Abstract
The patch-clamp technique allows currents to be recorded through single ion channels in patches of cell membrane in the tips of glass pipettes. When recording, voltage is typically applied across the membrane patch to drive ions through open channels and to probe the voltage-sensitivity of channel activity. In this study, we used video microscopy and single-channel recording to show that prolonged depolarization of a membrane patch in borosilicate pipettes results in delayed slow displacement of the membrane into the pipette and that this displacement is associated with the activation of mechanosensitive (MS) channels in the same patch. The membrane displacement, ≈1 μm with each prolonged depolarization, occurs after variable delays ranging from tens of milliseconds to many seconds and is correlated in time with activation of MS channels. Increasing the voltage step shortens both the delay to membrane displacement and the delay to activation. Preventing depolarization-induced membrane displacement by applying positive pressure to the shank of the pipette or by coating the tips of the borosilicate pipettes with soft glass prevents the depolarization-induced activation of MS channels. The correlation between depolarization-induced membrane displacement and activation of MS channels indicates that the membrane displacement is associated with sufficient membrane tension to activate MS channels. Because membrane tension can modulate the activity of various ligand and voltage-activated ion channels as well as some transporters, an apparent voltage dependence of a channel or transporter in a membrane patch in a borosilicate pipette may result from voltage-induced tension rather than from direct modulation by voltage.
Mechanosensitive (MS) ion channels can act as transducers for the senses of touch, hearing, balance, and proprioception and also seem to be involved in the regulation of cell volume (1–3). MS channels in patches of membrane in the tips of patch pipettes are readily activated when the membrane is stretched by the application of negative pressure to the shank of the patch pipette (4). The stretching of the membrane patch by negative pressure is thought to activate the channels by increased tension in the membrane (5). When the pressure is released, the channels then readily deactivate. In addition to stretch activation, some MS channels can also be activated by voltage (1). In this regard, depolarization of membrane patches from Xenopus oocytes activates endogenous MS channels in the patch after prolonged delays of typically 1–20 s (6). After stepping the voltage back to negative potentials, the MS channels then deactivate over several seconds. The delayed activation is observed for both on-cell and excised patches and typically occurs in a cooperative manner, with all channels activating over a short period of time compared with the duration of the delay to activation.
We have shown recently that the delayed activation of the MS channels is not observed in outside-out patches, in membrane outside of the patch pipette in whole-cell recordings from Xenopus oocytes, or when the patch pipettes are fabricated from or coated with soft glass (7). These observations suggest that the delayed cooperative activation of MS channels by voltage is not an intrinsic property of the channels but requires interactions between the patch of membrane and the patch pipette (7). Because MS channels are known to be activated by changes in membrane tension (5), the depolarization-induced delayed activation may arise from voltage-induced changes in membrane tension arising from membrane–pipette interactions. Such an explanation would account for the cooperative activation of the MS channels, because changes in membrane tension could be sensed by all the MS channels in a patch.
In this study, we used simultaneous video microscopy and single-channel recording to investigate how the membrane-pipette interactions might increase membrane tension to activate the channels. We showed that prolonged depolarization of a membrane patch in a borosilicate pipette results in the displacement of the membrane patch ≈1 μm into the pipette with each successive depolarization and that the displacement of the membrane patch is correlated with the activation of MS channels. Manipulations that prevent the voltage-induced displacement also prevent the activation of MS channels by voltage. Thus, the voltage-induced membrane displacement is associated with sufficient tension to activate MS channels. Because various ion channels and pumps have been shown to be modulated by membrane tension (8–19), it is possible that voltage modulation of some channels or transporters studied in patch pipettes may reflect changes in membrane tension rather than, or in addition to, the direct effects of voltage.
Materials and Methods
Single-Channel Recording.
