Abstract
The majority of studies examining activity-induced conformational changes in G protein-coupled receptors have focused on transmembrane helices or intracellular regions. Relatively few studies have examined the involvement of the extracellular region in general and the N-terminal region in particular in this process. To begin to address this, we generated a series of antibodies to the N-terminal region of opioid receptors. Characterization of these antibodies revealed that they differentially recognize activated receptors. Recently, we generated monoclonal antibodies that recognize regions proximal to glycosylation sites in the receptor N terminus. Characterization of these antibodies revealed that agonist treatment leads to a decrease in epitope recognition by the antibody presumably because of a movement of the region of the N terminus proximal to glycosylation sites. The time course of the decrease in antibody recognition suggested that it could be due to a post-activation-mediated event. Examination of the involvement of receptor residues in the C-tail and β-arrestin binding using site-directed mutagenesis and cells or tissues lacking β-arrestin 2 suggests a role for these desensitization-related mechanisms in governing antibody binding to the receptor. Thus, these N-terminally directed antibodies can differentially recognize post-activation-mediated changes in the C-terminal (intracellular) region of the receptor. Therefore, these conformation-sensitive antibodies represent powerful reagents to probe receptor activation states and provide a potential tool for identifying and characterizing new compounds of therapeutic interest.
G protein-coupled receptors (GPCRs)3 comprise one of the largest families of genes present in the mammalian genome. These receptors are activated in response to a number of signals ranging from neurotransmitters and peptide hormones, to odorant molecules and photons. Agonist binding to the receptor leads to the activation of second messenger signaling cascades via heterotrimeric G proteins and ultimately to a physiological effect. These include neurotransmission, cellular metabolism, secretion, growth, differentiation, inflammation, and immune responses among many others. Therefore, agonists or antagonists to GPCRs as well as agents that interfere with cellular pathways activated by these receptors are widely used in drug therapy (1). Because GPCRs are the primary targets for drug development, significant effort has been put toward understanding the structural changes occurring during receptor activation.
Studies examining how GPCRs are activated by agonists at the molecular level have suggested that small agonists bind to a pocket formed by the surrounding transmembrane helices, whereas peptide ligands contact additional determinants in extracellular loops and possibly the N-terminal tail (2). Binding of agonists, but not antagonists, leads to stabilization of the helical bundle into a conformation, which, in turn, induces the uncovering of a molecular determinant at the bottom of the core that is required for G protein binding and activation (reviewed in Ref. 2). Ideally, a comprehensive molecular mechanism for GPCR activation should include both the N- and C-terminal tails in addition to the helical transmembrane bundle. However, with the exception of glycoprotein hormone receptors, where the large N-terminal tail has been shown to be involved in high affinity and selective binding of receptor agonists (3) and of family C receptors where the very large extracellular N terminus is organized into a domain called the Venus flytrap module that contains the ligand-binding pocket (4, 5), most studies on GPCRs have focused on transmembrane segments and extracellular loops. Very little is known about the role of the N-terminal region in receptor activation. This could be because of a lack of tools, the variable nature of this region among GPCRs, and the difficulty in formulating a hypothesis on its folding.
We have recently used conformation-sensitive antibodies to show that the N-terminal region of a number of family A GPCRs undergoes conformational changes following receptor activation (6). These antibodies exhibit increased recognition of the agonist-treated (but not antagonist-treated) receptors. To begin to examine the molecular mechanism underlying agonist-mediated changes in the N-terminal region, we generated monoclonal antibodies (mAbs) to a defined region in the midportion of the μOR and δOR N-terminal tail. We identified a subset of antibodies to a region proximal to putative glycosylation sites that exhibited loss of recognition following agonist treatment (in contrast to the previously reported antibodies (6) that exhibited enhanced recognition) presumably because of the movement of glycosylated sugars near the epitope recognized by the antibodies. Using these antibodies, we show that mechanisms related to desensitization involving receptor C-terminal tail and β-arrestin binding play a role in the observed changes in receptor recognition by these antibodies.
EXPERIMENTAL PROCEDURES
Cell Culture and Transfection—CHO cells stably expressing either FLAG-tagged mouse μOR or δOR, C-terminal-truncated mutants of δOR lacking the last 15 (CT15) or 37 (CT37) amino acid residues, δOR phosphorylation site mutants S344G (CT2), T353A (CT3), T352A (CT4), T359A (CT5), T361A (CT6), or S363A (CT7) have been previously characterized (7, 8). These and the previously described HA-tagged μOR or μD164H mutant receptors (9) were grown in F12 medium as described (7-10). COS-7 cells were grown in Dulbecco's modified Eagle's medium containing 10% fetal bovine serum and 1% penicillin-streptomycin. Neuro 2A cells were grown in 50% Dulbecco's modified Eagle's medium and 50% F12 media containing 10% fetal bovine serum and 1% penicillin-streptomycin. COS-7 cells were transfected with either HA-tagged human μOR (2.5 μg) or HA-tagged human N40D μOR (5 μg). CHO cells stably expressing either FLAG-tagged mouse μOR or δOR were transfected with β-arrestin cDNA (2 μg). Neuro 2A cells were co-transfected with FLAG-tagged mouse μOR cDNA (2 μg) and β-arrestin cDNA (2 μg). CHO cells were transfected with 5 μg of C348A/C353A double mutant μOR cDNA (CC; 11). SK-N-SH cells endogenously expressing μOR and δOR were transfected with control siRNA or β-arrestin 2 siRNA as described previously (12). These transfections were carried out using Lipofectamine per the manufacturer's protocol (Invitrogen). Receptor expression levels were measured by ligand binding assays as described below. We have previously reported that expression of β-arrestin leads to up-regulation of β-arrestin levels, whereas expression of siRNA to β-arrestin leads to a significant decrease in the levels of the protein (12).
