Abstract
Microtubule-associated proteins (MAPs) ensure the fidelity of chromosome segregation by controlling microtubule (MT) dynamics and mitotic spindle stability. However, many aspects of MAP function and regulation are poorly understood in a developmental context. We show that mars, which encodes a Drosophila melanogaster member of the hepatoma up-regulated protein family of MAPs, is essential for MT stabilization during early embryogenesis. As well as associating with spindle MTs in vivo, Mars binds directly to protein phosphatase 1 (PP1) and coimmunoprecipitates from embryo extracts with minispindles and Drosophila transforming acidic coiled-coil (dTACC), two MAPs that function as spindle assembly factors. Disruption of binding to PP1 or loss of mars function results in elevated levels of phosphorylated dTACC on spindles. A nonphosphorylatable form of dTACC is capable of rescuing the lethality of mars mutants. We propose that Mars mediates spatially controlled dephosphorylation of dTACC, which is critical for spindle stabilization.
Introduction
Microtubule-associated proteins (MAPs) ensure the fidelity of chromosome segregation during cell division by controlling the formation and stability of spindle microtubules (MTs). Because disruption of spindle formation can promote genomic instability, an understanding of MAP function and regulation is central to dissecting basic mechanisms of tumorigenesis and would be invaluable in designing new therapies for the treatment of cancer. Although much progress has been made in understanding the functions of spindle-associated MAPs in the last few years, many aspects of their role or regulation remain to be fully elucidated. Human hepatoma up-regulated protein (HURP) has been described as a highly charged MAP that can bind directly to MTs in vitro and enhance their polymerization (Santarella et al., 2007). In vivo, HURP is part of a Ran-dependent complex that stabilizes mitotic MTs and is required for the formation and function of bipolar mitotic spindles (Koffa et al., 2006; Sillje et al., 2006; Wong and Fang, 2006). However, it is not known how HURP-associated proteins functionally interact with one another in a developmental context to support normal cellular function.
Mars, a D. melanogaster sequence homologue of HURP, was previously identified as a protein phosphatase 1 (PP1) binding protein, implicating reversible phosphorylation in the control of Mars or Mars-associated proteins (Bennett and Alphey, 2004; Yang et al., 2005). In this paper, we report the essential role of mars during early embryogenesis, its interactions with other MAPs, and its key role in promoting protein dephosphorylation on mitotic spindles to ensure spindle stability.
Results and discussion
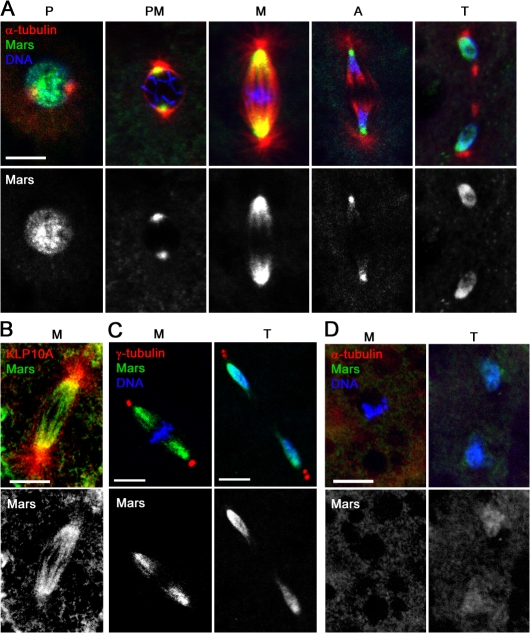
HURP is a component of the mitotic spindle apparatus. To determine the cell cycle distribution of Mars, we generated a Mars-specific antibody and used it to stain syncytial embryos undergoing nuclear division. In prophase, Mars antibody staining was predominantly around the centrosome. In metaphase and anaphase, Mars was localized on spindle MTs in a gradient along the pole-to-pole axis with more intense staining at the centrosome-proximal regions (Fig. 1 A). This is distinct from the distribution of HURP, which has been shown to be predominantly at chromatin-proximal regions until telophase, when levels sharply decline (Koffa et al., 2006; Sillje et al., 2006; Wong and Fang, 2006). In telophase, we observed a low level of discrete Mars staining at the midbody (not depicted), but the majority of Mars protein appeared to be spread over the nuclear envelope where it persisted during interphase (Fig. 1 A and Fig. S1, available at http://www.jcb.org/cgi/content/full/jcb.200712080/DC1). Double staining for Mars and either Klp10A, which is primarily localized at focused minus ends where it promotes depolymerization and poleward flux (Rogers et al., 2004), or γ-tubulin, which marks the face of the centrosome and nucleates MT polymerization (Jeng and Stearns, 1999), confirmed that Mars is localized at MT minus ends but not at the centrosome (Fig. 1, B and C). Mars' localization during mitosis was completely disrupted upon treatment with colchicine to depolymerize MTs, indicating that Mars associates with spindle MTs (Fig. 1 D).
Figure 1.
Mars localizes to spindle MTs. (A) Fixed wild-type embryos stained to reveal the distribution of Mars (green), α-tubulin (red), and DNA (blue) during mitosis. (B and C) Mars is concentrated at MT minus ends. Fixed wild-type embryos stained to reveal distribution of Mars (green) and either KLP10A (red; B) or γ-tubulin (red) and DNA (blue; C). (D) An embryo treated with 500 μg/ml colchicine before fixation to depolymerize MTs. Under these conditions, Mars staining (green) disappears from the spindle during metaphase but can be seen on the nuclear envelope at telophase. P, prophase; PM, prometaphase; M, metaphase; A, anaphase; T, telophase. Bars, 10 μm.