The methods used for single-channel recording from patches of membrane from oocytes obtained from Xenopus laevis have been described (6, 7). The neurons used for recording were cultured from the hippocampal region of embryonic (e15) rat brain as described (20). Patch pipettes were fabricated from borosilicate (hard) glass (equivalent to Corning 7740 glass; Clark Electromedical Instruments, Reading, U.K.), and the tips were heat polished. For some experiments, the tips of the borosilicate pipettes were coated with soft glass (Corning 8161; Garner Glass Company, Claremont, CA) by first melting soft glass on the heating filament of a microforge and then placing the tips of the borosilicate pipettes 20–30 μM from the filament for approximately 0.5–1 s, allowing the soft glass to evaporate from the filament and deposit on the tips. The pipettes were heated in the middle and bent about 45° so that the part distal to the bend would run parallel to the bottom of the recording chamber, which was made with a thin coverglass for optical clarity.
Experiments were performed with either the on-cell or the inside-out patch-clamp configuration (21), as indicated. On-cell patches were formed by pressing the tip of a heat-polished patch pipette against the membrane of the cell and then applying slight negative pressure to the patch pipette. In some experiments, patches were formed by applying negative voltage in place of negative pressure. Inside-out patches were formed by drawing the patch pipette away from the cell surface after establishing an on-cell patch, exposing the cytoplasmic face of the patch to the bath solution. Depolarizing steps were held for up to 120 s and involved symmetrical (about zero) voltage steps from negative holding potentials, which ranged from −150 to −50 mV, to positive potentials, which ranged from +50 to +150 mV.
The presence of an intact membrane patch was verified by a high-resistance (gigaohm) seal and also by the ability to record the activity of single channels from the patch. The channels monitored in patches from Xenopus oocytes were MS channels, as verified through activation of the channels by negative pressure applied to the pipette. The channels monitored in patches from neurons were not identified specifically, but seemed similar to large conductance Ca2+-activated K+ channels.
For experiments on oocytes, the pipette (extracellular) and bath (intracellular) solutions typically contained (in mM) 150 NaCl, 5 TES (N-Tris[hydroxymethyl]methyl-2-aminoethanesulfonic acid), and 2 EGTA. For some experiments, the pipette contained KF in place of the NaCl. Similar results were obtained with both pipette solutions. For experiments on cultured neurons, the pipette solution was as above with KF, and the bath solution contained (in mM) 121 NaCl, 2.68 KCl, 2.04 CaCl2, 1.48 MgCl2, 0.05 MgSO4, 0.83 NaH2PO4, 2.0 NaHC03, and 2.0 Hepes. All solutions were adjusted to pH 7.0. Current traces were typically low-pass filtered at 1–2 kHz (−3 dB) for analysis. Currents were recorded with an Axopatch 200A patch-clamp amplifier (Axon Instruments, Foster City, CA) and stored on VCR tape (Instrutech, Mineola, NY) for subsequent analysis. Experiments were performed at room temperature (19–23°C). Negative or positive pressure could be applied pneumatically to the back of the pipette through a port on the pipette holder (6).
Video Microscopy (22, 23).
Images were obtained with an inverted Nikon Diophot microscope typically by using a ×60 1.4-numerical aperture oil immersion objective or a long working distance ×40 0.5-numerical aperture dry objective. A ×5 projection lens was used to image the tip of the pipette onto a Dage–MTI (Michigan City, IN) 65SITX video camera. The images were displayed on a high-resolution monitor during the experiment and also recorded on a VCR. The images were later digitized at 30 Hz with a frame grabber (Dazzle Multimedia Lav-1000, Fremont, CA) and analyzed visually with coreldraw 5.0. Photos were prepared with adobe photoshop 4.0 and printed on a Kodak 8650 printer. Typically, 1–5 s worth of frames were averaged for each still photo. Although the still photos presented in this paper are adequate to show the position of the membrane patch, the dynamic displays of the images on high-resolution monitors and also with movies constructed with the frame grabber made identification and movement of the membrane patch much more obvious. Fluorescent images were obtained with the long working distance ×40 0.5-numerical aperture dry objective by using a 520-nm emission filter. The position of the membrane was delimited by adding 0.01–1 mM fluorescein (Molecular Probes) to the pipette solution.
Results
Prolonged Depolarization Displaces the Membrane Patch into the Patch Pipette.