Generation and Purification of Antibodies—The location of the peptides used for the generation of μOR (14SDPLAPASWSPAPGSWL30) and δOR (3LVPSARAELQSSPLV17) mAbs is indicated in Fig. 1. These peptides were synthesized as multiple antigenic peptides (MAPs, Research Genetics, Huntsville, AL) and used to generate monoclonal antibodies using a classical protocol (13). The hybridoma were screened by ELISA using either 1 μg of MAPs or CHO cells expressing receptors (1 × 105 cells/well) (13). These antibodies (4D6 for μOR and 5A2 for δOR) were subtyped as described (14) and found to be of the IgG2b subtype.4 These antibodies are highly receptor-specific and exhibit less than 1% cross-reactivity against other related receptors (13). NT1 antibodies (to an epitope in the N terminus of μOR close to TM1) and polyclonal antibodies (pAb) SA25 (to the epitope 14SDPLAPASWSPA25 of μOR) or LV17 (to the epitope 3LVPSARAELQSSPLV17 of δOR) were generated previously (6).
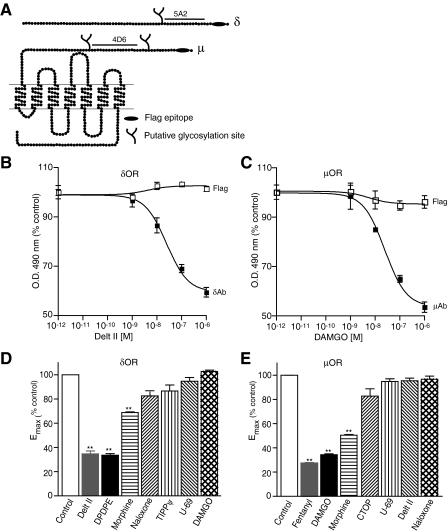
FIGURE 1.
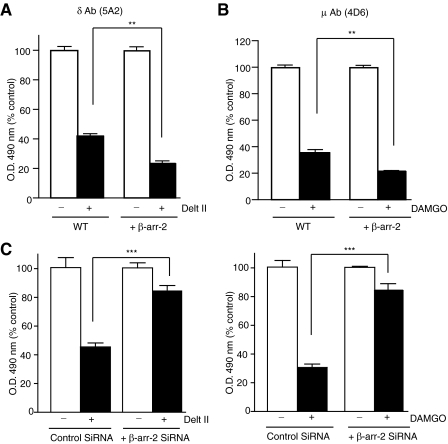
Agonist-induced conformational changes in the N terminus of μOR and δOR. A, schematic representation of μOR and δOR. The FLAG epitope (black ellipse), putative N-linked glycosylation sites (branches), and amino acid sequences used for the generation of antigenic peptides (black lines) are indicated. B and C, CHO cells stably expressing δOR (C) or μOR (B) were treated with indicated doses of deltorphin II or DAMGO in isotonic HEPES buffer, pH 7.4 and probed with 5A2, 4D6, and FLAG antibodies by ELISA as described under “Experimental Procedures.” Absorbance obtained with cells not treated with agonist (1.27 ± 0.04 for 5A2, 1.44 ± 0.02 for 4D6, 1.03 ± 0.01 for FLAG in CHOμOR and 1.15 ± 0.07 for FLAG in CHOδOR) following subtraction of signal with only secondary antibody were taken as 100%. Results are mean ± S.E. of three experiments in triplicate. D and E, CHO cells stably expressing δOR (D) or μOR (E) were treated without or with 1 μm different ligands in 50 mm Tris-Cl buffer, pH 7.5 and probed with μOR (4D6) and δOR (5A2) mAbs by ELISA as described under “Experimental Procedures.” Data with vehicle-treated cells were taken as 100%. Results are mean ± S.E. of three experiments in triplicate. **, p < 0.01 Dunnett's test.
Effect of Ligand Treatment on Receptor Recognition by mAbs—Cells expressing FLAG-tagged μOR or δOR, HA-tagged μOR (WT), D164H μOR, or CC μOR, were plated on poly-l-lysine-treated 24-well plates (2 × 105 cells/well). Cells were treated without or with ligands (0-10-6 m) in Tris buffer (50 mm Tris-Cl, pH 7.5) or in isotonic HEPES buffer (10 mm HEPES containing 300 mm sucrose and 0.2 mm EDTA, pH 7.4) for 30 min at 37 °C. The extent of receptor recognition was measured using ELISA with mAbs to μOR, δOR, or FLAG epitope essentially as described (13). Briefly, cells were quickly rinsed three times (within 5 min) with cold PBS and fixed with ice-cold methanol for 10 min at -20 °C. ELISA was carried out by incubating cells with 3% BSA in PBS for 1 h at 37 °C followed by overnight incubation at 4 °C with 1:500 dilution of primary antibodies in 1% BSA in PBS. The wells were then washed three times with 1% BSA in PBS (5 min each wash) followed by 1 h of incubation at 37 °C with 1:500 dilution (in 1% BSA in PBS) of secondary antibody coupled to horseradish peroxidase. The wells were washed three times with 1% BSA in PBS (5 min each wash) and color developed by addition of the substrate, o-phenylenediamine (5 mg/10 ml in 0.15 m citrate buffer pH 5 containing 15 μl of H2O2). Absorbance at 490 nm was measured with a Bio-Rad ELISA reader. A490 nm values obtained with anti-mouse secondary antibody coupled to horseradish peroxidase were 0.21 ± 0.01 with untransfected cells, 0.19 ± 0.01 for cells expressing μOR, 0.27 ± 0.04 for cells expressing δOR. A490 nm values obtained with untransfected cells (after subtraction of signal obtained with only secondary antibody) were 0.21 ± 0.04 for μOR, 0.26 ± 0.03 for δOR, and 0.23 ± 0.05 for FLAG mAbs. We verified that the results obtained in the ELISA assay were not an artifact of the methanol fixation step by carrying out ELISA in unfixed cells. In this case, to minimize cell loss, the assay was carried out in cells in suspension, and following each step, the supernatant was removed by centrifuging cells at 1,000 × g for 3 min. The level of receptor recognition obtained with δOR and μOR mAbs showed a linear relationship to the amount of receptor epitope present (supplemental Fig. S1) and was not an artifact of the methanol fixation step, because similar results were obtained with unfixed cells (supplemental Fig. S1). We find that the mAbs described in this study (that show decreased recognition of activated receptors) exhibit differences in EC50 for antibody recognition of activated receptors (25 nm for δOR mAb, 14 nm for μOR mAb) compared with previously described polyclonal antibodies (7.5 nm for δOR pAb and 2.2 nm for μOR pAb; supplemental Fig. S2) that could be a reflection of the higher affinity of binding of the polyclonal antibodies to their respective epitopes.
