Abstract
Promoter chromatin disassembly is a widely used mechanism to regulate eukaryotic transcriptional induction. Delaying histone H3/H4 removal from the yeast PHO5 promoter also leads to delayed removal of histones H2A/H2B, suggesting a constant equilibrium of assembly and disassembly of H2A/H2B, whereas H3/H4 disassembly is the highly regulated step. Toward understanding how H3/H4 disassembly is regulated, we observe a drastic increase in the levels of histone H3 acetylated on lysine-56 (K56ac) during promoter chromatin disassembly. Indeed, promoter chromatin disassembly is driven by Rtt109 and Asf1-dependent acetylation of H3 K56. Conversely, promoter chromatin reassembly during transcriptional repression is accompanied by decreased levels of histone H3 acetylated on lysine-56, and a mutation that prevents K56 acetylation increases the rate of transcriptional repression. As such, H3 K56 acetylation drives chromatin toward the disassembled state during transcriptional activation, whereas loss of H3 K56 acetylation drives the chromatin toward the assembled state.
Keywords: Asf1, K56 acetylation, transcription
The packaging of DNA together with histone proteins into chromatin is essential for regulating all of the activities of the eukaryotic genome, including repair, replication, and gene expression. The chromatin structure dynamically changes to facilitate these processes and regulate access to the DNA sequence. For example, nucleosomes are disassembled from many eukaryotic promoters during transcriptional activation to provide access to the general transcription machinery (1). The histone H3/H4 chaperone known as Asf1 contributes to the disassembly of histones H3/H4 from multiple budding yeast promoters during transcriptional activation (2–4).
Histone posttranslational modifications may also play an important role in regulating chromatin disassembly. Many of the best studied sites of histone posttranslational modifications map to the N-terminal tails of the histones that extend out from the globular core of the nucleosome and are unlikely to have a direct influence on the structure of the nucleosome. By contrast, the newly identified acetylation of lysine-56 within the globular core of histone H3 (H3 K56ac) (5, 6) is unique because it is predicted to break a DNA:histone interaction, potentially destabilizing the nucleosome. H3 K56 acetylation occurs predominantly on newly synthesized histones that are assembled into chromatin after DNA replication (6) and are rapidly deacetylated after S phase (7, 8). Functionally, K56 acetylation promotes survival after the exposure of cells to genotoxic agents because of its role in stabilizing the replisome (6, 9). The histone acetyltransferase (HAT) responsible for acetylation of K56 on newly synthesized histones is Rtt109 (10–12) and needs either of two histone chaperones, Asf1 or Vps75, for enzymatic activity (13).
Given the requirement for the histone chaperone Asf1 for H3 K56 acetylation and its role in chromatin disassembly from promoters during transcriptional activation, we investigated whether there was any role for H3 K56 acetylation in chromatin disassembly. We find that disassembly of H3/H4, not the disassembly of H2A/H2B, is the highly regulated step during transcriptional induction. Furthermore, K56 acetylation is required for expeditious promoter chromatin disassembly and transcriptional activation of the PHO5 gene. Conversely, loss of acetylated H3 K56 promotes promoter chromatin reassembly during transcriptional repression. These studies suggest that there is an equilibrium of H3/H4 disassembly and reassembly that can be tipped toward the assembled or disassembled state by the level of H3 K56 acetylation to facilitate rapid changes in gene expression.
Results
Delaying H3/H4 Removal from Promoter Regions Results in Delayed H2A/H2B Removal.