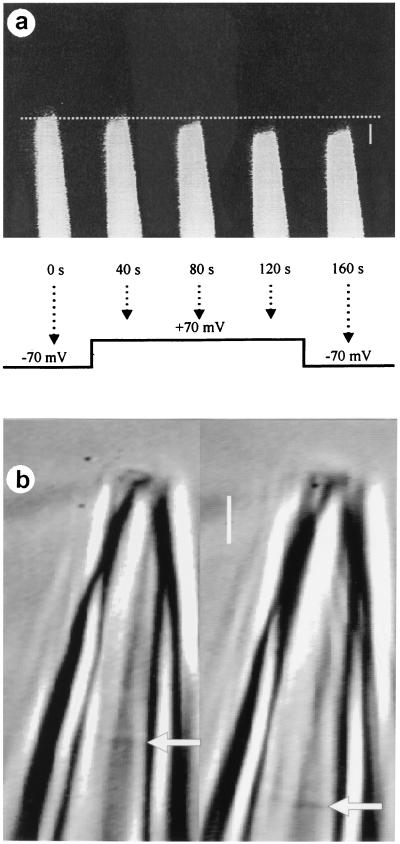
Fig. 1a shows fluorescent images of a patch pipette containing a membrane patch (after the formation of a gigaohm seal) from the surface membrane of a Xenopus oocyte. The pipette is in the on-cell configuration (21) such that the membrane in the pipette is contiguous with the surface membrane of the oocyte. The position of the membrane patch in the pipette was delimited by adding fluorescein to the solution in the pipette before forming the patch. After stepping the voltage across the patch from −70 mV to +70 mV, the membrane patch started to move into the pipette after an initial delay of ≈20 s, as indicated by the movement of the fluorescent boundary, and then continued to move over the next 80 s, for a total displacement of ≈3 μm. The membrane patch then remained at the displaced position when the voltage was stepped back to −70 mV. Visual comparisons of fluorescent images to images obtained with transmitted light (not shown) indicated that the fluorescent boundary was located at the same position as the increased density identified (see below) as the membrane patch with transmitted light.
Figure 1.
Depolarization-induced displacement of the membrane patch. (a) Fluorescent images of a patch pipette containing fluorescein dye to delimit the membrane patch shows depolarization-induced displacement of the membrane patch. The arrows indicate the points in time during the voltage step protocol when the images were obtained. The data were obtained from an on-cell patch on a Xenopus oocyte with a borosilicate glass pipette. (Bar = 4 μm.) (b) Depolarization-induced displacement of an excised membrane patch from a cultured hippocampal neuron. Depolarization of the membrane from −50 mV to +50 mV for 60 s induced 2.6-μm displacement of the patch membrane. The displacement of the membrane is shown before (Left) and after (Right) the voltage step. Arrows indicate the membrane patch. A borosilicate glass pipette was used. (Bar = 2 μm.)
Fig. 1b presents another example of depolarization-induced membrane movement. In this case, the patch of membrane was excised from a cultured rat hippocampal neuron, and the image was taken with transmitted light. Fig. 1b Left shows the position of the membrane patch at the holding potential of −50 mV, and Fig. 1b Right shows the position of the membrane patch 60 s after applying a depolarizing step to +50 mV. The membrane patch moved 2.6 μm into the pipette during the 60 s depolarization.
When borosilicate glass was used to fabricate the patch pipettes, as shown in Fig. 1, depolarization-induced displacement of the membrane into the pipette was observed in 38 of 40 examined patches from Xenopus oocytes, in 7 of 7 examined patches from cultured hippocampal neurons, and in 3 of 3 patches when the membrane was delimited with fluorescein. Displacement was observed with both excised patches and on-cell patches. In 26 patches from Xenopus oocytes in which the movement was measured quantitatively, the mean movement of the membrane patch during the depolarizing voltage steps was 0.9 μm, with a range of 0.2–3.0 μm. In only 4 of the 26 patches was there any movement back toward the tip of the pipette (−0.2 to −0.3 μm) after stepping back to the negative potentials. Repeated application of depolarizing voltage steps resulted in additional delayed displacement of the membrane patch into the pipette, except that the magnitude of the displacement could become less with successive applications of the depolarizing steps.