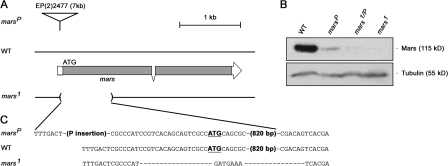
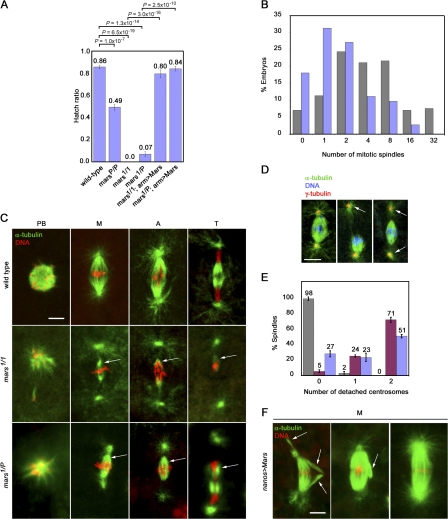
To determine the in vivo role of mars, we generated a null allele of mars, mars1, by imprecise excision of a P element transposon (referred to as marsP hereafter), which we found inserted in the mars 5′ untranslated region (Fig. 2, A and C).marsP flies express full-length Mars protein at a much lower level than wild type (Fig. 2 B). mars1 flies fail to produce Mars protein, which is consistent with molecular analysis revealing that ∼0.84 kb of the coding region, including the translation start site, is deleted in this mutant (Fig. 2, B and C). mars mutant flies are viable but female sterile. Notably, eggs laid by mars1, marsP, and mars1/P flies show a greatly reduced ability to hatch (Fig. 3 A). As we were able to visualize sperm tails in early-arrested embryos laid by mars mutant females (unpublished data), we conclude that the sterility observed in mars mutants is caused by arrest in embryogenesis after fertilization. The viability of embryos laid by mars mutant mothers was restored by moderate overexpression of marsWT (wild-type mars) in the female germline (Fig. 3 A), indicating that the failure of mars mutant embryos to develop is caused by disruption of the mars transcription unit.
Figure 2.
Molecular defects in mars mutants. (A) mars genomic region in marsP, wild type, and mars1. The coding region of mars is represented by shading. marsP contains a P element insertion, EP(2)2477, in the mars untranslated region. mars1 has a 0.84-kb deletion that removes the mars initiation codon and part of the coding region. (B) Mars protein produced in embryos from wild-type, marsP, mars1/P, and mars1 flies. Total protein was analyzed by immunoblotting using Mars antibody. marsP produced full-length Mars protein in a much lower amount, whereas mars1 failed to produce any detectable protein. Blotting with α-tubulin antibodies controlled for loading. (C) Genomic sequence of wild-type and mars mutant strains. The initiation codon (ATG) of mars is bold and underlined.
Figure 3.
mars is essential for spindle stability. (A) Hatch ratios, plotted as the mean ± standard error of five experiments, showing greatly reduced ability of embryos laid by mars mutant females to hatch compared with wild type. Ectopic expression of UASP-FM-marsWT under control of the arm-GAL4 driver (arm>Mars) rescues this phenotype. P-values determined from student's t tests are shown above the graph. (B) Graph showing significant reduction in mean number of mitotic spindles in 15–45-min embryos laid by mars1 mutant mothers (blue; n = 144 embryos) compared with wild-type mothers (gray; n = 185 embryos; P = 2.9 × 10−10). 81.9% of mars1 mutant embryos progress through meiosis to form one or more mitotic figure. (C) Images of mitotic spindles from embryos laid by wild-type, mars1, and mars1/P mothers showing small spindles in mars mutants (arrows) and loss of centrosome attachment to the spindle body. Loss of chromosome attachment to monopolar MTs is apparent in polar bodies. PB, polar body; M, metaphase; A, anaphase; T, telophase. (D) Images of spindles from mutant embryos, showing detached centrosomes (arrows). (E) Bar graph showing percentage of spindles with detached centrosomes from wild type (gray), mars1 (red), and mars1/P (blue), plotted as the mean ± standard error of three experiments. (F) Representative images showing effects of strong ectopic expression of marsWT with nanos-GAL4VP16. Compared with the wild type (C, top), these embryos display abnormally robust spindles, which often have aberrantly aligned MT fibers at metaphase (arrow). Bars, 10 μm.