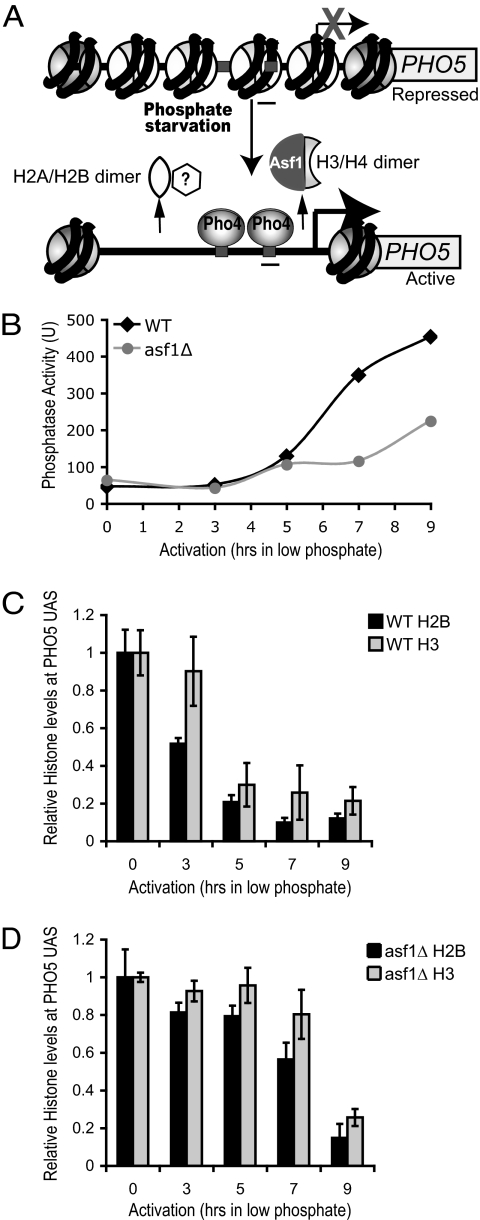
Chromatin assembly occurs via two distinct steps, where H3/H4 is assembled first to form the tetrasome, followed by the addition of H2A/H2B to complete the nucleosome in vivo (14). To investigate whether chromatin disassembly also occurs by a separable two-step process or in a concerted manner in vivo, we compared disassembly of H2B and H3 at the well characterized budding yeast PHO5 promoter. Specifically, we analyzed histone occupancy at nucleosome 2 of the PHO5 promoter (Fig. 1A) during transcriptional activation by using ChIP. Induction of the phosphatase encoded by the PHO5 gene is achieved by depletion of phosphate from the media (Fig. 1B). We found that H2B removal occurred in a very similar time frame as H3 removal in WT yeast (Fig. 1C).
Fig. 1.
Removal of H3/H4 is the rate-limiting step during chromatin disassembly. (A) Schematic of the PHO5 promoter region. Lighter circles represent nucleosomes that are disassembled upon transcriptional activation. The bar indicates the region amplified in the ChIP analyses. (B) Asf1 is required for the normal kinetics of PHO5 activation. Acid phosphatase levels were measured in strains JLY096 (WT) and SKW043 (asf1Δ) at the indicated times after transfer to low-phosphate media. (C and D) Histone H3 and H2B-HA levels over the PHO5 promoter relative to the GAL1 control region in WT yeast (C) and in asf1Δ yeast (D), from the same time course as in B.
Because H3/H4 disassembly and transcriptional induction from the PHO5 promoter is facilitated by the H3/H4 chaperone Asf1 during transcriptional induction (2), we examined whether H2A/H2B disassembly is also affected by the absence of Asf1. The requirement for Asf1 for PHO5 activation depends on the exact concentration of phosphate in the media, which in turn controls the nuclear concentration of the Pho4 activator (15). Because our earlier studies of PHO5 activation used undefined phosphate-depleted rich media, we empirically determined that a phosphate concentration of 0.15 mM revealed a requirement for Asf1 for the normal kinetics of PHO5 induction [supporting information (SI) Fig. S1]. As such, all inductions of PHO5 transcription in this study were achieved via a switch to 0.15 mM phosphate-containing synthetic media. The delayed induction of the PHO5 gene in the asf1Δ strain (Fig. 1B) is a consequence of the delayed chromatin disassembly from the promoter in this strain (Fig. 1D). We found that inactivation of Asf1 resulted in not only delayed disassembly of histone H3, but also delayed disassembly of H2B (Fig. 1D) as compared with WT (Fig. 1C). Because Asf1 is not known to be a chaperone for H2A/H2B, these data indicate that there is likely to be a constant equilibrium of H2A/H2B removal and replacement at the promoter during transcriptional induction and that, as long as H3/H4 remain on the promoter, there is no obstacle for the reassembly of H2A/H2B. These data also indicate that removal of H3/H4, not H2A/H2B, is the highly regulated step during the transition from a chromatin-packaged promoter to a nucleosome-depleted promoter during transcriptional induction.
