Abstract
Repeated exposure to a stimulus facilitates its processing. This is reflected in faster and more accurate identification, reduced perceptual identification thresholds, and more efficient classifications for repeated compared with novel items. Here, we test a hypothesis that this experience-based behavioral facilitation is a result of enhanced communication between distinct cortical regions, which reduces local processing demands. A magnetoencephalographic investigation revealed that repeated object classification led to decreased neural responses in the prefrontal cortex and temporal cortex. Critically, this decrease in absolute activity was accompanied by greater neural synchrony (a measure of functional connectivity) between these regions with repetition. Additionally, the onset of the enhanced interregional synchrony predicted the degree of behavioral facilitation. These findings suggest that object repetition results in enhanced interactions between brain regions, which facilitates performance and reduces processing demands on the regions involved.
Keywords: functional communication, learning, magnetoencepholography, synchrony, memory
Previous experience with a stimulus facilitates its subsequent processing and leads to a fundamental form of learning termed priming. This experience-based facilitation manifests in faster and more accurate responses for repeated stimuli relative to novel stimuli. Because priming is an elementary form of learning, revealing the cognitive and neural mechanisms that underlie repetition priming is critical to our complete understanding of how experience facilitates behavior.
Previous neuroimaging studies have consistently demonstrated reduced neural activity particularly in prefrontal cortex (PFC) and temporal cortex for repeated relative to novel stimuli during object-recognition and object-decision tasks (1–5). The regions that show response reductions are those that are critical to processing a stimulus in the context of the particular experimental paradigm (6). The findings of neural response reduction coupled with behavioral facilitation in repetition priming have led to the proposal that these neural reductions reflect more efficient stimulus processing and/or improvements in stimulus-related decision processes (3, 6–10). Specifically, repetition is hypothesized to induce local neural changes that speed access to relevant object knowledge thereby facilitating performance. However, recent work has demonstrated the importance of cross-cortical interactions, particularly top-down influences originating from the PFC, in object processing (11, 12). Furthermore, these interactions between top-down and bottom-up processes may be critical to the neural and behavioral manifestations of priming (3, 4, 6, 12, 13). Here, we present results consistent with a mechanism by which the interactions between cortical regions involved in identification and object representations in the temporal cortex (14, 15) and executive and selection processes in the PFC (16–18) are selectively optimized with repetition.
The importance of cross-cortical communication in priming has been suggested by previous research demonstrating that, during encoding, PFC-temporal interactions were stronger at stimulus onset for words that showed subsequent behavioral facilitation (priming) (19). Because the interaction between the PFC and temporal regions was anticipatory, this result suggests that attention may modulate PFC–temporal interactions during encoding (20). Moreover, disrupting PFC activity during encoding attenuates the subsequent behavioral gains and neural response reductions associated with priming in both the PFC and temporal cortex (4). These previous studies support the hypothesis that PFC–temporal cortex interactions are critical during the learning process (6, 9). However, these studies cannot directly address the mechanisms by which behavior is facilitated on subsequent exposures when repeated information is retrieved.
To examine the potential relationship between priming and cross-cortical communication, we examined the functional connectivity between PFC and temporal cortex during repetition. These regions were chosen based on prior results that showed that local activation reductions in these regions were associated with priming (1–3, 5, 21) and that disruption of PFC functioning has subsequent effects on the neural changes associated with priming in temporal cortex (4). Finally, we hypothesized that changes in functional connectivity would correlate with behavioral facilitation if cross-cortical communication were a critical component of priming.
We administered a standard priming task involving repeated size classifications of line drawings of everyday objects (1, 21) to test these hypotheses. We measured neural activity using high temporal-resolution magnetoencephalography (MEG) in 16 subjects while they engaged in study and test phases of the experiment. During the study phase, each object was repeated three times, immediately followed by a test phase in which the studied objects were presented for a fourth time (repeated objects) interspersed with an equal number of novel objects (“repeated” is used to refer to the fourth presentation of objects and “novel” is used to refer to the novel objects presented in the test phase of the experiment). During the entire experiment, the subject's task was to make size classifications (e.g., “is this object bigger than a shoebox?”) (1, 21). Repeated size classifications have been shown to lead to reductions in neural activity in PFC and temporal regions (1, 2, 22, 23).
To examine the relationship between cross-cortical communication and priming, we examined synchrony‖ of the MEG signal between the PFC and temporal cortex. Previous research has indicated that this measure can reflect an improvement in functional connectivity between specific cortical regions (24, 25). We examined cross-cortical synchrony between the PFC and temporal cortex for repeated and novel stimuli by calculating phase-locking values (PLVs) (26, 27). PLVs are a measure of the trial-by-trial variance in the phase relationship between two signals at a particular frequency. Larger PLVs reflect smaller trial-by-trial variance and therefore greater coupling between the phases of the signals, i.e., greater functional connectivity.