To examine whether the voltage-induced membrane displacement was related to the suction that was typically used to form a patch, we also obtained patches without using suction. In these experiments, touching the tip of the pipette to the cell and applying a voltage of −50 to −80 mV to the pipette for several minutes resulted in movement of the membrane into the pipette and the formation of a tight seal. (Applying negative potential to the shank of the pipette is equivalent to depolarization if a patch is present.) In the absence of negative pressure, membrane displacement and patch formation were not observed unless negative voltage was applied to the pipette. The depth of the membrane patch in the patch pipette after a tight (gigaohm) seal had formed was typically similar to the depth when the patch was formed by suction. For patches formed without suction, depolarization steps induced further displacement of the patches into the patch pipette, similar to that observed for patches initially formed by suction. Thus, suction is not necessary for tight-seal formation or for voltage-induced displacement of the membrane into the patch pipette.
The displacement of the membrane patch into the pipette by depolarization might be expected to lead to increases in membrane tension. Consistent with this idea, the following section shows that the depolarization-induced displacement is correlated with activation of MS channels in the patch.
Depolarization That Induces Membrane Displacement Also Activates MS Channels.
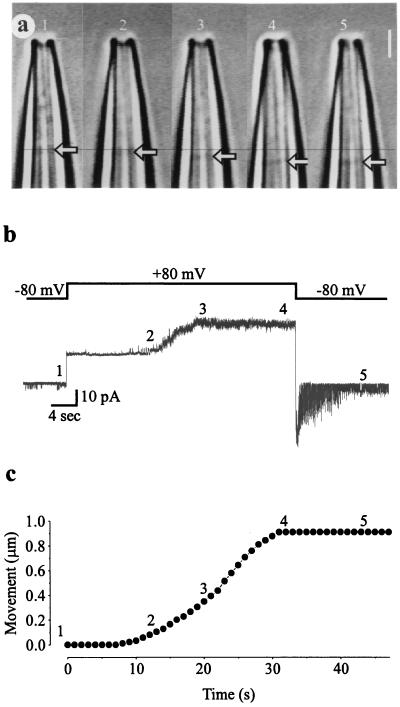
A simultaneous recording of video images of a membrane patch and currents through the same patch is shown in Fig. 2 for a patch of membrane excised from a Xenopus oocyte. The membrane potential was stepped from −80 to +80 mV for 37 s and then stepped back to −80 mV (Fig. 2b, Upper trace). Images of the membrane patch (arrows) in the pipette before, during, and after the depolarizing voltage step are shown in Fig. 2a, and the changes in the position of the membrane patch in the pipette are plotted against time after the depolarizing step in Fig. 2c. After the depolarizing step, the membrane started to move into the patch pipette after a delay of ≈8 s, reaching a displacement of ≈1 μm during the next 20 s. After repolarization, the membrane remained at its displaced position.
Figure 2.
Depolarization-induced displacement of the membrane patch is associated with activation of MS channels. (a) High-resolution video microscopy of a patch of membrane in a patch pipette. The membrane patch (arrows) moves into the pipette during depolarization. (Bar = 2 μm.) (b) After a voltage step from −80 to +80 mV (Upper trace), the nine MS channels in the membrane patch start to open after a delay of ≈10 s (Lower trace), with full activation at ≈20 s. When the voltage is stepped back to −80 mV, the MS channels then deactivate over a period of ≈10 s. Channel opening is indicated by upward current steps at positive membrane potentials and downward current steps at negative membrane potentials. The leakage current through the patch, indicated by the 18-pA current steps that coincide with the voltage steps, was not subtracted from the current record. (c) Plot of the distance moved by the membrane patch vs. time after the voltage step. The corresponding numbers 1–5 in the three parts of the figure indicate the time points for simultaneous measurement of video and current data from the same inside-out patch excised from a Xenopus oocyte with a borosilicate glass pipette.
The depolarizing voltage step also activated MS channels in the patch with a delay that lagged the movement of the membrane. The nine MS channels in the patch started to activate a few seconds after the membrane started to move, and reached maximum activation during the next 10 s of membrane movement (Fig. 2b, Lower trace). The MS channels then deactivated when the voltage was stepped back to the holding potential. This voltage-dependent delayed activation and deactivation of MS channels in patches of membrane excised from Xenopus oocytes is a characteristic feature of these channels (6, 7). Repeated application of depolarizing voltage steps resulted in additional delayed displacement of the membrane patch into the pipette, as mentioned in the previous section, and each additional delayed displacement of the membrane was accompanied by delayed activation of MS channels, similar to that shown in Fig. 2 a–c. In 15 of 15 membrane patches in which the maximum number of MS channels in a patch could be clearly determined because of their small number (1–3 channels), there was no change in the maximum number of MS channels activated by repeated depolarizing voltage steps.