An Inverse Correlation Between Levels of Acetylated H3 K56 and Histone Occupancy at the PHO5 Promoter.
Given the potential role of acetylation of lysine-56 on histone H3 (H3 K56ac) in destabilizing the nucleosome structure, we investigated whether H3 K56ac serves as a switch to regulate H3/H4 disassembly. By ChIP analysis, we observed that the levels of acetylated H3 K56 at the PHO5 promoter did not drastically change during promoter chromatin disassembly (Figs. S2 and S3). However, during this same time course, the levels of H3 drastically drop in WT yeast (Fig. 2B), such that, when we normalize the levels of K56Ac to the level of histone H3, it is apparent that there is a striking increase in the proportion of histone H3 carrying K56Ac that remains on the promoter during transcriptional induction (Fig. 2C). As such, there is a clear correlation between chromatin disassembly and levels of acetylated H3 K56.
Fig. 2.
H3 K56ac at the activating PHO5 promoter depends on Asf1 and its interaction with H3/H4 dimers. (A) Acid phosphatase levels were measured in strains BY4741 (WT) and BY4741asf1Δ (asf1Δ) at the indicated times after transfer to low-phosphate media. (B) Histone H3 levels over the PHO5 promoter relative to the GAL1 control region. Samples were taken from the same time course as in A. (C) Histone H3 K56ac levels, normalized to H3 levels, over the PHO5 promoter relative to the GAL1 control region. Samples were taken from the same time course as in A and B. (D) Location of Y112 and R145 residues (orange) of Asf1 (purple) in complex with histone H3 (turquoise) and H4 (green), derived from ref. 16. (E) Histone H3 levels over the PHO5 promoter in strains SKW047 (WT) and SKW048 (asf1Δ), normalized as in Fig. 1. (F) Histone H3 K56ac levels, normalized to H3 levels, over the PHO5 promoter region normalized as in Fig. 1. Samples were taken from the same time course in D and E. Although these data are from one experiment, the findings were highly reproducible.
Asf1 and Its Interaction with H3/H4 Dimers Is Required for the Increased Proportion of Histones Carrying Acetylated K56 During Transcriptional Induction.
To investigate the influence of Asf1 on the levels of H3 K56ac on the promoter, we examined the effect of deleting ASF1. In striking contrast to the situation in WT yeast, no increase in the proportion of histones carrying K56ac is seen at the PHO5 promoter in asf1Δ yeast (Fig. 2C). Furthermore, an Asf1 mutant (Y112A/R145E; Fig. 2D) that prevents its interaction with H3/H4 dimers and delays PHO5 induction (16) also disrupted chromatin disassembly (Fig. 2E) and prevented the increase in levels of K56Ac on the PHO5 promoter (Fig. 2F). These data indicate that the interaction between Asf1 and the histone dimer, which can occur only either during chromatin disassembly or before chromatin assembly, is essential for the increased proportion of histones carrying the H3 K56Ac mark at the PHO5 promoter during transcriptional activation.
H3 K56ac Acts as a Chromatin Disassembly Cue at the PHO5 Promoter.