The effect of different concentrations of antibody on recognition of agonist-treated receptors was examined in CHO cells expressing FLAG-tagged μOR. The cells were treated with 1 μm DAMGO for 30 min as described above. ELISA was carried out using mAbs to μOR or FLAG (1-50 μg/well) and a 1:2,000 dilution (in 1% BSA in PBS) of secondary antibody coupled to horseradish peroxidase.
In another set of experiments, CHO cells co-expressing μOR or δOR and β-arrestin 2, Neuro 2A cells co-expressing μOR and β-arrestin 2 or SK-N-SH cells endogenously expressing μOR and δOR transfected with control siRNA or β-arrestin 2 siRNA were treated without or with 1 μm DAMGO or deltorphin II for 30 min at 37 °C and the extent of receptor recognition measured by ELISA as described above.
Binding Assays—The expression level of individual receptors (FLAG-tagged μOR or δOR, HA-tagged μOR (WT), D164H μOR, or CC μOR) was determined by incubating cells with 10 nm [3H]diprenorphine, and nonspecific binding was determined in the presence of 1 μm cold diprenorphine as described previously (10). The expression level of μOR in the prefrontal cortex of WT and β-arrestin 2 knock-out (k/o) mice was determined by incubating cells with 1 nm[3H]DAMGO, and nonspecific binding was determined in the presence of 1 μm cold DAMGO as described previously (10).
Time Course of Ligand Treatment on Receptor Recognition by Abs—CHO cells stably expressing FLAG-tagged wild-type or C-terminal mutants of δOR (CT15, CT37, CT2, CT3, CT4, CT5, CT6, or CT7) were plated on poly-l-lysine-treated 24-well plates (2 × 105 cells/well). Cells were treated without or with deltorphin II (1 μm) in 50 mm Tris-Cl, pH 7.5 for different time periods (0-60 min) at 37 °C (under these conditions there is no receptor internalization), and ELISA was carried out with δOR (5A2) or FLAG mAb or δOR pAb (LV17) as described above. In a parallel experiment, cells expressing FLAG-tagged δOR were treated without or with deltorphin II (1 μm) in 50 mm Tris-Cl, pH 7.5 for 1 h at 37 °C. Cells were rinsed in assay buffer, incubated with the δOR antagonist, TIPPΨ (1 μm), for different time periods (0-60 min) at 37 °C, and ELISA was carried out with δOR mAb (5A2) or δOR pAb (LV17) as described above. We find that the 5A2 mAb exhibits a t½ ∼ 3.9 ± 1.8 min while the previously generated pAb (LV17) exhibits a t½ ∼ 3.5 ± 1.7 min for change in receptor recognition following agonist treatment (Fig. 3A and supplemental Fig. S3A). This change in recognition (decrease in the case of 5A2 and increase in the case of LV17) is reversed following treatment of cells with the δOR antagonist, TIPPΨ, with a t½ ∼ 2.1 ± 1.3 min for 5A2 mAb and ∼2.2 ± 1.8 min for LV17 pAb (supplemental Fig. S3, B and C).
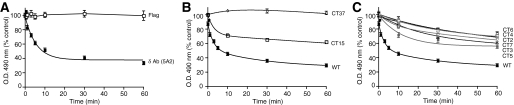
FIGURE 3.
The role of the C-terminal tail in agonist-induced decrease in antibody recognition. CHO cells stably expressing wild-type δOR (A), C-terminal-truncated mutants lacking the last 15 (CT15 δOR) or 37 (CT37 δOR) amino acid residues (B) or the phosphorylation site mutants (C) CT2 (S344G), CT3 (T353A), CT4 (T352A), CT5 (T359A), CT6 (T361A), or CT7 (S363A) were treated without or with 1 μm deltorphin II for different time periods (0-60 min) and probed with δOR (5A2) specific mAb by ELISA as described under “Experimental Procedures.” Data from vehicle-treated cells were taken as 100%. Results are mean ± S.E. of three experiments in triplicate.
Time Course of Phosphorylation of δOR—CHO cells stably expressing FLAG-tagged wild-type δOR were plated on poly-l-lysine-treated 24-well plates (2 × 105 cells/well). Cells were treated without or with deltorphin II (100 nm) for different time periods (0-60 min) at 37 °C, following which cells were lysed in 70 °C 1× Laemmli's sample buffer. Lysates were subjected to SDS-PAGE and Western blotting using polyclonal antibodies to phosphorylated δOR (1:100, Cell Signaling Technology) and anti-tubulin mAb (1:20,000: Sigma) as primary antibodies and anti-rabbit IR680 and anti-mouse IR800 (1:10,000, Li-Cor) as secondary antibodies. Blots were imaged using the Odyssey Imaging system (Li-Cor).
Effect of Pertussis Toxin Treatment on Receptor Recognition by μAb—For this, CHO cells expressing μOR (2 × 105 cells/well) were treated overnight with or without 50 ng of pertussis toxin (PTX). Cells were washed three times with PBS, treated without or with 100 nm DAMGO for 30 min at 37 °C, and subjected to ELISA using μOR and δOR mAbs as described above. Cells not treated with PTX were used as controls.
Effect of PNGase Treatment on Receptor Recognition by mAbs—CHO cells stably expressing FLAG-tagged μOR or δOR, COS-7 cells transiently expressing the isoforms N40 μOR or N40D μOR (2 × 105 cells/well) were incubated with or without 1 μm agonists, in the absence or presence of PNGase F (40 units) for 3 h at 37 °C in 50 mm Tris-Cl, pH 7.5. Cells were washed once in PBS and fixed in ice-cold methanol for 10 min. The extent of receptor recognition by μOR and δOR antibodies was determined using ELISA as described above.
