC1, dephosphorylates the lipid second messenger, phosphatidylinositol 3,4,5-trisphosphate. J. Biol. Chem. 27313375-13378. [DOI] [PubMed] [Google Scholar]
- 25.Mandriota, S. J., K. J. Turner, D. R. Davies, P. G. Murray, N. V. Morgan, H. M. Sowter, C. C. Wykoff, E. R. Maher, A. L. Harris, P. J. Ratcliffe, and P. H. Maxwell. 2002. HIF activation identifies early lesions in VHL kidneys: evidence for site-specific tumor suppressor function in the nephron. Cancer Cell 1459-468. [DOI] [PubMed] [Google Scholar]
- 26.Maxwell, P. H., M. S. Wiesener, G. W. Chang, S. C. Clifford, E. C. Vaux, M. E. Cockman, C. C. Wykoff, C. W. Pugh, E. R. Maher, and P. J. Ratcliffe. 1999. The tumour suppressor protein VHL targets hypoxia-inducible factors for oxygen-dependent proteolysis. Nature 399271-275. [DOI] [PubMed] [Google Scholar]
- 27.Mills, A. A., B. Zheng, X. J. Wang, H. Vogel, D. R. Roop, and A. Bradley. 1999. p63 is a p53 homologue required for limb and epidermal morphogenesis. Nature 398708-713. [DOI] [PubMed] [Google Scholar]
- 28.Mori, S., Y. Kadokawa, K. Hoshinaga, and T. Marunouchi. 2003. Sequential activation of Notch family receptors during mouse spermatogenesis. Dev. Growth Differ. 457-13. [DOI] [PubMed] [Google Scholar]
- 29.Nguyen, B. C., K. Lefort, A. Mandinova, D. Antonini, V. Devgan, G. Della Gatta, M. I. Koster, Z. Zhang, J. Wang, A. Tommasi di Vignano, J. Kitajewski, G. Chiorino, D. R. Roop, C. Missero, and G. P. Dotto. 2006. Cross-regulation between Notch and p63 in keratinocyte commitment to differentiation. Genes Dev. 201028-1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nickoloff, B. J., J. Z. Qin, V. Chaturvedi, M. F. Denning, B. Bonish, and L. Miele. 2002. Jagged-1 mediated activation of notch signaling induces complete maturation of human keratinocytes through NF-kappaB and PPARgamma. Cell Death Differ. 9842-855. [DOI] [PubMed] [Google Scholar]
- 31.Pastorekova, S., Z. Zavadova, M. Kostal, O. Babusikova, and J. Zavada. 1992. A novel quasi-viral agent, MaTu, is a two-component system. Virology 187620-626. [DOI] [PubMed] [Google Scholar]
- 32.Pedrero, J. M., D. G. Carracedo, C. M. Pinto, A. H. Zapatero, J. P. Rodrigo, C. S. Nieto, and M. V. Gonzalez. 2005. Frequent genetic and biochemical alterations of the PI 3-K/AKT/PTEN pathway in head and neck squamous cell carcinoma. Int. J. Cancer 114242-248. [DOI] [PubMed] [Google Scholar]
- 33.Podsypanina, K., L. H. Ellenson, A. Nemes, J. Gu, M. Tamura, K. M. Yamada, C. Cordon-Cardo, G. Catoretti, P. E. Fisher, and R. Parsons. 1999. Mutation of Pten/Mmac1 in mice causes neoplasia in multiple organ systems. Proc. Natl. Acad. Sci. USA 961563-1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pollard, P. J., B. Spencer-Dene, D. Shukla, K. Howarth, E. Nye, M. El-Bahrawy, M. Deheragoda, M. Joannou, S. McDonald, A. Martin, P. Igarashi, S. Varsani-Brown, I. Rosewell, R. Poulsom, P. Maxwell, G. W. Stamp, and I. P. Tomlinson. 2007. Targeted inactivation of fh1 causes proliferative renal cyst development and activation of the hypoxia pathway. Cancer Cell 11311-319. [DOI] [PubMed] [Google Scholar]
- 35.Rangarajan, A., C. Talora, R. Okuyama, M. Nicolas, C. Mammucari, H. Oh, J. C. Aster, S. Krishna, D. Metzger, P. Chambon, L. Miele, M. Aguet, F. Radtke, and G. P. Dotto. 2001. Notch signaling is a direct determinant of keratinocyte growth arrest and entry into differentiation. EMBO J. 203427-3436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Saito, K., S. Kawakami, Y. Okada, R. Takazawa, F. Koga, Y. Kageyama, and K. Kihara. 2006. Spatial and isoform specific p63 expression in the male human urogenital tract. J. Urol. 1762268-2273. [DOI] [PubMed] [Google Scholar]
- 37.Shao, X., S. Somlo, and P. Igarashi. 2002. Epithelial-specific Cre/lox recombination in the developing kidney and genitourinary tract. J. Am. Soc. Nephrol. 131837-1846. [DOI] [PubMed] [Google Scholar]