The inverse correlation between levels of H3 and H3 K56ac at the PHO5 promoter led us to ask whether K56ac is required for chromatin disassembly. A K56R mutant that mimics permanent deacetylation delayed PHO5 induction to the same extent as deletion of ASF1 (Fig. 3A), and this was at least partly due to a delay in chromatin disassembly from the PHO5 promoter (Fig. 3B and Figs. S4 and Fig. S5). A K56Q mutant that mimics permanent acetylation activated PHO5 transcription at least as well as WT, if not slightly better (Fig. 3A). Furthermore, chromatin disassembly occurred even faster in the K56Q strain than in the WT strain (Fig. 3B). These data demonstrate that H3 K56ac promotes chromatin disassembly from the PHO5 promoter during transcriptional induction.
Fig. 3.
H3 K56ac is required for normal activation of PHO5 and promoter chromatin disassembly. (A) Acid phosphatase levels were measured in strains that are WT, asf1Δ, K56Q, K56Q asf1Δ, K56R, and K56R asf1Δ versions of MSY421 at the indicated times after transfer to low-phosphate media. (B) Histone H3 levels at the PHO5 promoter, as in Fig. 1. Samples were taken from the same time course as in A. (C) Phosphatase levels were measured in strains BY4741 (WT), BY4741rtt109Δ (rtt109Δ), and BY4741asf1Δ (asf1Δ) at the indicated times after transfer to low-phosphate media. (D) Histone H3 levels at the PHO5 promoter normalized as in Fig. 1. Samples were taken from the same time course as in C. (E) Histone H3 K56ac levels, normalized to H3 levels, over the PHO5 promoter normalized as in Fig. 1. Samples were taken from the same time course as in C. (F) Rtt109-TAP levels at the PHO5 UAS and ORF in strain ZGY925 at the indicated times after switch to low-phosphate conditions. “Sepharose at UAS” gives an indication of background due to DNA binding to the Sepharose alone. “HA at UAS” represents the ChIP with an HA antibody. All data were normalized to the signal obtained at the GAL1 promoter.
Given that mimicking acetylated K56 via the K56Q mutation promoted chromatin disassembly, we asked whether the K56Q mutation could bypass the contribution of Asf1 toward chromatin disassembly from the PHO5 promoter. We found that the K56Qasf1Δ double mutant activated transcription better and disassembled chromatin better than the asf1Δ strain, but not quite as well as the K56Q mutant alone (Fig. 3 A and B). These results suggest that an important function of Asf1 during promoter chromatin disassembly is to promote the increase of H3 K56Ac levels on the promoter region during transcriptional induction.
We were concerned that substitution of lysine-56 for arginine may not allow the optimal histone–DNA contacts to be formed within the nucleosome and that this may explain why the defect in chromatin disassembly with the K56R mutant was less severe than that of the asf1 mutant. Therefore, we examined yeast lacking the K56 HAT Rtt109 to prevent acetylation of H3 K56 while maintaining the histone–DNA contacts formed by lysine-56 within the nucleosome. We found that the rtt109Δ strain had greatly delayed activation of PHO5, equivalent to that seen in the asf1Δ strain (Fig. 3C). The rate of transcriptional induction was no worse in an rtt109Δasf1Δ double mutant than either single mutant alone (data not shown). We also observed delayed chromatin disassembly from the PHO5 promoter in the rtt109Δ strain (Fig. 3D), which in turn is likely to be a consequence of the failure to increase relative levels of K56ac on the PHO5 promoter in the absence of Rtt109 (Fig. 3E and Fig. S6). This result further demonstrates that acetylated K56 is playing an important role in driving chromatin disassembly from promoters during transcriptional induction. Consistent with previous evidence that Rtt109 can acetylate only free histones but not histones on chromatin, (17), we were not able to detect recruitment of Rtt109 to the PHO5 promoter (Fig. 3F). Furthermore, in contrast to the prediction that acetylation of K56 enables a ubiquitin ligase complex containing Rtt101 to maintain genomic integrity (12), Rtt101 has no role in the K56ac-dependent activation of PHO5 transcription (Fig. S7). As such, the observed role of H3 K56 acetylation in chromatin disassembly during transcription may be via a mechanism distinct from its role in the maintenance of genome stability.