Effect of Ligand Treatment on Endogenous Receptors in Membranes from Wild-type or β-Arrestin K/O Animals—Male WT and β-arrestin 2 k/o mice (4-6 months old; 25-35 g weight) were used for these studies in accordance with the National Institutes of Health guidelines for the care and use of animals and with approved animal protocols from The Ohio State University Animal Care and Use Committee. Mice (3-6 per group) were injected intraperitoneally with either vehicle (0.9% physiological saline, 10 μl/g), morphine sulfate (10 mg/kg), naloxone hydrochloride (1 mg/kg), or naloxone + morphine, where naloxone was given 10 min prior to morphine treatment. Morphine sulfate was from the NIDA, National Institutes of Health drug supply program; naloxone hydrochloride was purchased from Tocris-Cookson. At 30-min post-treatment, mice were sacrificed by cervical dislocation. Whole brains were rapidly removed and frozen on top of dry ice, then wrapped in aluminum foil, and stored at -80 °C. Samples were shipped on dry ice overnight and stored at -80 °C until use. The prefrontal cortex was dissected out, and membranes were prepared as described previously (10). 1 μg of membrane containing protease inhibitor mixture (Sigma) along with 100 μg of HEK-293 membranes (for bulk) were placed in Eppendorf tubes, treated without (in the case of in vivo receptor activation, Fig. 5B, supplemental Fig. S4) or with 1 μm deltorphin II for 30 min at 37 °C (in the case of in vitro receptor activation, Fig. 5A). Ligands were removed by centrifugation, and membranes were subjected to ELISA using μOR and δOR antibodies in the presence of protease inhibitor mixture (Sigma) as described previously (6). The effect of overnight incubation at 4 °C on receptor integrity was measured by ligand binding (in the absence or presence of GTPγS) using [3H]diprenorphine as described above. The receptor binding was 98.3 ± 2.7% that observed following 30 min of treatment at 37 °C (in the absence of GTPγS) and 97.7 ± 1.8% in the presence of GTPγS.
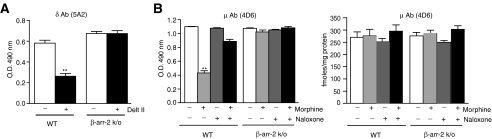
FIGURE 5.
The role of β-arrestin 2 in antibody recognition. A, PFC membranes from β-arrestin 2 knock-out mice (β-arr k/o) or their wild-type (WT) littermate controls were treated with 1 μm deltorphin II for 30 min at 37 °C and the extent of antibody recognition by δOR (5A2) mAb monitored by ELISA as described under “Experimental Procedures.” B, β-arrestin 2 knock-out mice (β-arr k/o) or their WT littermate controls were injected intraperitoneally with either 10 mg/kg morphine, 1 mg/kg naloxone, 10 mg/kg morphine + 1 mg/kg naloxone, or saline and sacrificed 30 min later. Membranes prepared from the PFC were probed by ELISA with the μOR (4D6) mAb as described under “Experimental Procedures.” Receptor levels were determined by incubating membranes (10 μg) with 1 nm [3H]DAMGO as described under “Experimental Procedures.” Nonspecific binding was determined in the presence of 1 μm DAMGO. Results are mean ± S.E. of three experiments from 3-4 animals. **, p < 0.01 versus control, Dunnett's test.
RESULTS
Agonist Treatment Leads to a Decrease in Recognition by Receptor-specific Antibodies—We have recently generated antibodies to the N-terminal region of μOR and δOR that exhibited increased recognition of activated receptors (6). To further probe these agonist-induced changes in the receptor that lead to changes in antibody binding, we generated additional monoclonal antibodies to an epitope in the N-terminal region of mouse μOR and δOR (to a region proximal to the region recognized by our previous antibodies). Interestingly, among the panel of anti-δOR mAb secreting clones, the 5A2 clone exhibited a decrease in receptor recognition following agonist treatment (Fig. 1B). Similarly, among μOR mAbs, the 4D6 clone exhibited decreased receptor recognition following agonist treatment (Fig. 1C). Both the mAbs displayed a high receptor type selectivity and recognized an epitope in membranes from the brain of wild-type but not from animals lacking the respective receptors (supplemental Fig. S5). Further analysis examining the FLAG epitope located at the N terminus of the receptor with the anti-FLAG antibody indicated that the decrease in antibody recognition was not caused by the loss of receptors from the cell surface (Fig. 1, B and C). In the case of μOR, the highest decrease in recognition (∼50%) was seen with 1 μm DAMGO; increasing the antibody concentration by 10-fold (to 50 μg/well) did not attenuate the extent of recognition suggesting that a population of receptors exhibit high affinity binding whereas a second population no longer binds antibody after activation.
Next, we examined the effect of different opioid ligands on the extent of receptor recognition by the δOR and μOR mAbs. We find that treatment with δOR selective agonists and not μOR or κOR selective agonists leads to a decrease in receptor recognition by the δOR mAb (Fig. 1D). Similarly, treatment with μOR selective ligands leads to a decrease in receptor recognition by the μOR mAb (Fig. 1E). Interestingly, the extent of loss of recognition appears to correlate with the efficacy of the ligand (Fig. 1, D and E). For example, morphine, a partial agonist with lower efficacy, exhibits a smaller decrease than [d-Pen2,d-Pen5]-enkephalin (DPDPE) and deltorphin II, potent full agonists, in receptor recognition by the δOR mAb (Fig. 1D). Taken together, these results suggest that treatment with selective agonists leads to a change in the conformation of the N-terminal region of μOR or δOR, and this change can be detected by the mAbs raised against μOR and δOR (4D6 and 5A2), respectively.
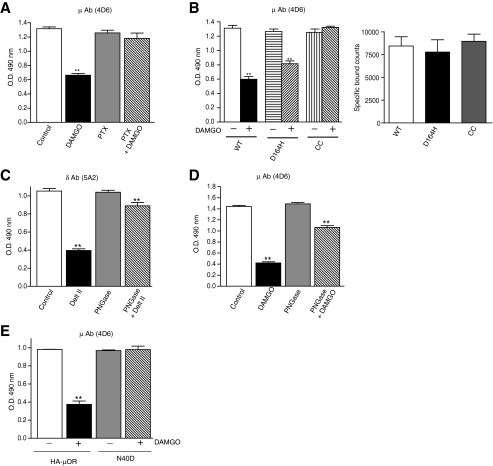
Role of Receptor Activity on Antibody Recognition—To examine the involvement of G proteins in the loss of receptor recognition we used PTX treatment prior to agonist binding. We find that PTX treatment impairs the agonist-mediated decrease in receptor recognition (but not basal recognition) in the case of both μOR (Fig. 2A) as well as δOR (not shown). This suggests that a functional G protein is required for the observed agonist-mediated decrease in recognition by the antibodies thereby indicating that these mAbs could differentiate between different activity states of the receptor.