Because MS channels can be activated by stretching the membrane (1–5), a simple explanation for the delayed activation of MS channels by depolarization (Fig. 2) is that the voltage step does not activate the MS channels directly but rather activates them indirectly through depolarization-induced movement of the membrane patch. If it is the movement of the membrane patch that activates MS channels, as suggested by the data presented in Fig. 2, then a number of predictions can be made: (i) activation of MS channels should correlate with movement of the membrane patch; (ii) movement should precede channel activation; (iii) factors that decrease the latency to movement should also decrease the latency to activation; and (iv) preventing movement of the membrane should prevent activation of MS channels. Each of these predictions was examined in turn.
Depolarization-Induced Membrane Displacement and MS Channel Activation Are Correlated.
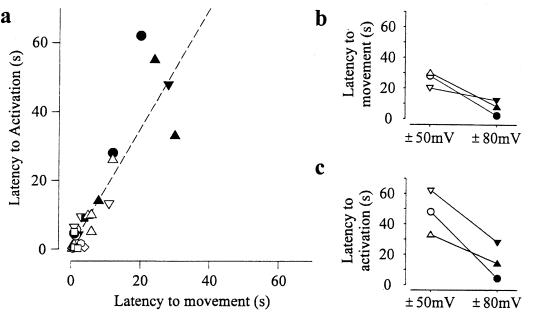
Latencies to both movement and activation are shown in Fig. 3a for 26 depolarizing steps obtained from 17 separate patches from Xenopus oocytes in which simultaneous recordings were made of both video images and single-channel currents through MS channels. The dashed line indicates a correlation between the latency to movement and the latency to activation (linear regression, r = 0.88). The observed slope of the regression line of 1.6 indicates that movement precedes activation. In individual measurements, movement preceded activation in 21 of the 26 measurements. However, for five measurements with brief latencies to both movement and activation, the measured latency to movement followed the measured latency to activation. Activation preceding movement at brief latencies may simply reflect the uncertainty in detecting the initial onset of movement. Alternatively, activation preceding movement for brief latencies may indicate that, in some cases, the depolarization-induced processes involved in displacing the membrane may increase membrane tension sufficiently to activate MS channels before significant movement can be detected.
Figure 3.
Correlation between depolarization-induced membrane displacement and channel activation. (a) Plot of the latency to the onset of movement of the membrane patch vs. the latency to the onset of channel openings. Latency is measured from the time of the voltage step. The linear regression (dashed line) had a correlation coefficient of 0.88 and a slope of 1.6, indicating that activation follows movement. Data from each patch are indicated by a different symbol. Holding potentials ranged from −80 to −50 mV, and the voltage was stepped to voltages ranging from +50 to +80 mV. (b) Three different patches (indicated by different symbols) in which depolarization steps between both ±50 mV and ±80 mV were applied for each patch. The latency to both membrane movement and channel activation was decreased with larger voltage steps. The latency to membrane movement was defined as the time from the onset of depolarization to the beginning of membrane movement. The beginning of movement was estimated by projecting a line drawn through the points between about 20% and 80% of the maximal movement to intercept the time axis. The latency to channel activation was defined as the time from the onset of depolarization to the beginning of channel activation and was estimated by using the same approach used for membrane movement. Borosilicate glass pipettes were used.
In three of three examined patches, decreasing the latency to membrane movement by increasing the magnitude of the depolarizing voltage step (Fig. 3b) also decreased the latency to activation (Fig. 3c). Thus, activation of MS channels is correlated with movement of the membrane patch.
Preventing Depolarization-Induced Membrane Displacement Prevents MS Channel Activation.