The Proportion of Histones Carrying the H3 K56Ac Mark at the PHO5 Promoter Drops During Transcriptional Repression.
To investigate whether loss of the H3 K56ac mark plays a role in chromatin reassembly, we investigated the levels of H3 K56ac at the PHO5 promoter during transcriptional repression. As we have previously shown, histones are rapidly reassembled onto the PHO5 promoter in response to phosphate addition, which signals for transcriptional repression (Fig. 4A) (18). During this same time course of PHO5 activation and repression, the absolute levels of H3 K56Ac do not change (Fig. 4B). However, upon normalizing for total histone H3 occupancy on the promoter a striking decrease in the proportion of histones carrying the H3 K56ac mark at the PHO5 promoter during transcriptional repression is apparent (Fig. 4C). To investigate whether the reduction in H3 K56Ac levels influences the rate of transcriptional repression, which is a direct consequence of the rate of chromatin reassembly (18), we examined PHO5 repression in the H3 K56R and K56Q mutants. We consistently observed that the K56R mutant that mimics no acetylation represses PHO5 transcription faster than normal (Fig. 4D and Figs. S8–S11). Collectively, these data indicate that there exists an equilibrium of H3/H4 chromatin assembly and disassembly that is tipped toward the chromatin disassembled state by H3 K56ac and is tipped toward the chromatin assembled state by unacetylated H3 K56 (Fig. 4E).
Fig. 4.
Loss of H3 K56ac promotes transcriptional repression. (A) Histone H3 levels over the PHO5 promoter relative to the GAL1 control region; addition of phosphate promotes repression. (B) H3 K56ac levels over the PHO5 promoter relative to the GAL1 control region. (C) Histone H3 K56ac levels, normalized to H3 levels, over the PHO5 promoter relative to the GAL1 control region. Samples were taken from the same time course in A–C. (D) Acid phosphatase levels were measured in strains that are WT, K56Q, and K56R versions of MSY421. (E) Model for the role of H3 K56 acetylation in promoter chromatin disassembly during transcriptional induction, and loss of K56 acetylation in chromatin reassembly during transcriptional repression.
Discussion
Although there has been much recent excitement around acetylation of K56 on H3 given the potential role of this modification in breaking a histone–DNA contact in the globular core of the nucleosome (19), the molecular function of this modification in vivo was unclear. In this work we demonstrate that the rate-limiting step during chromatin disassembly is the removal of H3/H4 from the DNA and that acetylation of K56 drives chromatin disassembly from the PHO5 promoter during transcriptional activation in vivo. Conversely, unacetylated K56 drives chromatin reassembly during transcriptional repression in vivo. As such, the modification state of K56 on histone H3 plays an important role in enabling rapid transcriptional changes.
Thus far, the only studies linking H3 K56ac and transcription have been reports of increased levels of K56ac on active promoters and ORFs (5, 20, 21). In addition, it has been shown that K56 acetylation promotes the recruitment of the Snf5 subunit of the ATP-dependent chromatin remodeler and the subsequent transcriptional activation of SUC2 and the genes encoding H2A/H2B (5). This role of K56 acetylation for recruitment of Snf5 fits perfectly well with our conclusion that K56 acetylation drives chromatin disassembly, because we recently showed that stable recruitment of SWI/SNF is not achieved until after promoter chromatin disassembly (4).