FIGURE 2.
Effect of receptor activity and deglycosylation on antibody recognition. A, cells expressing μOR, pretreated overnight with 50 ng of PTX, were treated without or with 100 nm DAMGO for 30 min at 37 °C and probed by ELISA with μOR (4D6) and δOR (5A2) mAbs as described under “Experimental Procedures.” Control cells were not subjected to PTX treatment. B, cells expressing μOR (WT) or CAM mutants (D164H μOR and CC μOR) were treated without or with 100 nm DAMGO and probed by ELISA with μOR (4D6) mAbs as described under “Experimental Procedures.” The expression level of individual receptors was determined using [3H]diprenorphine as described under “Experimental Procedures.” C and D, CHO cells stably expressing FLAG-tagged δOR (C) or μOR (D) were incubated with or without 1 μm deltorphin II or DAMGO, in the absence or presence of PNGase F and probed with δOR (5A2) or μOR (4D6) specific mAbs by ELISA as described under “Experimental Procedures.” Control cells were not subjected to PNGase F treatment. E, COS-7 cells transiently expressing HA-tagged human μOR or HA-tagged human N40D μOR (N40D) were incubated with or without 1 μm DAMGO in the absence or presence of PNGase F and probed with μOR (4D6) specific mAb by ELISA as described under “Experimental Procedures.” Results are mean ± S.E. of three experiments in triplicate. **, p < 0.01 versus control, Dunnett's test.
Effect of Constitutively Active Mutants on Antibody Recognition—Next we examined if μOR mAbs could differentially recognize constitutively active mutants (CAM) of the μOR. For this we used two CAM of the μOR, a double cysteine mutant (C348A/C353A) that exhibits significant spontaneous activity (11), and the D164H mutant that exhibits a high degree of structural instability (9). We find an attenuation in the loss of recognition of agonist-treated CAM receptors as compared with wild-type receptors when these receptors are expressed at similar levels (Fig. 2B). The greatest attenuation was observed with the double cysteine mutant suggesting that these antibodies could be used in future studies for an in-depth characterization of a variety of mutant receptors that differ in the extent of their coupling and activation.
Involvement of Glycosylation in Antibody Recognition of Activated Receptors—We tested the possibility that the agonist-induced decrease in antibody recognition is due to a movement of the oligosaccharide side chain in the N terminus, because these mAbs recognize a region proximal to a putative glycosylation site (Fig. 1A). We tested this possibility by treating the receptors with PNGase F to remove the oligosaccharide side chain. This treatment led to a significant recovery of receptor recognition both in the case of δOR and μOR mAbs (Fig. 2, C and D). There is only a 10-20% loss of recognition of deglycosylated receptors as compared with a 60-70% loss of recognition seen with non-treated (glycosylated) receptors (Fig. 2, C and D) suggesting that, following receptor activation, the epitopes recognized by these mAbs are masked by the adjacent oligosaccharide side chains. This is further supported by results obtained with a naturally occurring glycosylation site mutant isoform of human μOR (N40D) where a potential glycosylation site Asn-40 is substituted with non-glycosylatable Asp. When cells expressing the substituted (Asp-40) μOR are treated with DAMGO, there is no significant decrease in recognition by the 4D6 antibody; this is in contrast to cells expressing non-substituted (Asn-40) μOR where agonist treatment leads to a significant decrease (Fig. 2E). These results are consistent with the notion that the oligosaccharide side chain at Asn-40 plays a role in masking the epitope involved in the recognition of the activated glycosylated μOR.
Effect of C-terminal Truncations on Antibody Recognition—Next, we used δOR as a model and examined the time course of change in receptor recognition by δOR mAb. We find that agonist treatment led to a time-dependent decrease in receptor recognition (t½ ∼3.9 ± 1.8 min), which paralleled the time course of increase in the extent of receptor phosphorylation (Fig. 3A and supplemental Fig. S3, D and E). Previous studies have shown that this time course also parallels the time course of receptor desensitization (15). The decrease in receptor recognition was not due to a loss of receptors from the cell surface, because we did not see a time-dependent change in receptor recognition with the FLAG antibody (Fig. 3A). In addition, we find that this decrease in recognition is reversed following treatment of cells with the δOR antagonist, TIPPΨ (t½ ∼ 2.1 ± 1.3 min; supplemental Fig. S3). Taken together, these results suggest that post-activation events such as those involving receptor desensitization could result in conformational changes leading to a loss of receptor recognition.
To test the involvement of the receptor C-terminal tail in antibody recognition we used C-terminal truncations lacking 15 (CT15 δOR) or 37 (CT37 δOR) amino acid residues. There was a significant attenuation of the agonist-induced decrease in recognition of CT15 δOR by the δOR mAb (Fig. 3B). Further truncation (CT37 δOR) led to a complete attenuation of the decrease in recognition (Fig. 3B). These results indicate that the C-terminal tail is critical to the observed agonist-mediated effects.
The C-terminal region of δOR contains several phosphorylation sites and many of these have been thought to play a role in receptor desensitization (16). We used point mutants of phosphorylation sites in the C terminus of δOR to examine the role of these residues in the observed decrease in recognition by the mAb. We find that all of these mutants exhibited significant impairments in the extent of recognition as compared with the wild-type δOR, indicating that mutation of individual phosphorylation sites reduces the agonist-induced change of recognition of the receptors by the antibody (Fig. 3C). Taken together, these results indicate that phosphorylatable residues in the receptor C-terminal tail play an important role in mediating the agonist-induced conformational change of the N terminus.
Effect of β-Arrestin 2 Expression on Antibody Recognition—Because the post-activation event includes arrestin binding to the phosphorylated C-tail, we examined the involvement of arrestin in the agonist-mediated loss of antibody recognition. We find that overexpression of β-arrestin 2 in cells stably expressing δOR or μOR leads to a greater decrease in recognition of agonist activated receptors (Fig. 4A). We also find that reducing the levels of β-arrestin 2 by transfecting cells with β-arrestin 2 siRNA (12) leads to an attenuation of the decrease in recognition by the antibody (Fig. 4C). Taken together, these results suggest that β-arrestin 2 binding to the C-terminal tail of δOR plays an important role in determining the degree of loss of recognition of activated receptors by the δOR mAb.