Synchrony analysis was focused on the α and β frequency bands because these frequency bands have shown the most reliable relationship with learning (19), semantic analysis (28), and visual attention (29). Additionally, theoretical and experimental work suggests that the lower-frequency bands, such as α and β, are more critical for long-range communication than the higher-frequency bands, which predominate for local communication (11, 24, 30, 31).
In addition to examining the cross-cortical interactions resulting from priming, it is important to determine the direction of information flow in the cortex during priming. Recent studies have suggested that repetition-related reductions in object-knowledge regions result from top-down modulation from PFC (4). The question of whether the cortical interactions potentially underlying priming are mainly from the PFC to the temporal cortex or from the temporal cortex to the PFC can be examined by using a method for determining the direction of information flow between two synchronous signals (32). Specifically, direction is determined by calculating the directional PLV (dPLV), which is a measure of how strong relatively earlier time windows of one signal is phase-locked to relatively later time windows of a second signal. If the past phase of the signal from one cortical region is phase-locked to the current phase of the signal in a second region, then we may infer that the first region directly or indirectly influences the measured response in the second neural region.
Results
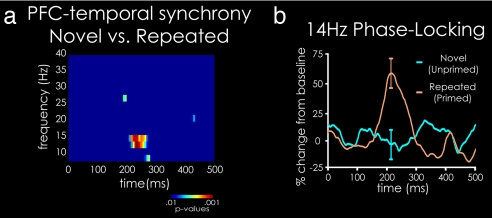
Priming resulted in reaction times that were 145 ms shorter for repeated objects than for novel stimuli [repeated (788 ms) < novel (933 ms), T15 = 6.33, P < 0.001]. This behavioral facilitation was accompanied by a significant decrease in the magnitude of the event-related, evoked MEG activity (1–5), as measured by dynamic statistical parameter mapping power, in the PFC from 333 ms and the temporal cortex from 406 ms after stimulus onset [see supporting information (SI) Fig. S1]. Critically, synchrony between the signals from the PFC and temporal cortex was significantly greater than during 500 ms of prestimulus baseline for repeated stimuli in the low β (≈12–14 Hz) frequency band and failed to reach significance for novel stimuli (Fig. 1 and Fig. S2). Synchrony between the PFC and temporal cortex was significantly stronger for repeated than for novel stimuli during the time period from 190 ms to 270 ms after stimulus onset (P = 0.018 corrected for multiple comparisons) and peaked at 215 ms (Fig. 2 a and b), a time approximately coinciding with or preceding the onset of reduced MEG activity during repetition priming (Fig. S1)(5, 21, 33–35). The regions of PFC and temporal cortex revealing significant synchronous activity were consistent with those that show response reductions in fMRI using the same paradigms used here (1) and other, similar visual object priming paradigms (2–4). Additionally, across individual subjects, significantly greater, P < 0.05, synchrony was seen for repeated compared with novel stimuli in 13 of 16 subjects at some point between 100 and 300 ms, in 9 of 16 subjects at 215 ms, and in 8 of 16 subjects across the entire 190- to 270-ms time window (with an additional three subjects showing a P < 0.1 trend over this time window). The probability that 9 of 16 subjects show P < 0.05 is <0.00001 and for 8 of 16 subjects P < 0.0001 when a Bernouli distribution, corrected for multiple comparisons, is used.
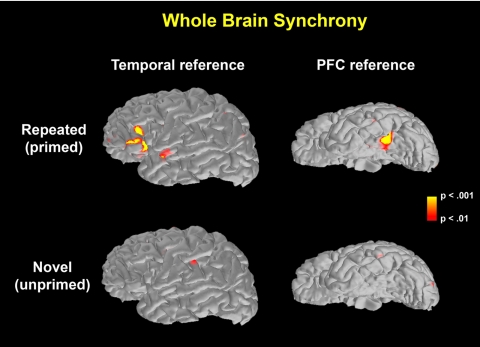
Fig. 1.
Peak synchrony (14 Hz, 215 ms, see Fig. 2) between a single reference ROI and the entire brain relative to prestimulus baseline synchrony. For a reference ROI in the temporal cortex, significant synchrony is seen in a relatively localized region of the PFC for primed, but not novel, objects. For a reference ROI in the PFC, significant synchrony is seen in a relatively localized region of the temporal cortex for primed, but not novel, objects. These results demonstrate in a reciprocal manner that there is significant synchrony between relatively restricted portions of the PFC and temporal cortex for primed, but not novel, objects. Note that the images on the left side of the figure are lateral views of the left hemisphere, and the images on the right side are ventral views. Other views across both hemispheres demonstrate that the left PFC and temporal cortex are the loci of significant synchrony (see Fig. S2 for other views of the brain).