There are clear correlations between the proportion of histones carrying K56ac and chromatin assembly/disassembly at the PHO5 promoter in vivo. A high proportion of histone H3 bears K56ac when the promoter is undergoing chromatin disassembly during transcriptional activation (Fig. 2 and Fig. S12), whereas a low proportion of H3 bears K56ac when the promoter is undergoing chromatin assembly during transcriptional repression (Fig. 3). Furthermore, mutants that block K56 acetylation (rtt109Δ, asf1Δ, and K56R) result in delayed chromatin disassembly and delayed transcriptional activation at the PHO5 promoter (Figs. 2 and 3). We suspect that the more rapid chromatin disassembly during PHO5 induction that is seen with the K56R mutant as compared with either rtt109Δ or asf1Δ (Fig. 2B) is a consequence of the weaker interaction that arginine-56 would make with DNA, as compared with lysine-56, within the nucleosome. Indeed, chromatin carrying an arginine rather than a lysine at residue 56 of H3 has a more accessible chromatin structure in vivo (22). Further evidence that H3 K56 acetylation drives chromatin disassembly was provided by the fact that the K56Q mimic of permanent acetylation disassembles chromatin even faster than normal (Fig. 2B). Conversely, the K56R mutant that prevents acetylation repressed transcription even faster than normal (Fig. 4D), which is presumably a consequence of enhanced chromatin reassembly. These data indicate that there exists a permanent equilibrium of assembly and disassembly of histones H3/H4 at the PHO5 promoter that is influenced by H3 K56 acetylation to achieve rapid transcriptional activation or repression (Fig. 4E). This is consistent with recent analyses showing a significant level of replication-independent histone H3/H4 exchange throughout the yeast genome (21, 23–25), and specifically at the repressed yeast PHO5 promoter (21, 23).
The increased proportion of histones carrying K56ac on the PHO5 promoter during chromatin disassembly is a consequence of deposition of previously acetylated H3 K56, rather than the acetylation of K56 on the chromatin for the following reasons: (i) the increase in H3 K56 acetylation on the PHO5 promoter requires the interaction between Asf1 and the H3/H4 dimer (Fig. 2), which cannot occur in the context of an intact nucleosome; (ii) Rtt109 has been shown to acetylate only free histones, not nucleosomal histones (17); (iii), Rtt109 is not recruited to the PHO5 promoter during transcriptional activation (Fig. 3); and (iv) the interaction of K56 with DNA within the nucleosome structure (19) physically blocks the accessibility of a HAT to this residue. Entirely consistent with this model, there exists a high degree of replication-independent exchange of newly synthesized histones that carry K56ac into many chromatin-packaged promoter regions (21). The general conclusion from the work of Nourani and coworkers (21) was that K56ac and Asf1 drive chromatin assembly. In contrast to their study, which examined histone exchange, i.e., the replacement of preexisting histones with new histones where it is impossible to differentiate between a defect in chromatin assembly or disassembly (21), our study examines dynamic situations where either chromatin assembly or chromatin disassembly predominates. Our data clearly show that Asf1 and K56ac drive chromatin disassembly.
Upon receiving the stimulus for transcriptional activation, we propose that the equilibrium of chromatin assembly and disassembly is tipped toward the disassembled state because the incorporation of histones acetylated on H3 K56 generates weaker nucleosomes that are more easily disassembled (Fig. 4E). Consistent with this idea, mononucleosomes bearing the K56Q mutation that mimics permanent acetylation have an 80% increase in nucleosome sliding rate and an 18% increased unfolding rate relative to the unacetylated nucleosome in vitro (26). Although H3 K56Ac is required to tip this equilibrium to the chromatin disassembled state, it is clearly not sufficient, because the H3 K56Q mutation that mimics permanent acetylation does not cause chromatin disassembly at the repressed PHO5 promoter (Fig. 2). We predict that during induction the increased local concentration of HATs and ATP-dependent chromatin remodelers cooperates with the looser K56ac nucleosomes to drive promoter chromatin disassembly (Fig. 4E). As a consequence of the enhanced chromatin disassembly at the promoter, there are more naked DNA gaps for new histones that carry the H3 K56ac mark to be deposited, which in turn are rapidly disassembled because they are less stable in the coactivator-rich environment of the activating promoter (Fig. 4E). This model accounts for the drastic increase in the proportion of histones carrying H3 K56ac on the PHO5 promoter during transcriptional activation, concomitant with the drastic decrease in bulk histone levels.