FIGURE 4.
The effect of β-arrestin 2 expression on receptor recognition. CHO cells stably expressing FLAG-tagged δOR (A) or μOR (B) transfected with β-arrestin 2 cDNA were treated with 1 μm deltorphin II or DAMGO for 30 min at 37 °C, and the extent of antibody recognition by δOR (5A2) and μOR (4D6) mAbs monitored by ELISA as described under “Experimental Procedures.” C, SK-N-SH cells endogenously expressing μOR or δOR transfected with control siRNA or β-arrestin 2 siRNA were treated with 1 μm DAMGO or deltorphin II for 30 min at 37 °C and the extent of antibody recognition by μOR (4D6) and δOR (5A2) mAbs monitored by ELISA as described under “Experimental Procedures.” Results are mean ± S.E. of three experiments in triplicate. **, p < 0.01; ***, p < 0.001, Dunnett's test.
Next, we examined the role of endogenous β-arrestin 2 on the recognition of agonist-activated receptors by these mAbs. For this, we used prefrontal cortical membranes from WT or β-arrestin 2 k/o animals and examined the effect of deltorphin II treatment on the level of recognition of endogenous receptors. We find that treatment of membranes from WT animals leads to a decrease in recognition by the mAb, whereas this is not seen with membranes from mice lacking β-arrestin 2 (Fig. 5A). Next, we examined the physiological significance of β-arrestin 2 recruitment in vivo using β-arrestin 2 k/o mice. For this, mice (WT or β-arrestin 2 k/o) were administered with morphine, naloxone, or a combination of both. Brains were collected 30 min following drug administration and analyzed for receptor expression and antibody binding. We find that drug administration has no significant effects on receptor levels in either WT or β-arrestin 2 k/o mice (Fig. 5B). In contrast, in WT animals systemic administration of morphine leads to a loss of receptor recognition by the μOR mAb that is decreased by co-administration of naloxone (naloxone alone has no effect, Fig. 5B). The decreased recognition is also not observed with an antibody targeting an epitope of the μOR that is close to the first transmembrane region (Ref. 6 and supplemental Fig. S4) or with the δOR mAb (supplemental Fig. S4). In animals lacking β-arrestin 2, systemic administration of morphine had no effect on the recognition of the receptor by the antibody (Fig. 5B). Taken together, these results suggest that morphine administration leads to changes in the conformation of the N-terminal region of μOR in the brain, and that β-arrestin 2 plays a crucial role in this process. Thus these antibodies could serve as useful tools to probe post-activation-mediated conformational changes in the N-terminal region of endogenous receptors.
DISCUSSION
In these studies we show, for the first time, that activation-mediated changes at the C terminus of GPCRs (because of phosphorylation and arrestin recruitment) are transmitted to the N terminus, and this can be detected using N-terminally directed conformation-sensitive antibodies. The majority of studies examining activity induced conformational changes in GPCRs have focused, thus far, on transmembrane helices or intracellular regions (17, 18). Relatively few studies have examined the involvement of the extracellular region in general and the N-terminal region in particular in this process (19). Studies with receptors with a long N-terminal tail such as family C receptors (4, 5) have led to the proposal that the N terminus is organized into a Venus flytrap module that undergoes specific ligand-induced conformational changes (4, 5). Such a model is not applicable to members of family A GPCRs (such as opioid receptors) that have fairly short N termini where the ligand-binding domain is not confined to the N-terminal tail. In the case of these receptors, a combination of mutagenesis and functional assays have been used to propose a role for the N terminus in receptor activation (20, 21). Our studies with antibodies support the notion that the N terminus undergoes conformational changes following receptor activation. This involves the movement of a structured N-terminal tail leading to the unmasking of the epitope(s) recognized by conformation-sensitive antibodies. Recent findings that the N-terminal region of rhodopsin, a prototypical family A GPCR, is highly structured is consistent with such a notion (22, 23).
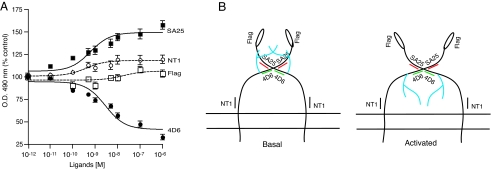
In these studies, we show that a specific region within the N terminus undergoes a substantial change in conformation in response to receptor activation. We show that the 4D6 antibody to the midportion of μOR exhibits decreased recognition of the activated receptor, whereas the M1 antibody to the FLAG epitope tag at the N terminus does not (Fig. 1). In a previous study, we showed that SA25 (a μOR antibody recognizing a region adjacent to the region recognized by 4D6) exhibits increased recognition of activated receptors while the NT1 antibody to a region proximal to transmembrane 1 of μOR does not (Ref. 6 and Fig. 6A). Therefore, using antibodies to four distinct regions within the N terminus, we show that the region in the midportion of μOR that is proximal to glycosylation sites undergoes significant movement whereas distal regions do not (Fig. 6A). Furthermore, these studies show that receptor activation leads to the movement of the glycosylated side chains leading to a decrease in recognition by one set of antibodies (4D6 or 5A2) and increase in recognition by the other (SA25 or LV17). Because the epitopes for both sets of antibodies are in the N terminus and adjacent to each other, one could envision that this portion of the N terminus undergoes a dynamic change (a schematic depiction is shown in Fig. 6B). According to this model, in the basal state, the N termini of the two protomers (of a dimeric receptor) are in close proximity at the region proximal to the glycosylation site. The involvement of glycosylated side chains in the positioning of the N-terminal domain is supported by recent crystallographic studies (23); these studies demonstrate that the polysaccharide side chains of individual protomers (within a dimer) wrap around each other in the basal state (23). It can be envisioned that agonist treatment leads to the movement of these N termini away from each other causing the glycosylated side chains to mask one epitope (4D6) and unmask another (SA25); such a movement of a portion of the N terminus had not been demonstrated thus far in the case of opioid receptor or any other GPCR.
FIGURE 6.