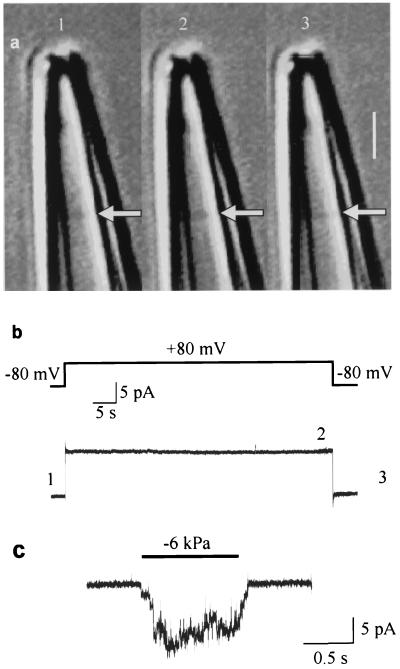
Two different experimental approaches were used to test whether preventing movement of the membrane also prevented activation of MS channels. The first approach was based on an observation made in a previous study: depolarization-induced delayed activation of MS channels no longer occurs when the tips of the borosilicate glass pipettes are coated with soft glass (7). Perhaps this lack of activation with soft glass arose from a lack of movement of the membrane patch, because soft glass has a different composition and charge density that may interact with the membrane differently than hard glass (24). To examine this possibility, the tip of a borosilicate pipette was coated with soft glass (see Materials and Methods); a membrane patch was formed, and a depolarizing voltage step was applied while the membrane patch was observed. The patch no longer moved (Fig. 4a), and delayed activation of MS channels no longer occurred (Fig. 4b). This lack of depolarization-induced activation was not due to the absence of MS channels in the membrane patch, because applying negative pressure to the pipette activated MS channels in the same patch (Fig. 4c). A lack of both membrane movement and activation of MS channels by depolarization was observed in seven of seven experiments in which the tips of the pipettes were coated with soft glass.
Figure 4.
Depolarization does not produce membrane displacement or activate MS channels when the tip of a borosilicate pipette is coated with soft glass. The simultaneous video images in a and current recordings in b indicate that a 60-s depolarizing step from −80 to +80 mV does not displace the membrane patch (arrows) in the pipette or activate MS channels. There is also a lack of movement and activation when the potential is stepped back to −80 mV. The numbers 1–3 in a and b indicate the times of the records. (Bar = 2 μm.) (c) MS channels were present in the same patch, as indicated by their activation by negative pressure (−6 kPa) applied to the patch pipette (downward current steps at the −80 mV potential).
The second approach used to prevent movement of the membrane patch was to apply positive pressure (≈0.5 kPa) to the back of the patch pipette. In three of five patches in which the applied positive pressure was sufficient to prevent depolarization-induced movement of the patch, depolarization did not activate the MS channels in the patch. In the remaining two patches, the pressure applied to prevent movement activated the MS channels directly; thus, it was not possible to examine whether a depolarizing step would activate the channels. The results in this section show, then, that preventing the depolarization-induced displacement of the membrane patch by either of two rather different mechanisms, soft glass or pressure, prevented the depolarization-induced activation of MS channels.
Discussion
High-resolution images of membranes in patch pipettes have been presented previously (22, 23, 25, 26), and it has been shown that rapid changes in voltage can cause rapid submicrometer movements of the suspended part of the membrane patch (27–30). Our study shows that prolonged depolarization of a membrane patch in a borosilicate pipette can lead to substantial displacement of the membrane patch into the pipette and that this displacement correlates in time with the activation of MS channels in the same patch. Decreasing the delay to displacement of the membrane patch decreased the delay to activation, and blocking the displacement by either of two different means prevented activation of the MS channels. Because increased membrane tension can activate MS channels (5), a possible explanation for how the depolarization-induced displacement activates MS channels would be through movement-associated increases in the tension in the patch.
Further support that activation of MS channels is associated with the depolarization-induced movement rather than the direct effect of voltage on the MS channels is our previous observation that depolarizing voltage steps applied to whole Xenopus oocytes activate MS channels in patched membrane on the same oocyte but do not activate MS channels in membrane outside of the patch pipette (7). Activation through depolarization-induced increases in membrane tension associated with displacement of the patch would also account for the apparent cooperative delayed activation of the MS channels in the patch described previously (6), because MS channels distributed in a patch of membrane could all sense changes in tension simultaneously in the patch.