In our model, during repression the equilibrium of H3/H4 assembly/disassembly is tipped toward reassembly (Fig. 4E). Even though histones are clearly reassembled onto the promoter during repression, the amount of K56ac on the promoter does not increase (Fig. 4). This suggests that, during repression, either the newly incorporated histones are rapidly deacetylated or the newly incorporated histones are old histones that were never acetylated on H3 K56. To differentiate between these possibilities, it will be interesting to determine whether the histone chaperone Spt6 that mediates the reassembly of chromatin onto promoters during transcriptional repression (18) has a preference for old or unacetylated histones.
Given the requirement for Asf1 in acetylation of free H3, it is tempting to speculate that the contribution of Asf1 to chromatin disassembly is reflecting its role in acetylating the free histones on K56, whose subsequent incorporation into chromatin drives chromatin disassembly. The other alternative is that Asf1 has a bona fide role in disassembling H3/H4 from chromatin to allow the incorporation of the newly synthesized H3 that is acetylated on K56. The fact that the K56Q mutation that mimics permanent K56 acetylation only partially bypasses the requirement of Asf1 for chromatin disassembly (Fig. 2) does suggest that Asf1 may make additional contributions to chromatin disassembly that are separate from its role in K56 acetylation. In agreement with this idea, the eviction of old unacetylated histone H3 from chromatin is significantly reduced upon deletion of ASF1 (21).
Given the insight that we have gained into H3 K56ac function during transcriptional regulation, we can extend our predictions to DNA replication. We predict that the purpose of depositing histones carrying K56ac after replication is to allow pliability to the newly forming chromatin structure, enabling optimal positioning of nucleosomes in response to the influence of sequence-specific DNA binding factors, electrostatic repulsion between adjacent nucleosomes, ATP-dependent remodeling factors, and DNA sequence. Once the nucleosomes have attained their optimal positions on the newly formed chromatin strands after DNA replication, the rapid deacetylation of the H3 K56 mark would stabilize the nucleosomes into their favored positions.
Materials and Methods
Yeast Strains and Media.
All experiments were performed by growing yeast in synthetic high (13.4 mM) phosphate-containing media, then shifting the log phase cultures to low (0.15 mM) phosphate media to achieve PHO5 induction and addition of phosphate back to 13.4 mM to achieve PHO5 repression. The genotypes of yeast strains used in this study are provided in Table S1.
ChIP Analysis.
Complete details of the ChIP analyses can be found in SI Methods. Amplification of the GAL1 promoter was used as a normalization control. Values shown are averages of three independent experiments; error bars indicate the 95% confidence interval.
Supplementary Material
Acknowledgments.
We are grateful to Jason Feser and Seth Noone for critical reading of the manuscript. We thank David Allis (Rockefeller University, New York), Michael Lichten (National Cancer Institute, Bethesda, MD), David Stillman (University of Utah, Salt Lake City), David Toczyski (University of California, San Francisco), and Zhigou Zhang (Mayo Clinic, Rochester, MN) for providing us with reagents for this study. This work was supported by National Institutes of Health Grant GM64475 (to J.K.T.). J.K.T. is a Scholar of the Leukemia and Lymphoma Society.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0800057105/DCSupplemental.