Fig. 2.
Phase-locking analysis, demonstrating greater synchrony between prefrontal and temporal cortical regions for repeated vs. novel visual objects. (a) T test on PLVs across subjects from 8–40 Hz and 0–500 ms after stimulus for repeated (fourth presentation) vs. novel trials, demonstrating significant differential phase synchrony concentrated in the low β frequency band (≈12–14 Hz). (b) PLV time course at 14 Hz, indicating significantly greater phase locking for repeated vs. novel trials 190–270 ms after stimulus onset.
The dPLV analysis demonstrated that the phase of the PFC activity at relatively earlier times was, in fact, predictive of the later phase of the temporal activity (mean = 29.5 ms, SE = 10.7 ms, T15 = 2.76, P = 0.015), suggesting that information was projected from the PFC to the temporal cortex.
We tested the specificity of these synchrony results to interactions between the PFC and temporal cortex by examining whether phase locking was due to global synchronization. First examined was the parietal cortex. Inconsistent with the possibility of global synchronization, PLVs failed to reach statistical significance between the PFC and parietal cortex (approximately corresponding to the left intraparietal region) (T15 = 0.85, P = 0.41) and between the temporal and parietal cortices (T15 = 1.14, P = 0.27) for the frequency and time period of significant PFC–temporal synchrony (190–270 ms, ≈12–14 Hz). PLVs failed to reach statistical significance between the PFC and parietal cortex or between the temporal and parietal cortices across all time windows from 0 to 500 ms. Additionally, a whole brain analysis of the synchrony from the temporal cortex to the rest of the brain and from the PFC to the rest of the brain also supported the idea that the cross-cortical interaction is specific to these two regions (Fig. 1 and Fig. S2).
To rule out the possibility that the increased synchrony could be explained by coincidental phase-locking to the stimulus onset, a trial-shuffle method (26, 27) was used. The results of this analysis demonstrate that synchrony was not due to coincidental phase locking to the stimulus onset both within subjects (15 of 16 subjects, P < 0.05) and across subjects (P < 0.01 for every time point between 190 and 270 ms). This trial-shuffled result confirms that the synchrony increase was due to the PFC phase-locking to the temporal cortex and not due to each region being independently phase-locked to the stimulus onset.
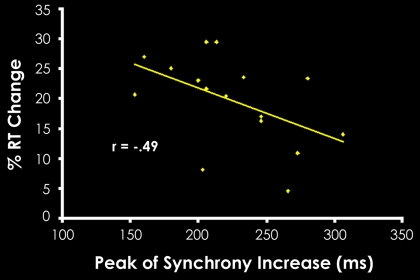
Correlating the synchrony results with behavioral facilitation in object classification revealed a connection between PFC–temporal synchrony and improved behavioral performance in repetition priming. Specifically, subjects that showed greater behavioral priming also had a trend for an earlier peak in learning-related synchrony (defined as the time point of maximum repeated PLV − novel PLV), between the PFC and temporal cortex (r = −.49, P = 0.052; Fig. 3). Because the difference between repeated and novel PLV reflects how learning affects neural interactions, this result demonstrates that the earlier that learning-related neural synchrony manifests, then the greater is the facilitation of performance. The increase of synchrony for repeated relative to novel stimuli, as well as the correlation between the latency of synchrony and behavior, supports the hypothesis that optimized cortical communication induced by learning plays a critical role in priming-related behavioral facilitation.
Fig. 3.
Correlation between behavioral priming levels and the latency of peak synchrony increase across subjects. The peak of synchrony increase was defined as the time point at which synchrony for repeated stimuli minus synchrony for novel stimuli was the greatest. These results demonstrate that those subjects whose peak synchrony increase for repeated items occurred earlier in time also demonstrated greater performance advantages for these items relative to novel items.
Discussion
This study examined the critical importance of experience-induced changes in communication across neural regions for priming and improved performance. The findings we report here demonstrate that there is increased cross-cortical synchrony for repeated images between the PFC and temporal cortex, two central cortical areas where decreases in activation are typically observed. Additionally, the timing of the increased cross-cortical synchrony correlated with behavioral facilitation, demonstrating the importance of synchrony as a neural correlate of priming. This correlation suggests that earlier cross-cortical communication leads to greater facilitation in behavioral performance during priming. These results support a mechanism for the performance improvement associated with repetition priming whereby repeated experience optimizes communication between the PFC and temporal cortex and may have broader implications for neural basis of other forms of learning as well.