To determine the cause of the lethality of mars mutant embryos, we fixed embryos from wild-type and mars mutant females and examined the distribution of nuclei and MTs. Embryos lacking maternal mars arrested during early embryogenesis after no more than five nuclear divisions. 81.9% (n = 144) of 15–45-min embryos laid by mars1 mothers exhibited at least two discrete DNA-containing regions (Fig. 3, B and C). The first of these was localized to the embryonic cortex and resembled a polar body, most likely containing the unused products of meiosis II (Wilson and Borisy, 1998). One or more additional DNA-containing regions, each surrounded by a bipolar spindle, were also observed more centrally, indicating that the vast majority of mutant embryos pass through meiosis to form one or more mitotic figure. Notably, the spindle structures in mars1 mutant embryos were very small and weak, albeit still bipolar (Fig. 3 C). Most spindles had at least one detached centrosome, possibly because of weakened spindle–centrosome interactions (Fig. 3, C–E). We also observed unaligned chromosomes in the mars1 mutant, which is indicative of insufficient MT attachment or tension (Fig. S2 A, available at http://www.jcb.org/cgi/content/full/jcb.200712080/DC1). Embryos laid by mars1/P mothers resembled those from mars1 mothers, except the phenotypes were somewhat less severe (Fig. 3, C–E). Quantitation of α-tubulin staining revealed no significant difference between astral MTs in embryos laid by wild-type and mars1/P mothers (P = 0.416), whereas cold-resistant kinetochore MTs were destabilized in embryos laid by mars1/P mothers (Fig. S2 B). Approximately 51% of embryos laid by marsP homozygous mothers (n = 264) failed to develop beyond embryogenesis. Many showed terminal phenotypes at or shortly after gastrulation, presumably as a consequence of primary defects during the early cleavage divisions. Collectively, analysis of loss-of-function mutants suggest that mars has a role in spindle MT stabilization. HURP has bundling activity in vitro and in vivo (Koffa et al., 2006; Sillje et al., 2006; Wong and Fang, 2006). Correspondingly, strong overexpression of marsWT in syncytial embryos resulted in enlarged spindles with ectopic MT fibers (Fig. 3 F), suggesting that mars is limiting for MT bundling.
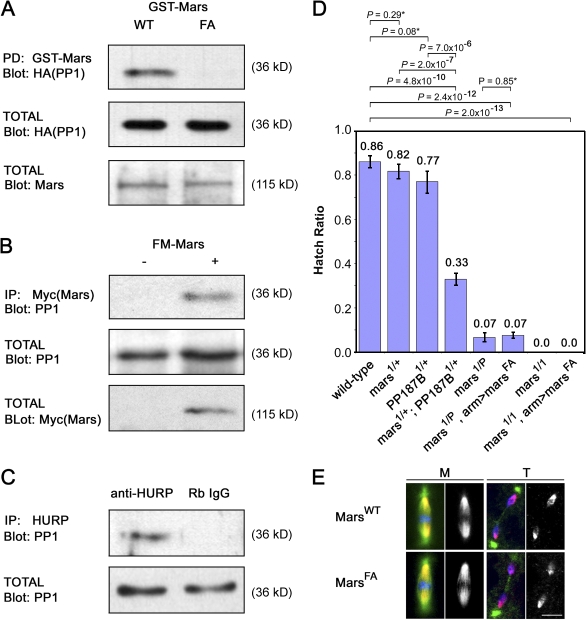
mars was originally isolated from a two-hybrid screen for putative PP1 binding proteins and contains a canonical PP1 binding motif (K/R,x,V,x,F; Bennett and Alphey, 2004; Bennett et al., 2006). To verify interactions with PP1, we performed pulldown experiments between Mars and PP1α87B, the major D. melanogaster PP1 isoform (Dombrádi et al., 1990; Baksa et al., 1993; Kirchner et al., 2007). We found that GST-tagged full-length MarsWT interacted efficiently with HA-tagged PP1α87B from crude D. melanogaster embryo extracts (Fig. 4 A), whereas cells expressing GST alone did not bind (not depicted). We also observed efficient binding between endogenous PP1 and FLAG-Myc (FM)–tagged MarsWT by coimmunoprecipitation from D. melanogaster embryo extracts (Fig. 4 B). To assess the importance of the putative PP1 binding motif for interaction with PP1, we tested a mutant form of Mars in which phenylalanine 839 was replaced with alanine (MarsF839A) in our pulldown assay. The ability of MarsF839A to bind PP1 was greatly reduced compared with wild-type Mars (Fig. 4 A), indicating that Phe839 is crucial for interaction with PP1. Immunoprecipitation with an HURP antibody followed by immunoblotting with PP1 antibody showed that endogenous human PP1 and HURP also interacted efficiently with each other in HeLa cell extracts (Fig. 4 C), suggesting that binding to PP1 is an evolutionarily conserved property of HURP proteins.
Figure 4.
Binding to PP1 is essential for Mars function. (A) Mars binds to PP1α87B in a GST pulldown (PD) assay. Binding requires Phe839 of Mars. arm-GAL4 UAS-HA-PP1α87B fly extracts were incubated with lysates from Escherichia coli expressing GST-MarsWT or MarsF839A. Mars complexes were purified on glutathione-Sepharose and immunoblotted with HA to test for coprecipitation of PP1α87B. Blots of total extracts confirmed equal levels of HA-PP1 and GST-Mars in these experiments. (B) Mars coprecipitates PP1 from D. melanogaster embryonic nuclear extracts. arm-GAL4 (−) and arm-GAL4 UASP-FM-mars (+) embryo extracts were subjected to immunoprecipitation (IP) with Myc antibodies followed by immunoblotting with PP1 antibodies. Blots of total extracts confirmed levels of PP1 and FM-tagged Mars. (C) HURP coprecipitates PP1 from HeLa cell nuclear extracts. Immunoprecipitation of extracts with rabbit HURP antibody or with rabbit random IgG (Rb IgG) was followed by immunoblotting with PP1 antibodies to test for binding. (D) Hatch ratios, plotted as the mean ± standard error of seven experiments. Loss of one copy of PP1α87B enhances mars1/+ to semilethality. Ectopic marsFA fails to rescue lethality of embryos laid by mars mutant mothers. P-values indicated by an asterisk are not statistically significant. (E) Ectopic MarsWT and MarsF839A have identical distributions on mitotic spindles. Fixed embryos from arm>marsWT or arm>marsF839A mothers were stained to reveal the distribution of α-tubulin (green), FLAG-tagged Mars (red and greyscale), and DNA (blue) during mitosis. M, metaphase; T, telophase.