- 38.Shen, T., Z. Zhuang, D. J. Gersell, and F. A. Tavassoli. 2000. Allelic deletion of VHL gene detected in papillary tumors of the broad ligament, epididymis, and retroperitoneum in von Hippel-Lindau disease patients. Int. J. Surg. Pathol. 8207-212. [DOI] [PubMed] [Google Scholar]
- 39.Shin, K. H., J. M. Kim, K. S. Rho, K. H. Park, J. E. Oh, and B. M. Min. 2002. Inactivation of the PTEN gene by mutation, exonic deletion, and loss of transcript in human oral squamous cell carcinomas. Int. J. Oncol. 21997-1001. [PubMed] [Google Scholar]
- 40.Soriano, P. 1999. Generalized lacZ expression with the ROSA26 Cre reporter strain. Nat. Genet. 2170-71. [DOI] [PubMed] [Google Scholar]
- 41.Stambolic, V., M. S. Tsao, D. Macpherson, A. Suzuki, W. B. Chapman, and T. W. Mak. 2000. High incidence of breast and endometrial neoplasia resembling human Cowden syndrome in pten+/− mice. Cancer Res. 603605-3611. [PubMed] [Google Scholar]
- 42.Suh, E. K., A. Yang, A. Kettenbach, C. Bamberger, A. H. Michaelis, Z. Zhu, J. A. Elvin, R. T. Bronson, C. P. Crum, and F. McKeon. 2006. p63 protects the female germ line during meiotic arrest. Nature 444624-628. [DOI] [PubMed] [Google Scholar]
- 43.Tachibana, M., M. Shibakita, S. Ohno, S. Kinugasa, H. Yoshimura, S. Ueda, T. Fujii, M. A. Rahman, D. K. Dhar, and N. Nagasue. 2002. Expression and prognostic significance of PTEN product protein in patients with esophageal squamous cell carcinoma. Cancer 941955-1960. [DOI] [PubMed] [Google Scholar]
- 44.Takahashi, K., M. Murakami, and S. Yamanaka. 2005. Role of the phosphoinositide 3-kinase pathway in mouse embryonic stem (ES) cells. Biochem. Soc. Trans. 331522-1525. [DOI] [PubMed] [Google Scholar]
- 45.Thoma, C. R., I. J. Frew, C. R. Hoerner, M. Montani, H. Moch, and W. Krek. 2007. pVHL and GSK3beta are components of a primary cilium-maintenance signalling network. Nat. Cell Biol. 9588-595. [DOI] [PubMed] [Google Scholar]
- 46.Vivanco, I., N. Palaskas, C. Tran, S. P. Finn, G. Getz, N. J. Kennedy, J. Jiao, J. Rose, W. Xie, M. Loda, T. Golub, I. K. Mellinghoff, R. J. Davis, H. Wu, and C. L. Sawyers. 2007. Identification of the JNK signaling pathway as a functional target of the tumor suppressor PTEN. Cancer Cell 11555-569. [DOI] [PubMed] [Google Scholar]
- 47.Vortmeyer, A. O., M. G. Tran, W. Zeng, S. Glasker, C. Riley, M. Tsokos, B. Ikejiri, M. J. Merrill, M. Raffeld, Z. Zhuang, R. R. Lonser, P. H. Maxwell, and E. H. Oldfield. 2006. Evolution of VHL tumourigenesis in nerve root tissue. J. Pathol. 210374-382. [DOI] [PubMed] [Google Scholar]
- 48.Vortmeyer, A. O., Q. Yuan, Y. S. Lee, Z. Zhuang, and E. H. Oldfield. 2004. Developmental effects of von Hippel-Lindau gene deficiency. Ann. Neurol. 55721-728. [DOI] [PubMed] [Google Scholar]
- 49.Wang, S., A. J. Garcia, M. Wu, D. A. Lawson, O. N. Witte, and H. Wu. 2006. Pten deletion leads to the expansion of a prostatic stem/progenitor cell subpopulation and tumor initiation. Proc. Natl. Acad. Sci. USA 1031480-1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang, X. D., C. C. Leow, J. Zha, Z. Tang, Z. Modrusan, F. Radtke, M. Aguet, F. J. de Sauvage, and W. Q. Gao. 2006. Notch signaling is required for normal prostatic epithelial cell proliferation and differentiation. Dev. Biol. 29066-80. [DOI] [PubMed] [Google Scholar]
- 51.Wilson, J. D. 1978. Sexual differentiation. Annu. Rev. Physiol. 40279-306. [DOI] [PubMed] [Google Scholar]
- 52.Yang, A., R. Schweitzer, D. Sun, M. Kaghad, N. Walker, R. T. Bronson, C. Tabin, A. Sharpe, D. Caput, C. Crum, and F. McKeon. 1999. p63 is essential for regenerative proliferation in limb, craniofacial and epithelial development. Nature 398714-718. [DOI] [PubMed] [Google Scholar]