The proposed hypothesis, that improved performance in priming results from optimized communication between the PFC and temporal cortex, leads to a unique prediction. Specifically, disrupting more abstract processing in the PFC will attenuate priming not only in the PFC, but also in the “lower-level,” stimulus-driven temporal cortex because these two regions act cooperatively to facilitate behavior. In contrast, most previous hypotheses regarding the neural mechanisms of behavioral facilitation and neural-response reduction in repetition priming have suggested that processing efficiency is enhanced through local changes in neural populations (8). These local models do not predict that a higher-level disruption would effect lower-level processing. Previous findings have demonstrated attenuation of the priming induced neural response reductions in the prefrontal and temporal cortices and attenuation of the behavioral facilitation in priming when PFC activity was disrupted by transcranial magnetic stimulation (TMS) during encoding (4). If activity reductions in temporal cortex were the result of exclusively local stimulus-dependent mechanisms, then these previous results would not be possible. Instead, our current results and these prior findings (4) are predicted by a mechanism in which priming takes place as a result of facilitated interactions between cortical regions.
The results of the directionality analysis suggest that object information required for the classification task is accessed by the influence of selection and control processes in the PFC acting on object processing in the temporal cortex. The hypothesis that priming behavior depends heavily on feedback PFC processes is supported by MEG findings demonstrating that repetition-induced neural-response changes generally occur earlier in PFC than temporal cortex regions (5, 11, 21, 33, 34) and by the TMS results described previously (4). Finally, several studies demonstrate that PFC-activity reductions are a more reliable predictor of behavioral facilitation during priming paradigms than neural-response reductions in posterior temporal and inferotemporal regions (1, 36).
One hypothesis that arises from the present result is that increased synchrony may reflect a process whereby neural and computational efficiency are enhanced by selectively promoting only the cross-cortical interactions critical to individuals' cognitive objectives. This optimized communication is based on the idea that the interactions that successfully integrate information reflected in synchronous, phase-locked electrophysiological activity (24). Establishment of these synchronous interactions that optimize goal-directed behavior could reflect a Hebbian-like learning process (37), whereby synchronous activity perseveres, and asynchronous activity falls away, resulting in selective reinforcement of the cortical interactions critical to successful task completion. Alternatively, these interactions could reflect the development of stimulus–decision associations mediated by the medial temporal lobe (described in refs. 1, 38, and 39). In either case, by selectively optimizing only critical neural interactions, top-down and bottom-up processes more readily converge on an interpretation of the input stimulus (e.g., the object decision), which, in turn, improves behavioral performance (1, 3, 6, 12, 13, 38, 39). Indeed, recent modeling work demonstrates computationally how reduced activity with increased synchrony can result in more efficient processing (40).** Therefore, this hypothesis would account not only for reduced neural activity through asynchronous activity falling away but also for increased synchrony between the PFC and temporal cortex through the selective reinforcement of critical cross-cortical interactions.
This study reveals that repetition priming is associated with increased synchrony between frontal and temporal cortical regions and that the earlier subjects exhibit this increased synchrony, then the greater is the benefit to behavioral performance. Given these and earlier findings, we have described an interactions-based mechanism in which optimized communication between neural regions, rather than purely locally confined neural mechanisms, facilitates both neural processing and behavioral performance. These results underscore the critical role of further examining the dynamics of the interactions across large-scale brain networks in learning and cognition.
Methods
See SI Methods for more details on the methods.
Subjects.
Sixteen young native speakers of English (2 male, 14 female), with normal or corrected vision took part in the experiment.
Recording.
MEG signals were recorded by using a 306-channel neuromagnetometer (Vectorview; Neuromag) system in a three-layer magnetically shielded room (Imedco) that contained 102 identical sensor triplets, two orthogonal planar gradiometers, and one magnetometer, covering the entire head of the subject.
Behavioral Task.
Stimuli.
Four hundred eight colored line drawings of common animate and inanimate objects were selected from commercially available clip-art collections [CD ROM from Corel Mega Gallery (1997); Corel Corporation]. Pictures reflected varying orientations and visual size. The stimuli were presented through a back-projection system into the MEG chamber, and objects were presented centrally on a screen ≈3 feet from the MEG recording device.
Procedure.
The data for this analysis were a subset of a larger study examining specificity effects in object priming (21). The common repetition trials used here were counterbalanced across subjects for the order in which they were presented among other conditions. The behavioral task in the larger study was the same as what is described below (i.e., a size judgment). The experiment spanned two runs, each of which consisted of a study and test phase. There were a total of 50 trials in both the novel and repeated conditions per subjects across the two test runs.