Binding to PP1 prompted us to test functional interactions between mars and PP1 in vivo. The ability of embryos laid by mars or PP1a87B heterozygotes to hatch resembled that of the wild type (Fig. 4 D). Embryos laid by flies transheterozygous for mars and PP1a87B showed a significantly reduced hatch ratio (Fig. 4 D). PP1 is a pleiotropic enzyme. To test the specific role of Mars-bound PP1, we introduced FM-tagged MarsF839A into flies to create Mars complexes lacking PP1. Ectopic expression of marsF839A at comparable levels to those of marsWT failed to rescue the embryonic lethality of embryos laid by mars1 or mars1/P mothers (Fig. 4 D). Collectively, these data suggest that binding to PP1 is critical for mars function. Identical distributions of MarsF839A and MarsWT, as determined by FLAG antibody staining, indicate that PP1 binding is not necessary for normal Mars localization on the mitotic spindle (Fig. 4 E).
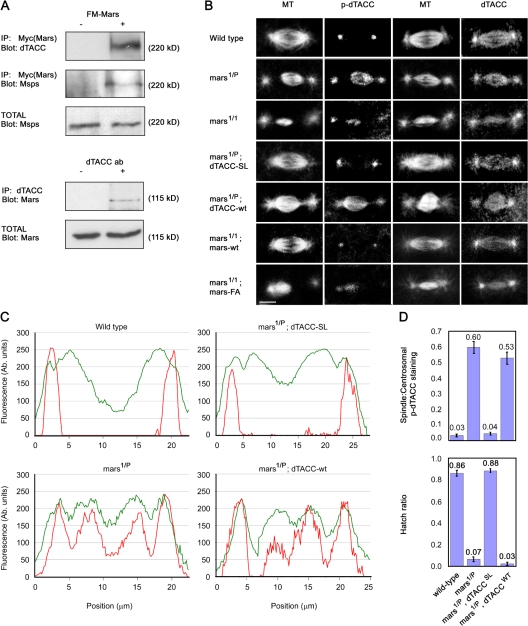
HURP has been shown to interact with other MAPs, including TPX2 and XMAP215 in Xenopus laevis (Koffa et al., 2006), suggesting that its role in the stabilization of spindle MTs may be partly mediated via interactions with other spindle assembly factors. In immunoprecipitation assays, we found that Mars associates with two proteins that are known to cooperate with each other to stabilize MTs during cell division (Lappin et al., 2002): the D. melanogaster XMAP215 homologue encoded by minispindles (msps) and Drosophila transforming acidic coiled-coil (dTACC) protein (Fig. 5 A). In syncytial embryos, dTACC is concentrated at centrosomes during mitosis but is also found on spindle MTs (Gergely et al., 2000) where it colocalizes with Mars (unpublished data). Mars staining was not affected in embryos that produce no detectable dTACC protein (Fig. S3 A, available at http://www.jcb.org/cgi/content/full/jcb.200712080/DC1; Lee et al., 2001). Conversely, the ability of dTACC to associate with MTs was largely unaffected in mars1/P mutant embryos (Fig. 5 B). Therefore, although Mars and dTACC associate with one another, they do not appear to be dependent on each other for their localization.
Figure 5.
An essential role of mars is to promote dTACC dephosphorylation. (A) Mars coprecipitates dTACC and Msps from D. melanogaster embryonic nuclear extracts. Immunoprecipitation of arm-GAL4 (−) and arm>marsWT (+) embryo extracts with Myc antibody, followed by immunoblotting with dTACC and Msps antibodies, showed binding to FM-tagged Mars. Immunoprecipitation with dTACC antibody followed by immunoblotting with Mars antibody confirmed dTACC binding. (B) Distribution of dTACC and p-dTACC on mitotic spindles in embryos from mothers of different genotypes, as indicated. In wild type, p-dTACC is only found at the centrosome but in mars mutants, p-dTACC abnormally accumulates on mitotic spindles. Total dTACC staining is largely unaffected in mars mutants. A nonphosphorylatable mutant form of dTACC (dTACCSL), but not wild-type dTACC (dTACCWT), restores spindle structure and normal distribution of p-dTACC in a mars1/P mutant background. Similarly, arm>marsWT, but not arm>marsFA, restores normal spindle structure and p-dTACC staining in a mars1 background. Bar, 10 μm. (C) Linescans of fluorescence intensity (arbitrary units) across spindles from embryos of different genotypes, as indicated. The distribution of p-dTACC (red trace) is shown relative to α-tubulin (green trace). (D) Top graph shows quantification of ratio of spindle/centrosomal p-dTACC staining. dTACCSL, but not dTACCWT, restores a normal p-dTACC ratio in embryos from mars1/P mothers. Bottom graph shows that dTACCSL, but not dTACCWT, rescues lethality of embryos laid by mars1/P mothers. Hatch ratios are plotted as the mean ± standard error from n = 5 experiments.