A comparison of agonist-induced conformational changes in the N terminus of μOR. A, cells stably expressing μOR were treated with indicated doses of DAMGO and probed with 4D6 (this study), SA25 (6), NT1 (6), and M1 (anti-FLAG) antibodies by ELISA as described under “Experimental Procedures.” Data with vehicle-treated cells were taken as 100%. Results are mean ± S.E. of three experiments in triplicate. B, model depicting agonist-mediated changes in the N terminus that are differentially detected by conformation-sensitive μOR antibodies. In the inactive state (basal), the epitope for 4D6 antibody (green lines) is accessible, while the epitope for SA25 antibody (red lines) is masked. Upon receptor activation, the N-terminal region undergoes a conformational change such that the epitope for the 4D6 antibody (green lines) is masked, presumably because of the movement of the glycosylated chain (blue branched lines); simultaneously, the epitope for SA25 antibodies is revealed (red lines). The accessibility of the antibody to the NT1 epitope (black line) or the FLAG epitope (open ellipse) are not significantly altered upon activation.
Another important finding in this study is that agonist-induced changes in the receptor attributed to post-activation events (involving phosphorylation of the C-tail and binding of arrestin) could be easily detected with our antibodies. Because tools to directly examine the activity states of native receptors were not available until now, the majority of the previous studies examining receptor activity used indirect methods to address this question (24-26). In the present study, we directly explore the activity states of native receptors using conformation-sensitive antibodies. We find that antibody recognition is able to correlate well with agonist efficacy (in that the highly efficacious agonist, DAMGO causes a higher loss of receptor recognition as compared with the less efficacious agonist, morphine). In addition, overexpression of β-arrestin 2 leads to an augmentation of the response whereas reduction in β-arrestin 2 levels leads to an attenuation indicating that our monoclonal antibodies that recognize phosphorylated, arrestin-bound receptors can be used as probes to detect the post-activation-mediated changes in the N-terminal region of opioid receptors in response to a variety of paradigms.
Finally, we demonstrate that changes in μOR in the brain following peripheral morphine administration can be investigated using our antibodies. Because these antibodies recognize conformational changes resulting from β-arrestin binding, changes in response to morphine administration imply that receptor activation by morphine leads to β-arrestin recruitment in vivo. Previous studies showing a lack of receptor endocytosis in response to morphine treatment (26-28) led to the proposal that morphine administration does not lead to efficient phosphorylation of the receptor resulting in poor recruitment of β-arrestin (29). Such an idea was further supported by β-arrestin overexpression, which was found to increase morphine-induced receptor endocytosis (29, 30). In the present study, using membranes from animals lacking β-arrestin 2 and antibodies sensitive to β-arrestin-bound receptor, we provide evidence for β-arrestin 2 recruitment to receptor upon activation by morphine. Our results are consistent with a recent report that found that treatment of striatal neurons with morphine leads to efficient endocytosis of μOR that can be blocked by overexpression of a dominant-negative mutant of β-arrestin 2 (31). It is possible that differences in the levels of β-arrestin in different cell types govern the extent of β-arrestin recruitment and ultimately desensitization of μOR, which could directly impact on the development of tolerance to morphine.
Activity state-sensitive antibodies represent powerful reagents that can be used to study endogenous receptors as well as to screen for ligands that modulate GPCR activity. We recently used antibodies to the N terminus of CB1 cannabinoid receptors (that exhibit increased recognition of agonist-treated receptors) to screen for peptidic ligands to this receptor. This led to the identification of hemopressin as a selective peptidic inverse agonist of CB1 receptors (32). Using this peptide antagonist we have been able to demonstrate the activity of endogenous CB1 receptors in an intracellular compartment (33). Thus, these antibodies in addition to being useful for immunoisolation of endogenous receptor-interacting complexes (34) serve as powerful tools for the identification of novel exogenous and endogenous modulators of GPCRs.
In summary, using conformation-sensitive antibodies, we have shown that a portion of the opioid receptor N terminus is masked, and another portion is unmasked upon agonist-induced receptor activation. In addition, our results suggest that morphine administration leads to arrestin binding to the μOR, thus challenging the dogma in the field that morphine treatment does not lead to efficient arrestin recruitment. These findings were possible by the availability of these conformation-sensitive antibodies that differentially detect β-arrestin binding to receptors. Thus, these antibodies represent new and useful reagents that can be used to (i) examine the duration and extent of activation of endogenous receptors, (ii) isolate and characterize CAM receptors, and (iii) screen and/or identify drugs that are allosteric modulators of family A GPCRs, which could be of potential therapeutic value.
Supplementary Material
Acknowledgments
We thank Drs. L. Liu-Chen for the gift of CHO cells expressing HA-tagged mu receptors, D. Massotte for the gift of μ CAM CC mutant, L. Yu for the gift of the N40D mutant, and J. Pintar for brains from μ k/o and δ k/o mice. We would also like to thank R. Blitzer, J. Moron, and N. Abul-Husn for critical reading of the manuscript.
This work was supported in part by National Institutes of Health Grants DA08863, DA19521, and NS053751 (to L. A. D.) and DA14600 and DA18860 (to L. M. B.). The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. S1-S5.
Footnotes
The abbreviations used are: GPCR, G-protein coupled receptor; Ab, antibody; BSA, bovine serum albumin; DAMGO, [d-Ala2,NMe-Phe4,Gly-ol5]-enkephalin; ELISA, enzyme-linked immunosorbent assay; mAb, monoclonal antibody; MAP, multiple antigenic peptide; pAb, polyclonal antibody; PBS, phosphate-buffered saline; HA, hemagglutinin; WT, wild type; GTPγS, guanosine 5′-3-O-(thio)triphosphate; OR, opioid receptor; CAM, constitutively active mutants; CHO, Chinese hamster ovarian cells; siRNA, small interfering RNA; k/o, knockout; PTX, pertussis toxin.