During the study phase, participants made size judgments of a total of 75 items presented in pseudorandom order, consisting of 25 items repeated three times. Participants were asked to make a size judgment by deciding whether the real-life object depicted in the picture was “bigger than a shoe box” and to indicate their decision by pushing a “yes” or a “no” key with the index and middle fingers of their right hand, respectively. Each test phase consisted of 50 randomly ordered pictures. Twenty-five of these were repeated from the study phase and 25 were novel objects being seen for the first time.
The behavioral task was identical in the study and test phases of the experiment. Pictures were presented every 2 sec. Approximately 8 min separated the first repetition of an image at study and the fourth repetition in the test phase of the experiment.
All analyses and results reported were performed on the data gathered in the test phase, and therefore, “repeated” refers to the fourth presentation of the stimuli and “novel” to the new stimuli presented in the test phase.
Phase-Locking Analysis.
To determine the trial-by-trial phase locking between neural regions, we used the spectral dynamic statistical parametric mapping method (27), a method to measure phase synchrony between signals projected onto the cortical surface. This method employs the anatomically constrained minimum-norm estimate (MNE) inverse solution (5, 41) to determine PLVs (26) between regions on the cortical surface.
To calculate PLVs, the MEG sensor data were filtered by using a continuous, width-five Morlet wavelet transform at each frequency of interest. The wavelet representation of each trial was then mapped from the sensors onto the cortex by using the MNE inverse solution. The phase was then extracted from the wavelet data averaged across all dipoles in each region of interest for each trial about each time point and at each frequency of interest. The a priori frequencies of interest were in the α and β frequency bands (8–20 Hz) based on previous studies (19, 29, 31), although all even frequencies from 8 to 40 Hz were reported to fully categorize the results. The PLV was then determined by using the following formula:
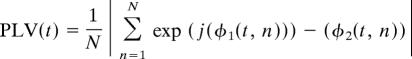 |
where φ(t, n) is the phase of the signals from the two ROIs at time t and trial n. PLVs range from 0 to 1, where 1 indicates perfect synchrony across trials, and 0 indicates that the phase relationship is completely random.
Statistical Testing.
The cumulative distribution function was estimated for the PLVs by using the Raleigh test (27), and statistical significance was determined by using a t test across subjects both by determining significance of the PLVs compared with 500 ms of prestimulus baseline and between experimental conditions.
Nonparametric statistics were then used to compare conditions and control for multiple comparisons (42). All of the time-frequency points that were P < 0.05 were determined and clustered on the basis of temporal and frequency adjacency. Cluster-level statistics were calculated by determining the sum of the t values within each cluster (cluster mass), and the maximum cluster mass across the data was determined. Permutations were then created by collecting the data from the conditions across the 16 subjects in a single set. Half of these collected trials were placed into subset one, and the remaining were placed into subset two. The maximum cluster mass was then determined for all possible permutations of the data (216 partitions for 16 subjects). The proportion of these permutations that had a smaller maximum cluster mass than the nonpermuted data were the P value calculated by using a complete permutation test. Because of the global null hypothesis used for the cluster mass test, this method inherently controls for multiple comparisons.
For individual subject analyses, a similar statistical test was used, with permutations created across trials instead of across subjects. The time-frequency points that showed P < 0.05 in each individual were determined. A Bernouli distribution was used to find the probability that the proportion of subjects showing P < 0.05 at each time-frequency point could occur by chance. A Bonferroni adjustment was then applied to this probability to correct for multiple comparisons.
Directional Phase-Locking.
Directional phase-locking is based on the theory that if information from “X” can predict future information about “Y,” it is likely that X is driving Y. Phase-locking values measure how predictive the phase of X in a particular time window is of the phase of Y in the same time window and vice versa. Therefore, to calculate directional phase-locking, PLVs are determined between all possible time shifts of the two signals. This measure determines how predictive the past phase of X is of the future phase of Y and vice versa. We infer that the direction of information flow is from X to Y if the past phase of X is more predictive of the phase of Y than the present phase of X and that X is more predictive of Y than chance (chance distribution taken from the prestimulus baseline) (32).
The dPLV is defined as the maximal PLV over all shuffles of the signals about a particular time window
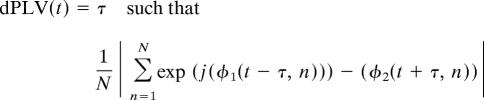 |
is maximal for all possible time shuffles τ. Therefore, dPLV reflects the amount that the first signal should be shuffled into the past for maximum predictability of the future phase of the second signal. To minimize the possibility of finding spurious directional interactions (i.e., false-positives), dPLVs are calculated only for time-windows where the underlying PLVs are significant relative to baseline. Statistical values are established by calculating t values across subjects of the dPLV relative to the expected value of the dPLV (i.e., zero).
Region of Interest (ROI) Selection.