Phosphorylation of dTACC on Ser863 is critical for stabilization of the minus ends of centrosome-associated MTs during mitosis (Barros et al., 2005). Although dTACC is found on both the centrosome and mitotic spindle, phosphorylated dTACC (p-dTACC) is tightly localized to the centrosomes (Barros et al., 2005), suggesting that once phosphorylated, p-dTACC is either unable to exchange with the soluble pool of dTACC or is rapidly dephosphorylated when it leaves the centrosome. The role of dephosphorylated TACC is not known, but it may function to stabilize MTs through lateral interactions with MTs or interactions with MT plus ends. The localization of Mars toward the minus ends of spindle MTs and association with both dTACC and PP1 prompted us to examine the involvement of Mars in maintaining low levels of p-dTACC on the spindle.
To examine the effect of Mars on dTACC phosphorylation, we stained mars mutant embryos with an antibody that specifically recognizes dTACC phosphorylated at Ser 863 (p-dTACC). mars mutant embryos showed increased levels of p-dTACC on the mitotic spindles compared with the wild type (Fig. 5, B–D). On careful examination of these mutants, we noticed some spindles that looked normal but possessed elevated levels of p-dTACC (Fig. S3 B), indicating that increased p-dTACC was not simply a secondary consequence of aberrant spindle structure. Levels and distribution of total dTACC appeared normal in mars mutants (Fig. 5 B), although we cannot rule out that global ratios of DTACC/p-DTACC are affected. We used marsF839A to examine whether Mars promotes the dephosphorylation of dTACC by binding to PP1. Embryos with moderate levels of ectopic marsWT in embryos laid by either mars1 or mars1/P mothers were essentially wild type in appearance and had little or no p-dTACC staining on mitotic spindles. In contrast, mars mutant embryos ectopically expressing marsF839A at comparable levels to those of ectopic marsWT retained elevated p-dTACC staining on spindles (Fig. 5 B and not depicted).
To test whether promoting the dephosphorylation of dTACC is a critical function of mars, we examined whether the sterility of mars mutants could be rescued by a nonphosphorylatable form of dTACC (dTACCS863L) expressed under control of the dTACC promoter (Barros et al., 2005). dTACCS863L, but not dTACCWT(wild-type dTACC), restored a normal distribution of p-dTACC staining on mitotic spindles (Fig. 5, B–C). Quantification of p-dTACC staining confirmed that dTACCS863L restored a normal ratio of spindle/centrosomal p-dTACC staining in mars mutant embryos (Fig. 5 D). When we examined hatching of these embryos, we found that the lethality of embryos laid by mars1/P mothers was rescued by dTACCS863L but not dTACCWT (Fig. 5 D). Collectively, these data indicate that dephosphorylation of dTACC on the spindle is an essential function of mars. Homozygous mars1 mutants were not rescued by dTACCS863L (unpublished data), suggesting that residual Mars protein in mars1/P embryos may play a dTACC-independent role, such as MT bundling, or that the level of ectopic dTACCS863L was insufficient to compensate for elevated p-dTACC in a mars1 background.
In summary, we have shown that mars, which encodes a D. melanogaster sequence homologue of HURP, is critical for mitotic spindle structure and chromosome segregation during early embryogenesis. The primary defect in mars mutants appears to be loss of spindle MT stability, whereas overexpression of mars leads to the production of enlarged spindles with ectopic MTs. These data are consistent with a role for mars in MT bundling/stabilization similar to that described for its human homologue HURP. However, our identification of Mars as an interacting subunit of PP1 suggests a novel mechanism by which this family of proteins can maintain normal spindle structure in vivo. Binding of PP1 to Mars implicates PP1 in dephosphorylation of Mars or a Mars-associated protein. Although dTACC may be a substrate of PP1, it is also possible that Mars-bound PP1 may indirectly stimulate dephosphorylation of dTACC on the spindle by activating another protein phosphatase or inactivating a dTACC kinase such as Aurora-A, a known target of PP1 during mitosis (Katayama et al., 2001).
Our genetic experiments indicate that promoting dephosphorylation of dTACC on mitotic spindles is an essential role of Mars. Why is it important to maintain dephosphorylated TACC on the spindle? One possibility is that mars functions to ensure that MT stabilization mediated by p-dTACC only occurs at the centrosome, allowing a more dynamic spindle. This is hard to test however, because the mechanism by which p-dTACC stabilizes MTs is unclear. The effect of dTACC on MT assembly appears to be mediated by effector proteins, such as Msps, as TACC has not been described to possess MT stabilizing activity on its own (Kinoshita et al., 2005). Phosphorylation of dTACC might help activate effectors because dTACC mutated at Ser863 is able to recruit Msps to the centrosome but not promote MT assembly (Barros et al., 2005). However, it is not known which aspect of Msps activity is affected by dTACC phosphorylation or to what extent the effect of dTACC phosphorylation is context dependent. It is conceivable that dTACC stabilizes spindle MTs by establishing lateral interactions with MTs or interactions with plus ends and that these functions of dTACC are impaired when phosphorylated at Ser863.
Is mars a functional homologue of HURP? We have confirmed that various aspects of mars and HURP function are conserved, including spindle stabilization and binding of Mars to Msps and PP1. However, Mars and HURP display apparently distinct spindle localizations, suggesting that there may be differences in how these proteins are used during cell division. This may reflect a wider difference in the organization of MAPs that control MT stability and the formation of bipolar spindles in flies and humans.