A. Gupta and L. A. Devi, unpublished data.
References
- 1.Hill, S. J. (2006) Br. J. Pharmacol. 147 S27-S37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gether, U. (2000) Endocr. Rev. 21 90-113 [DOI] [PubMed] [Google Scholar]
- 3.Vassart, G., Pardo, L., and Costagliola, S. (2004) Trends Biochem. Sci. 29 119-126 [DOI] [PubMed] [Google Scholar]
- 4.Kunishima, N., Shimada, Y., Tsuji, Y., Sato, T., Yamamoto, M., Kumasaka, T., Nakanishi, S., Jingami, H., and Morikawa, K. (2000) Nature 407 971-977 [DOI] [PubMed] [Google Scholar]
- 5.Silve, C., Petrel, C., Leroy, C., Bruel, H., Mallet, E., Rognan, D., and Ruat, M. (2005) J. Biol. Chem. 280 37917-37923 [DOI] [PubMed] [Google Scholar]
- 6.Gupta, A., Decaillot, F. M., Gomes, I., Tkalych, O., Heimann, A. S., Ferro, E. S., and Devi, L. A. (2007) J. Biol. Chem. 282 5116-5124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Trapaidze, N., Keith, D. E., Cvejic, S., Evans, C. J., and Devi, L. A. (1996) J. Biol. Chem. 271 29279-29285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cvejic, S., and Devi, L. A. (1997) J. Biol. Chem. 272 26959-26964 [DOI] [PubMed] [Google Scholar]
- 9.Li, J., Huang, P., Chen, C., de Riel, J. K., Weinstein, H., and Liu-Chen, L. Y. (2001) Biochemistry 40 12039-12050 [DOI] [PubMed] [Google Scholar]
- 10.Gomes, I., Filipovska, J., Jordan, B. A., and Devi, L. A. (2002) Methods 27 358-365 [DOI] [PubMed] [Google Scholar]
- 11.Brillet, K., Kieffer, B. L., and Massotte, D. (2003) BMC Pharmacol. 3 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rozenfeld, R., and Devi, L. A. (2007) FASEB J. 21 2455-2465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gupta, A., and Devi, L. A. (2006) Mt. Sinai J. Med. 73 673-681 [PubMed] [Google Scholar]
- 14.Ey, P. L., Prowse, S. J., and Jenkin, C. R. (1978) Immunochemistry 15 429-436 [DOI] [PubMed] [Google Scholar]
- 15.Law, P. Y., Kouhen, O. M., Solberg, J., Wang, W., Erickson, L. J., and Loh, H. H. (2000) J. Biol. Chem. 275 32057-32065 [DOI] [PubMed] [Google Scholar]
- 16.Trapaidze, N., Cvejic, S., Nivarthi, R. N., Abood, M., and Devi, L. A. (2000) DNA Cell Biol. 19 93-101 [DOI] [PubMed] [Google Scholar]
- 17.Jensen, A. D., Guarnieri, F., Rasmussen, S. G., Asmar, F., Ballesteros, J. A., and Gether, U. (2001) J. Biol. Chem. 276 9279-9290 [DOI] [PubMed] [Google Scholar]
- 18.Ballesteros, J. A., Jensen, A. D., Liapakis, G., Rasmussen, S. G., Shi, L., Gether, U., and Javitch, J. A. (2001) J. Biol. Chem. 276 29171-29177 [DOI] [PubMed] [Google Scholar]
- 19.Lecat, S., Bucher, B., Mely, Y., and Galzi, J. L. (2002) J. Biol. Chem. 277 42034-42048 [DOI] [PubMed] [Google Scholar]
- 20.Decaillot, F. M., Befort, K., Filliol, D., Walker, P., and Kieffer, B. L. (2003) Nat. Struct. Biol. 10 629-636 [DOI] [PubMed] [Google Scholar]
- 21.Levin, M. C., Marullo, S., Muntaner, O., Andersson, B., and Magnusson, Y. (2002) J. Biol. Chem. 277 30429-30435 [DOI] [PubMed] [Google Scholar]
- 22.Palczewski, K., Kumasaka, T., Hori, T., Behnke, C. A., Motoshima, H., Fox, B. A., Le Trong, I., Teller, D. C., Okada, T., Stenkamp, R. E., Yamamoto, M., and Miyano, M. (2000) Science 289 739-745 [DOI] [PubMed] [Google Scholar]
- 23.Salom, D., Lodowski, D. T., Stenkamp, R. E., Le Trong, I., Golczak, M., Jastrzebska, B., Harris, T., Ballesteros, J. A., and Palczewski, K. (2006) Proc. Natl. Acad. Sci. U. S. A. 103 16123-16128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gainetdinov, R. R., Premont, R. T., Bohn, L. M., Lefkowitz, R. J., and Caron, M. G. (2004) Annu. Rev. Neurosci. 27 107-144 [DOI] [PubMed] [Google Scholar]
- 25.Kovoor, A., Celver, J. P., Wu, A., and Chavkin, C. (1998) Mol. Pharmacol. 54 704-711 [PubMed] [Google Scholar]
- 26.Keith, D. E., Murray, S. R., Zaki, P. A., Chu, P. C., Lissin, D. V., Kang, L., Evans, C. J., and von Zastrow, M. (1996) J. Biol. Chem. 271 19021-19024 [DOI] [PubMed] [Google Scholar]
- 27.Alvarez. V. A., Arttamangkul, S., Dang, V., Salem, A., Whistler, J. L., von Zastrow, M., Grandy, D. K., and Williams, J. T. (2002) J. Neurosci. 22 5769-5776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Borgland, S. L. (2001) Clin. Exp. Pharmacol. Physiol. 28 147-15411207668 [Google Scholar]
- 29.Zhang, J., Ferguson, S. S., Barak, L. S., Bodduluri, S. R., Laporte, S. A., Law, P. Y., and Caron, M. J. (1998) Proc. Natl. Acad. Sci. U. S. A. 95 7157-7162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Whistler, J. L., and von Zastrow, M. (1998) Proc. Natl. Acad. Sci. U. S. A. 95 9914-9919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Haberstock-Debic, H., Kim, K.-A., Yu, Y. J., and von Zastrow, M. (2005) J. Neurosci. 25 7847-7857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Heimann, A. S., Gomes, I., Dale, C. S., Pagano, R. L., Gupta, A., de Souza, L. L., Luchessi, A. D., Castro, L. M., Giorgi, R., Rioli, V., Ferro, E. S., and Devi, L. A. (2007) Proc. Natl. Acad. Sci. U. S. A. 104 20588-720593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rozenfeld, R., and Devi, L. A. (2008) FASEB J. in press
- 34.Gomes, I., Gupta, A., Filipovska, J., Szeto, H. H., Pintar, J. E., and Devi, L. A. (2004) Proc. Natl. Acad. Sci. U. S. A. 101 5135-5139 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.