For the whole-brain analyses (Fig. 1), functional ROIs were created by first determining the region in the PFC that demonstrated the strongest power for the third presentation of the stimuli (43). This region was used as a reference region, and the phase-locking between this region and the entire brain was determined for the fourth presentation. For novel objects (in Fig. 1), the reference region was determined by finding the portion of the PFC that demonstrated the strongest power for these novel objects. This ROI selection was used because the use of the response for novel objects to determine the reference ROI gives the greatest chance of seeing synchrony in this condition, if there is any. Therefore, this is an extremely conservative ROI selection criterion, biased against our effects of interest. The same process was repeated for the reference ROIs in the temporal cortex.
For the ROI analysis (Figs. 2 and 3), two regions were needed, a reference region and a target region. The reference region was found by locating the PFC region that showed maximal power for the third presentation (same as for Fig. 1). We then determined the target region in the temporal cortex that demonstrated maximal phase-locking to this PFC reference region in the third presentation. ROI search was restricted to subregions of the PFC and temporal cortex that have been previously demonstrated to show response reductions (1–5). These ROIs were then used to compare the phase-locking for novel versus repeated (fourth presentation) stimuli (Fig. 2) and for the correlation analysis (Fig. 3). This method for determining ROIs for individual subjects avoids issues with statistical circularity because the data used for ROI selection (i.e., the third presentation) was collected independently of the data used in the analysis (i.e., the fourth presentation and novel stimuli). This method of ROI selection can be compared favorably with the independent cross-validation method used in fMRI analysis (44). It is important to note that this ROI selection is also completely independent of the timing of the synchrony, which is a main result of the present work. The same process was also reversed, i.e., a reference ROI in the temporal cortex and a target ROI in the PFC were determined, and the results hold for this reversal as well. The frontal region included the anterior and posterior inferior frontal gyri, the inferior frontal sulcus, and the anterior portion of the precentral sulcus. The temporal region included the fusiform gyrus, the occipitotemporal sulcus, and the collateral sulcus.
Supplementary Material
Acknowledgments.
We thank L. Nichols for data collection; M. S. Hämäläinen, D. Handwerker, and F.-H. Lin for assistance with analysis; and E. Aminoff, N. Gronau, K. S. Kassam, K. Kverega, A. Martin, W. K. Simmons, and G. Wig for critical readings of the manuscript. This work was supported by National Institutes of Health Grant K23MH64004 (to D.M.S.), National Institute of Mental Health Grant R01 MH073982–01A1 (to I.G.D.), National Institute of Neurological Disorders and Stroke Grants R01 NS44319–01 and R01 NS050615 (to M.B.), National Center for Research Resources Regional Resource Grant P41RR14075, and the M.I.N.D. Institute.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0710674105/DCSupplemental.
Following the convention in cognitive neuroscience, we use the term “synchrony” as a general term indicating coupling between neural signals. Though the term “synchrony,” strictly speaking, applies only to coupled activity with zero phase difference (i.e. truly synchronous activity), here it refers to phase locked activity with any phase-lag.
The computational work described in (40) concentrated on reduced activity with increased local synchrony and would require modification to account for the cross-cortical synchrony seen in the present work.
References
- 1.Dobbins IG, Schnyer DM, Verfaellie M, Schacter DL. Cortical activity reductions during repetition priming can result from rapid response learning. Nature. 2004;428:316–319. doi: 10.1038/nature02400. [DOI] [PubMed] [Google Scholar]
- 2.Buckner RL, et al. Functional-anatomic correlates of object priming in humans revealed by rapid presentation event-related fMRI. Neuron. 1998;20:285–296. doi: 10.1016/s0896-6273(00)80456-0. [DOI] [PubMed] [Google Scholar]