Spindle defects caused by lack of TACC phosphorylation or by alterations in TACC or HURP protein levels may lead to genetic instability and are implicated in cancer progression (Raff, 2002; Barros et al., 2005; Brittle and Ohkura, 2005). Our data indicate that spatially controlled dephosphorylation also plays a positive role in TACC function, suggesting that deregulation of either phosphorylation or dephosphorylation of TACC may also be involved in the molecular pathology of cancer by compromising the fidelity of chromosome segregation.
Materials and methods
Fly strains
EP(2)2477, referred to here as marsP, is a homozygous viable P element insertion in the 5′ untranslated region of mars. GFP-dTACCWT and GFP-dTACCS863L (gift from J. Raff, The Gurdon Institute, Cambridge, England, UK) have been previously described (Barros et al., 2005). Other fly stains are described in FlyBase (http://www.flybase.org).
Isolation and characterization of a mars-null allele
Isolation of a null allele of mars by P element excision from marsP was performed as follows. Jumpstarter y w/Y; isogenic marsP/CyO, P(Delta2-3) males were crossed with y w; Tft/CyO females. From each cross, only one w revertant male, y w; mars*/CyO, in which the P element was excised, was individually crossed back to y w; Tft/CyO females. To determine the molecular lesion in the mars1 mutant, genomic DNA surrounding the original marsP insertion site was amplified from mars1 homozygotes by PCR using flanking primers and sequenced.
Statistical analysis
We used unpaired two-tailed t tests to compare mean hatch ratios of eggs from different strains and unpaired one-tailed t tests to compare mean number of mitotic spindles in wild-type and mars1 mutant embryos.
Site-directed mutagenesis and ectopic expression
marsF839A was constructed by PCR-based site-directed mutagenesis. For ectopic expression in flies, full-length marsWT and marsF839A were subcloned into pPFMW (Drosophila Genomics Resource Center, Indiana University, USA), a modified version of pUASP (Rorth, 1998) that contains an N-terminal 3xFLAG 6xMyc (FM) tag. UASP-FM-mars flies were made by P element–mediated germline transformation into a w1118 stain by Genetic Services, Inc. Embryos were provided with moderate levels of tagged MarsWT and MarsF839A by ectopic expression of UASP-FM-mars transgenes in the germline using arm-GAL4 (Sanson et al., 1996) or nanos-GAL4VP16 (Van Doren et al., 1998).
Immunoprecipitation and GST-pulldown experiments
We subjected lysates from arm-GAL4 or arm-GAL4 UASP-FM-mars flies to immunoprecipitation with dTACC (gift from J. Raff; Gergely et al., 2000) or Myc antibody (A14 rabbit polyclonal; Santa Cruz Biotechnology, Inc.). After adsorption on protein G bound to GammaBind Plus Sepharose (GE Healthcare), we analyzed immunoprecipitates and total cell extracts by immunoblotting with Myc (9E10 mouse monoclonal; Santa Cruz Biotechnology, Inc.), α-tubulin (DM1a; Sigma-Aldrich), Msps (gift from H. Ohkura, University of Edinburgh, Edinburgh, Scotland, UK; Cullen et al., 1999), PP1α87B (gift from P.T. Cohen, University of Dundee, Dundee, Scotland, UK; Helps et al., 2001), or dTACC (Gergely et al., 2000) antibodies. For GST-pulldown experiments, full-length mars ORF was subcloned into pDEST-15 (Invitrogen) for expression in E. coli in frame with an N-terminal GST tag. marsF839A was made by PCR-based site-directed mutagenesis and subcloned into pDEST-15 in the same way. Constructs were sequenced to confirm that they contained no sequence errors. To test binding to PP1, bacterial cell lysates expressing GST-tagged MarsWT or MarsF839A were incubated with arm-GAL4 UAS-HA-PP1α87B (Vereshchagina et al., 2004) D. melanogaster embryo extracts, and GST-labeled protein was precipitated with GST Bind Resin (EMD). Precipitates were examined by immunoblotting with HA antibody (12CA5; Roche). To test binding between HURP and PP1, HeLa cell nuclear extracts were subjected to immunoprecipitation with HURP antibody (gift from I.W. Mattaj, European Molecular Biology Laboratory, Heidelberg, Germany; Koffa et al., 2006). Precipitates were analyzed by immunoblotting with PP1 antibody (Helps et al., 2001).
Mars antibodies and immunofluorescence
Anti-peptide antibodies against Mars were raised by Eurogentec in rabbits by simultaneous immunization with two peptides: LVPEGTKTPPRRESN (residues 512–526) and TLRNRRVNLRPSSEFM (residues 906–921). Embryos were fixed with either methanol or formaldehyde and were processed for immunofluorescence as described previously (Huang and Raff, 1999). Colchicine treatment of embryos before fixation was as previously described (Gergely et al., 2000). Antibodies used for indirect immunofluorescence were as follows: FLAG (rabbit polyclonal; Sigma-Aldrich), KLP10A (gift from D. Sharp, Albert Einstein College of Medicine, Bronx, NY; Rogers et al., 2004), dTACC (Gergely et al., 2000), p-dTACC (gift from J. Raff; Barros et al., 2005), α-tubulin (DM1a; Sigma-Aldrich) and γ-tubulin (rabbit polyclonal or GTU88 monoclonal; Sigma-Aldrich). Secondary antibodies conjugated with Alexa Fluor 488 (Invitrogen), Cy3, or Cy5 (Jackson ImmunoResearch Laboratories) were used at 1:500–1,000 dilutions. DNA was counterstained with 1 μg/ml propidium iodide (Sigma-Aldrich).