The nature of the depolarization-induced forces that give rise to membrane displacement is not known, but the displacement of the patch would be expected to be associated with the flow of lipid along the wall of the pipette (23). The observation that depolarization-induced membrane displacement does not occur when the borosilicate pipette is coated with soft glass indicates that the membrane movement is either directly related to or affected by the interactions between the membrane and the glass of the pipette. Coupling of the lipid bilayer to the glass of a borosilicate pipette supports tensions on the order of 0.5–4.0 dyne/cm (refs. 31 and 32; 1 dyne = 10 μN). Perhaps voltage changes the adhesion of the membrane to the borosilicate glass at the site of seal formation or where the patch of membrane contacts the patch pipette, enabling membrane displacement and development of tension. Voltage-induced flexing of the membrane patch arising from the converse flexoelectric effect (28, 30, 33) might possibly contribute in some way toward moving the membrane or relaxing the contact between the membrane and the glass, allowing the membrane to move. There is also the possibility that cytoskeletal elements might be involved in some way. Although it is unclear which forces are involved in the depolarization-induced membrane movement and apparent increase in membrane tension, these forces dissipate with a time course of several seconds after the depolarization is removed, because the MS channels deactivate with this time course when the depolarization is removed.
Displacement of the membrane patch into the pipette by depolarization would be expected to increase the area of the patch because of the increasing diameter of our tapered patch pipettes. Thus, possible changes in some parameters arising from depolarization-induced changes in the area of membrane patches during an experiment might be attributed mistakenly to direct effects of voltage rather than to changes in the area of the patch.
Because membrane tension (stretch) can modulate a diversity of voltage and ligand gated ion channels in addition to stretch-activated channels (8–17, 34) as well as some ion transporters (18, 19), our findings suggest that some actions of depolarization on inside-out and on-cell patches of membrane formed with borosilicate pipettes may reflect voltage-induced changes in membrane tension rather than the direct effects of voltage.
Acknowledgments
We thank D. Nonner and J. Barrett for providing tissue-cultured neurons and F. Sachs for suggesting that voltage might move the membrane. This work was funded in part by Grant 93-00061 from the US–Israel Binational Science Foundation, by funds from the Zlotowski Center for Neuroscience (to S.D.S.), by National Institutes of Health Grant AR32805, and by a grant from the Muscular Dystrophy Association (to K.L.M.). Z.G. is a recipient of the Kreitman doctoral fellowship and the Folks Foundation graduate fellowship.
Abbreviation
- MS
mechanosensitive
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Morris C E. J Membr Biol. 1990;113:93–107. doi: 10.1007/BF01872883. [DOI] [PubMed] [Google Scholar]
- 2.Sackin H. Annu Rev Physiol. 1995;57:333–353. doi: 10.1146/annurev.ph.57.030195.002001. [DOI] [PubMed] [Google Scholar]
- 3.Hamill O P, McBride D W., Jr Topics Neurosci. 1996;19:258–261. doi: 10.1016/S0166-2236(96)30009-X. [DOI] [PubMed] [Google Scholar]
- 4.Guharay F, Sachs F. J Physiol. 1984;352:685–701. doi: 10.1113/jphysiol.1984.sp015317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sukharev S I, Sigurdson W J, Kung C, Sachs S. J Gen Physiol. 1999;113:525–539. doi: 10.1085/jgp.113.4.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Silberberg S D, Magleby K L. J Physiol. 1997;505:551–569. doi: 10.1111/j.1469-7793.1997.551ba.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gil Z, Magleby K L, Silberberg S D. Biophys J. 1999;76:3118–3127. doi: 10.1016/S0006-3495(99)77463-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hisada T, Ordway R W, Kirber M T, Singer J J, Walsh J V., Jr Pflügers Arch. 1990;417:493–499. doi: 10.1007/BF00370945. [DOI] [PubMed] [Google Scholar]