- 3.Zago L, Fenske MJ, Aminoff E, Bar M. The rise and fall of priming: How visual exposure shapes cortical representations of objects. Cereb Cortex. 2005;15:1655–1665. doi: 10.1093/cercor/bhi060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wig GS, Grafton ST, Demos KE, Kelley WM. Reductions in neural activity underlie behavioral components of repetition priming. Nat Neurosci. 2005;8:1228–1233. doi: 10.1038/nn1515. [DOI] [PubMed] [Google Scholar]
- 5.Dale AM, et al. Dynamic statistical parametric mapping: Combining fMRI and MEG for high-resolution imaging of cortical activity. Neuron. 2000;26:55–67. doi: 10.1016/s0896-6273(00)81138-1. [DOI] [PubMed] [Google Scholar]
- 6.Henson RN. Neuroimaging studies of priming. Prog Neurobiol. 2003;70:53–81. doi: 10.1016/s0301-0082(03)00086-8. [DOI] [PubMed] [Google Scholar]
- 7.Desimone R. Neural mechanisms for visual memory and their role in attention. Proc Natl Acad Sci USA. 1996;93:13494–13499. doi: 10.1073/pnas.93.24.13494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grill-Spector K, Henson R, Martin A. Repetition and the brain: neural models of stimulus-specific effects. Trends Cognit Sci. 2006;10:14–23. doi: 10.1016/j.tics.2005.11.006. [DOI] [PubMed] [Google Scholar]
- 9.Schacter DL, Wig GS, Stevens WD. Reductions in cortical activity during priming. Curr Opin Neurobiol. 2007;171:171–176. doi: 10.1016/j.conb.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 10.Wiggs CL, Martin A. Properties and mechanisms of perceptual priming. Curr Opin Neurobiol. 1998;8:227–233. doi: 10.1016/s0959-4388(98)80144-x. [DOI] [PubMed] [Google Scholar]
- 11.Bar M, et al. Top-down facilitation of visual recognition. Proc Natl Acad Sci USA. 2006;103:449–454. doi: 10.1073/pnas.0507062103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kveraga K, Ghuman AS, Bar M. Top-down predictions in the cognitive brain. Brain Cognit. 2007;65:145–168. doi: 10.1016/j.bandc.2007.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Friston K. A theory of cortical responses. Philos Trans R Soc London Ser B. 2005;360:815–836. doi: 10.1098/rstb.2005.1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Martin A, Wiggs CL, Ungerleider LG, Haxby JV. Neural correlates of category-specific knowledge. Nature. 1996;379:649–652. doi: 10.1038/379649a0. [DOI] [PubMed] [Google Scholar]
- 15.Tanaka K. Inferotemporal cortex and object vision. Annu Rev Neurosci. 1996;19:109–139. doi: 10.1146/annurev.ne.19.030196.000545. [DOI] [PubMed] [Google Scholar]
- 16.Badre D, Poldrack RA, Pare-Blagoev EJ, Insler RZ, Wagner AD. Dissociable controlled retrieval and generalized selection mechanisms in ventrolateral prefrontal cortex. Neuron. 2005;47:907–918. doi: 10.1016/j.neuron.2005.07.023. [DOI] [PubMed] [Google Scholar]
- 17.Thompson-Schill SL, et al. Verb generation in patients with focal frontal lesions: A neuropsychological test of neuroimaging findings. Proc Natl Acad Sci USA. 1998;95:15855–15860. doi: 10.1073/pnas.95.26.15855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wagner AD, Pare-Blagoev EJ, Clark J, Poldrack RA. Recovering meaning: Left prefrontal cortex guides controlled semantic retrieval. Neuron. 2001;31:329–338. doi: 10.1016/s0896-6273(01)00359-2. [DOI] [PubMed] [Google Scholar]
- 19.Düzel E, et al. Early, partly anticipatory, neural oscillations during identification set the stage for priming. NeuroImage. 2005;25:690–700. doi: 10.1016/j.neuroimage.2004.11.038. [DOI] [PubMed] [Google Scholar]
- 20.Yi DJ, Chun MM. Attentional modulation of learning-related repetition attenuation effects in human parahippocampal cortex. J Neurosci. 2005;25:3593–3600. doi: 10.1523/JNEUROSCI.4677-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schnyer DM, Dobbins IG, Nicholls L, Schacter D, Verfaellie M. Washington, D.C.: Society for Neuroscience; 2005. Rapid Decision Learning Alters the Repetition N400 Components in Left Frontal and Temporal Regions: Evidence from MEG Recordings During Repetition Priming. [Google Scholar]
- 22.Koutstaal W, et al. Perceptual specificity in visual object priming: functional magnetic resonance imaging evidence for a laterality difference in fusiform cortex. Neuropsychologia. 2001;39:184–199. doi: 10.1016/s0028-3932(00)00087-7. [DOI] [PubMed] [Google Scholar]
- 23.Simons JS, Koutstaal W, Prince S, Wagner AD, Schacter DL. Neural mechanisms of visual object priming: Evidence for perceptual and semantic distinctions in fusiform cortex. NeuroImage. 2003;19:613–626. doi: 10.1016/s1053-8119(03)00096-x. [DOI] [PubMed] [Google Scholar]
- 24.Engel AK, Fries P, Singer W. Dynamic predictions: Oscillations and synchrony in top-down processing. Nat Rev Neurosci. 2001;2:704–716. doi: 10.1038/35094565. [DOI] [PubMed] [Google Scholar]