Image acquisition and processing
Fixed embryos, mounted in 85% glycerol and 2.5% n-propylgallate in PBS, were examined using a microscope (Eclipse E800; Nikon) with either a 40× 1.3 NA Plan Fluor or a 60× 1.4 NA Plan Apo objective and a scanning confocal system (Radiance Plus; Bio-Rad Laboratories) equipped with LaserSharp 2000 software (Bio-Rad Laboratories). Images were imported to Photoshop (Adobe) and adjusted for brightness and contrast uniformly across entire fields. Quantitation of astral MTs was performed by making maximum intensity projections of 8–12 image stacks that were taken at 0.5-μm intervals from 15–90-min methanol-fixed embryos stained with α-tubulin, γ-tubulin, and DNA. The projections were imported into AQM Advance 6 software (Kinetic Imaging Ltd.), and mean α-tubulin intensities were measured within a circle around each centrosome from spindles in metaphase. The mean pixel intensity of astral MTs from at least 25 embryos of each genotype was calculated. All measurements were corrected for variation in staining conditions by subtracting the background intensity. A similar approach was taken to quantify p-dTACC staining, except that measurements were also taken at four points on the spindle, both proximal and distal to the centrosome. Linescans were generated using LSM510 software (Carl Zeiss, Inc.). The mean intensity through the center of the unprocessed spindle images, parallel to the long axis of the structure, was calculated for each channel of fluorescence by the software.
Online supplemental material
Fig. S1 shows individual channel images of Mars distribution during mitosis. Fig. S2 shows both misaligned chromosomes on mitotic spindles in embryos laid by mars mutant mothers and reduction of cold-resistant kinetochore MTs in embryos laid by mars1/P mothers. Fig. S3 shows both the spindle localization of Mars in dTACC mutant embryos and elevated p-dTACC spindle staining on a normal looking spindle from an embryo laid by mars1/P. Online supplemental material is available at http://www.jcb.org/cgi/content/full/jcb.200712080/DC1.
Supplementary Material
Acknowledgments
We thank P.T. Cohen for PP1α87B antibody, I.W. Mattaj for HURP antibody, T. Murphy and the Drosophila Genomics Resource Center for plasmid vectors, H. Ohkura for Msps antibody, J. Raff for dTACC fly strains and antibodies, D. Sharp for KLP10A antibody, and K. Clifton and E. Taylor for technical assistance. We thank the Centre for Cell Imaging at Liverpool University for assistance with image quantitation and members of the Bennett laboratory, M. Bollen, J-Y. Huang, and J. Wakefield, for advice and critical comments on the manuscript.
This work was funded by grant BBS/B/11869 from the Biotechnology and Biological Sciences Research Council to D. Bennett, with additional support from Cancer Research UK and the Royal Society. E. Lyulcheva was supported by an Oxford University European Scatcherd Scholarship and a Phizackerley Senior Scholarship at Balliol College, Oxford. D. Bennett was supported by a Todd-Bird Research Fellowship at New College, Oxford.
Abbreviations used in this paper: dTACC, Drosophila transforming acidic coiled coil; FM, FLAG-Myc; HURP, hepatoma up-regulated protein; MAP, microtubule-associated protein; Msps, minispindles; MT, microtubule; p-dTACC; phosphorylated dTACC; PP1, protein phosphatase 1.
References
- Baksa, K., H. Morawietz, V. Dombrádi, M. Axton, H. Taubert, G. Szabo, I. Torok, A. Udvardy, H. Gyurkovics, and B. Szöor. 1993. Mutations in the protein phosphatase 1 gene at 87B can differentially affect suppression of position-effect variegation and mitosis in Drosophila melanogaster.Genetics. 135:117–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barros, T.P., K. Kinoshita, A.A. Hyman, and J.W. Raff. 2005. Aurora A activates D-TACC–Msps complexes exclusively at centrosomes to stabilize centrosomal microtubules. J. Cell Biol. 170:1039–1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett, D., and L. Alphey. 2004. Cloning and expression of mars, a novel member of the guanylate kinase associated protein family in Drosophila. Gene Expr. Patterns. 4:529–535. [DOI] [PubMed] [Google Scholar]
- Bennett, D., E. Lyulcheva, and L. Alphey. 2006. Towards a comprehensive analysis of the protein phosphatase 1 interactome in Drosophila. J. Mol. Biol. 364:196–212. [DOI] [PubMed] [Google Scholar]
- Brittle, A.L., and H. Ohkura. 2005. Centrosome maturation: Aurora lights the way to the poles. Curr. Biol. 15:R880–R882. [DOI] [PubMed] [Google Scholar]
- Cullen, C.F., P. Deak, D.M. Glover, and H. Ohkura. 1999. mini spindles: A gene encoding a conserved microtubule-associated protein required for the integrity of the mitotic spindle in Drosophila. J. Cell Biol. 146:1005–1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dombrádi, V., J.M. Axton, H.M. Barker, and P.T.W. Cohen. 1990. Protein phosphatase activity in Drosophila mutants with abnormalities in mitosis and chromosome condensation. FEBS Lett. 275:39–43. [DOI] [PubMed] [Google Scholar]