- 9.Bear C E. Biochim Biophys Acta. 1991;1069:267–272. doi: 10.1016/0005-2736(91)90134-t. [DOI] [PubMed] [Google Scholar]
- 10.Bear C E, Li C. Am J Physiol. 1991;261:C1018–C1024. doi: 10.1152/ajpcell.1991.261.6.C1018. [DOI] [PubMed] [Google Scholar]
- 11.Chang W, Loretz C A. J Exp Biol. 1992;169:87–104. [Google Scholar]
- 12.Kirber M T, Ordway R W, Clapp L H, Walsh J V, Jr, Singer J J. FEBS Lett. 1992;297:24–28. doi: 10.1016/0014-5793(92)80319-c. [DOI] [PubMed] [Google Scholar]
- 13.Langton P D. J Physiol. 1993;471:1–11. doi: 10.1113/jphysiol.1993.sp019887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Taniguchi J, Takeda M, Yoshitomi K, Imai M. J Membr Biol. 1994;140:123–132. doi: 10.1007/BF00232900. [DOI] [PubMed] [Google Scholar]
- 15.Vandorpe D H, Small D L, Dabrowski A R, Morris C E. Biophys J. 1994;66:46–58. doi: 10.1016/S0006-3495(94)80749-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mienville J M, Barker J L, Lange G D. J Membr Biol. 1996;153:211–216. doi: 10.1007/s002329900124. [DOI] [PubMed] [Google Scholar]
- 17.Marunaka Y, Shintani Y, Downey G P, Niisato N. J Gen Physiol. 1997;110:327–336. doi: 10.1085/jgp.110.3.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sasaki N, Mitsuiye T, Wang Z, Noma A. Circ Res. 1994;75:887–895. doi: 10.1161/01.res.75.5.887. [DOI] [PubMed] [Google Scholar]
- 19.Wright A R, Rees S A, Vandenberg J I, Twist V W, Powell T. J Physiol. 1995;488:293–301. doi: 10.1113/jphysiol.1995.sp020967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nonner D, Barrett E F, Barrett J N. J Neurosci. 1996;16:6665–6675. doi: 10.1523/JNEUROSCI.16-21-06665.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hamill O P, Marty A, Neher E, Sakmann B, Sigworth F J. Pflügers Arch. 1981;391:85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- 22.Sokabe M, Sachs F. J Cell Biol. 1990;111:599–606. doi: 10.1083/jcb.111.2.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sokabe M, Sachs F, Jing Z. Biophys J. 1991;59:722–728. doi: 10.1016/S0006-3495(91)82285-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Corey D P, Stevens C F. In: Single-Channel Recording. Sakmann B, Neher E, editors. New York: Plenum; 1983. pp. 53–68. [Google Scholar]
- 25.Ruknudin A, Song M J, Sachs F. J Cell Biol. 1991;112:125–134. doi: 10.1083/jcb.112.1.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Horber J K H, Mosbacher J, Haberle W, Ruppersberg J P, Sakmann B. Biophys J. 1995;68:1687–1693. doi: 10.1016/S0006-3495(95)80346-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kalinec F, Holley M C, Iwasa K H, Lim D J, Kachar B. Proc Natl Acad Sci USA. 1992;89:8671–8675. doi: 10.1073/pnas.89.18.8671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Todorov A T, Petrov A G, Fendler J H. J Phys Chem. 1994;98:3076–3079. [Google Scholar]
- 29.Gale J E, Ashmore J F. Nature (London) 1997;389:63–66. doi: 10.1038/37968. [DOI] [PubMed] [Google Scholar]
- 30.Masbacher J, Langer M, Horber J K H, Sachs F. J Gen Physiol. 1998;111:65–74. doi: 10.1085/jgp.111.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Opsahl L R, Webb W W. Biophys J. 1994;66:71–74. doi: 10.1016/S0006-3495(94)80751-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Akinlaja J, Sachs F. Biophys J. 1998;75:247–254. doi: 10.1016/S0006-3495(98)77511-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Petrov A G, Miller B A, Hiristova K, Usherwood P N. Eur Biophys J. 1993;22:289–300. doi: 10.1007/BF00180263. [DOI] [PubMed] [Google Scholar]
- 34.Tabarean I V, Juranka P, Morris C E. Biophys J. 1999;77:758–774. doi: 10.1016/S0006-3495(99)76930-4. [DOI] [PMC free article] [PubMed] [Google Scholar]