- 25.Varela F, Lachaux JP, Rodriguez E, Martinerie J. The brainweb: Phase synchronization and large-scale integration. Nat Rev Neurosci. 2001;2:229–239. doi: 10.1038/35067550. [DOI] [PubMed] [Google Scholar]
- 26.Lachaux JP, Rodriguez E, Martinerie J, Varela FJ. Measuring phase synchrony in brain signals. Hum Brain Mapp. 1999;8:194–208. doi: 10.1002/(SICI)1097-0193(1999)8:4<194::AID-HBM4>3.0.CO;2-C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lin FH, et al. Spectral spatiotemporal imaging of cortical oscillations and interactions in the human brain. NeuroImage. 2004;23:582–595. doi: 10.1016/j.neuroimage.2004.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.von Stein A, Rappelsberger P, Sarnthein J, Petsche H. Synchronization between temporal and parietal cortex during multimodal object processing in man. Cereb Cortex. 1999;9:137–150. doi: 10.1093/cercor/9.2.137. [DOI] [PubMed] [Google Scholar]
- 29.Gross J, et al. Modulation of long-range neural synchrony reflects temporal limitations of visual attention in humans. Proc Natl Acad Sci USA. 2004;101:13050–13055. doi: 10.1073/pnas.0404944101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kopell N, Ermentrout GB, Whittington MA, Traub RD. Gamma rhythms and beta rhythms have different synchronization properties. Proc Natl Acad Sci USA. 2000;97:1867–1872. doi: 10.1073/pnas.97.4.1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.von Stein A, Sarnthein J. Different frequencies for different scales of cortical integration: from local gamma to long range alpha/theta synchronization. Int J Psychophysiol. 2000;38:301–313. doi: 10.1016/s0167-8760(00)00172-0. [DOI] [PubMed] [Google Scholar]
- 32.Ghuman AS, Lin FH, Bar M. Dynamical Neuroscience XIV: Frontiers in Neural Signal Processing. GA: Atlanta; 2006. Directional Phase-Locking: A Method for Measuring Phase Causalitya. [Google Scholar]
- 33.Dhond RP, Buckner RL, Dale AM, Marinkovic K, Halgren E. Spatiotemporal maps of brain activity underlying word generation and their modification during repetition priming. J Neurosci. 2001;21:3564–3571. doi: 10.1523/JNEUROSCI.21-10-03564.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Marinkovic K, et al. Spatiotemporal dynamics of modality-specific and supramodal word processing. Neuron. 2003;38:487–497. doi: 10.1016/s0896-6273(03)00197-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Puce A, Allison T, McCarthy G. Electrophysiological studies of human face perception. III: Effects of top-down processing on face-specific potentials. Cereb Cortex. 1999;9:445–458. doi: 10.1093/cercor/9.5.445. [DOI] [PubMed] [Google Scholar]
- 36.Maccotta L, Buckner RL. Evidence for neural effects of repetition that directly correlate with behavioral priming. J Cognit Neurosci. 2004;16:1625–1632. doi: 10.1162/0898929042568451. [DOI] [PubMed] [Google Scholar]
- 37.Hebb DO. The Organization of Behavior. New York: Wiley; 1949. [Google Scholar]
- 38.Schacter DL, Dobbins IG, Schnyer DM. Specificity of priming: A cognitive neuroscience perspective. Nat Rev Neurosci. 2004;5:853–862. doi: 10.1038/nrn1534. [DOI] [PubMed] [Google Scholar]
- 39.Schnyer DM, Dobbins IG, Nicholls L, Schacter DL, Verfaellie M. Rapid response learning in amnesia: Delineating associative learning components in repetition priming. Neuropsychologia. 2006;44:140–149. doi: 10.1016/j.neuropsychologia.2005.03.027. [DOI] [PubMed] [Google Scholar]
- 40.Gotts SJ. Psychology. Pittsburgh: Carnegie Mellon Univ; 2003. Mechanisms underlying efficiency in neural systems. [Google Scholar]
- 41.Hamalainen MS, Hari R. Magnetoencephalographic characterization of dynamic brain activation: Basic principles and methods of data collection and source analysis. In: Toga AV, Mazziotta JC, editors. Brain Mapping: The Methods. San Diego: Academic; 2002. pp. 227–254. [Google Scholar]
- 42.Maris E, Oostenveld R. Nonparametric statistical testing of EEG- and MEG-data. J Neurosci Methods. 2007;164:177–190. doi: 10.1016/j.jneumeth.2007.03.024. [DOI] [PubMed] [Google Scholar]
- 43.Gross J, et al. Dynamic imaging of coherent sources: Studying neural interactions in the human brain. Proc Natl Acad Sci USA. 2001;98:694–699. doi: 10.1073/pnas.98.2.694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Baker CI, Hutchison TL, Kanwisher N. Does the fusiform face area contain subregions highly selective for nonfaces? Nat Neurosci. 2007;10:3–4. doi: 10.1038/nn0107-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.