- Gergely, F., D. Kidd, K. Jeffers, J.G. Wakefield, and J.W. Raff. 2000. D-TACC: a novel centrosomal protein required for normal spindle function in the early Drosophila embryo. EMBO J. 19:241–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helps, N.R., P.T. Cohen, S.M. Bahri, W. Chia, and K. Babu. 2001. Interaction with protein phosphatase 1 Is essential for bifocal function during the morphogenesis of the Drosophila compound eye. Mol. Cell. Biol. 21:2154–2164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang, J., and J.W. Raff. 1999. The disappearance of cyclin B at the end of mitosis is regulated spatially in Drosophila cells. EMBO J. 18:2184–2195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeng, R., and T. Stearns. 1999. Gamma-tubulin complexes: size does matter. Trends Cell Biol. 9:339–342. [DOI] [PubMed] [Google Scholar]
- Katayama, H., H. Zhou, Q. Li, M. Tatsuka, and S. Sen. 2001. Interaction and feedback regulation between STK15/BTAK/Aurora-A kinase and protein phosphatase 1 through mitotic cell division cycle. J. Biol. Chem. 276:46219–46224. [DOI] [PubMed] [Google Scholar]
- Kinoshita, K., T.L. Noetzel, L. Pelletier, K. Mechtler, D.N. Drechsel, A. Schwager, M. Lee, J.W. Raff, and A.A. Hyman. 2005. Aurora A phosphorylation of TACC3/maskin is required for centrosome-dependent microtubule assembly in mitosis. J. Cell Biol. 170:1047–1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirchner, J., S. Gross, D. Bennett, and L. Alphey. 2007. Essential, overlapping and redundant roles of the Drosophila protein phosphatase 1 alpha and beta genes. Genetics. 176:273–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koffa, M.D., C.M. Casanova, R. Santarella, T. Kocher, M. Wilm, and I.W. Mattaj. 2006. HURP is part of a Ran-dependent complex involved in spindle formation. Curr. Biol. 16:743–754. [DOI] [PubMed] [Google Scholar]
- Lappin, T.R., R.N. Mullan, J.P. Stewart, N.A. Morgan, A. Thompson, and A.P. Maxwell. 2002. AINT/ERIC/TACC: an expanding family of proteins with C-terminal coiled coil domains. Leuk. Lymphoma. 43:1455–1459. [DOI] [PubMed] [Google Scholar]
- Lee, M.J., F. Gergely, K. Jeffers, S.Y. Peak-Chew, and J.W. Raff. 2001. Msps/XMAP215 interacts with the centrosomal protein D-TACC to regulate microtubule behaviour. Nat. Cell Biol. 3:643–649. [DOI] [PubMed] [Google Scholar]
- Raff, J.W. 2002. Centrosomes and cancer: lessons from a TACC. Trends Cell Biol. 12:222–225. [DOI] [PubMed] [Google Scholar]
- Rogers, G.C., S.L. Rogers, T.A. Schwimmer, S.C. Ems-McClung, C.E. Walczak, R.D. Vale, J.M. Scholey, and D.J. Sharp. 2004. Two mitotic kinesins cooperate to drive sister chromatid separation during anaphase. Nature. 427:364–370. [DOI] [PubMed] [Google Scholar]
- Rorth, P. 1998. Gal4 in the Drosophila female germline. Mech. Dev. 78:113–118. [DOI] [PubMed] [Google Scholar]
- Sanson, B., P. White, and J.P. Vincent. 1996. Uncoupling cadherin-based adhesion from wingless signalling in Drosophila.Nature. 383:627–630. [DOI] [PubMed] [Google Scholar]
- Santarella, R.A., M.D. Koffa, P. Tittmann, H. Gross, and A. Hoenger. 2007. HURP wraps microtubule ends with an additional tubulin sheet that has a novel conformation of tubulin. J. Mol. Biol. 365:1587–1595. [DOI] [PubMed] [Google Scholar]
- Sillje, H.H., S. Nagel, R. Korner, and E.A. Nigg. 2006. HURP is a Ran-importin beta-regulated protein that stabilizes kinetochore microtubules in the vicinity of chromosomes. Curr. Biol. 16:731–742. [DOI] [PubMed] [Google Scholar]
- Van Doren, M., A.L. Williamson, and R. Lehmann. 1998. Regulation of zygotic gene expression in Drosophila primordial germ cells. Curr. Biol. 8:243–246. [DOI] [PubMed] [Google Scholar]
- Vereshchagina, N., D. Bennett, B. Szoor, J. Kirchner, S. Gross, E. Vissi, H. White-Cooper, and L. Alphey. 2004. The essential role of PP1beta in Drosophila is to regulate nonmuscle myosin. Mol. Biol. Cell. 15:4395–4405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson, P.G., and G.G. Borisy. 1998. Maternally expressed gamma Tub37CD in Drosophila is differentially required for female meiosis and embryonic mitosis. Dev. Biol. 199:273–290. [DOI] [PubMed] [Google Scholar]
- Wong, J., and G. Fang. 2006. HURP controls spindle dynamics to promote proper interkinetochore tension and efficient kinetochore capture. J. Cell Biol. 173:879–891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, C.P., M.S. Chen, G.J. Liaw, S.F. Chen, G. Chou, and S.S. Fan. 2005. Using Drosophila eye as a model system to characterize the function of mars gene in cell-cycle regulation. Exp. Cell Res. 307:183–193. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.