References
- 1.Williams SK, Tyler JK. Transcriptional regulation by chromatin disassembly and reassembly. Curr Opin Genet Dev. 2007;17:88–93. doi: 10.1016/j.gde.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 2.Adkins MW, Howar SR, Tyler JK. Chromatin disassembly mediated by the histone chaperone Asf1 is essential for transcriptional activation of the yeast PHO5 and PHO8 genes. Mol Cell. 2004;14:657–666. doi: 10.1016/j.molcel.2004.05.016. [DOI] [PubMed] [Google Scholar]
- 3.Schwabish MA, Struhl K. Asf1 mediates histone eviction and deposition during elongation by RNA polymerase II. Mol Cell. 2006;22:415–422. doi: 10.1016/j.molcel.2006.03.014. [DOI] [PubMed] [Google Scholar]
- 4.Adkins MW, Williams SK, Linger J, Tyler JK. Chromatin disassembly from the PHO5 promoter is essential for the recruitment of the general transcription machinery and coactivators. Mol Cell Biol. 2007;27:6372–6382. doi: 10.1128/MCB.00981-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xu F, Zhang K, Grunstein M. Acetylation in histone H3 globular domain regulates gene expression in yeast. Cell. 2005;121:375–385. doi: 10.1016/j.cell.2005.03.011. [DOI] [PubMed] [Google Scholar]
- 6.Masumoto H, Hawke D, Kobayashi R, Verreault A. A role for cell-cycle-regulated histone H3 lysine 56 acetylation in the DNA damage response. Nature. 2005;436:294–298. doi: 10.1038/nature03714. [DOI] [PubMed] [Google Scholar]
- 7.Celic I, et al. The sirtuins hst3 and Hst4p preserve genome integrity by controlling histone h3 lysine 56 deacetylation. Curr Biol. 2006;16:1280–1289. doi: 10.1016/j.cub.2006.06.023. [DOI] [PubMed] [Google Scholar]
- 8.Maas NL, Miller KM, DeFazio LG, Toczyski DP. Cell cycle and checkpoint regulation of histone H3 K56 acetylation by Hst3 and Hst4. Mol Cell. 2006;23:109–119. doi: 10.1016/j.molcel.2006.06.006. [DOI] [PubMed] [Google Scholar]
- 9.Han J, Zhou H, Li Z, Xu RM, Zhang Z. Acetylation of lysine 56 of histone H3 catalyzed by RTT109 and regulated by ASF1 is required for replisome integrity. J Biol Chem. 2007;282:28587–28596. doi: 10.1074/jbc.M702496200. [DOI] [PubMed] [Google Scholar]
- 10.Driscoll R, Hudson A, Jackson SP. Yeast Rtt109 promotes genome stability by acetylating histone H3 on lysine 56. Science. 2007;315:649–652. doi: 10.1126/science.1135862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Han J, et al. Rtt109 acetylates histone H3 lysine 56 and functions in DNA replication. Science. 2007;315:653–655. doi: 10.1126/science.1133234. [DOI] [PubMed] [Google Scholar]
- 12.Collins SR, et al. Functional dissection of protein complexes involved in yeast chromosome biology using a genetic interaction map. Nature. 2007;446:806–810. doi: 10.1038/nature05649. [DOI] [PubMed] [Google Scholar]
- 13.Tsubota T, et al. Histone H3–K56 acetylation is catalyzed by histone chaperone-dependent complexes. Mol Cell. 2007;25:703–712. doi: 10.1016/j.molcel.2007.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gruss C, Wu J, Koller T, Sogo JM. Disruption of the nucleosomes at the replication fork. EMBO J. 1993;12:4533–4545. doi: 10.1002/j.1460-2075.1993.tb06142.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Korber P, et al. The histone chaperone Asf1 increases the rate of histone eviction at the yeast PHO5 and PHO8 promoters. J Biol Chem. 2006;281:5539–5545. doi: 10.1074/jbc.M513340200. [DOI] [PubMed] [Google Scholar]
- 16.English CM, Adkins MW, Carson JJ, Churchill MEA, Tyler JK. Structural basis for the histone chaperone activity of Asf1. Cell. 2006;127:495–508. doi: 10.1016/j.cell.2006.08.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Han J, Zhou H, Li Z, Xu RM, Zhang Z. The Rtt109-Vps75 histone acetyltransferase complex acetylates non-nucleosomal histone H3. J Biol Chem. 2007;282:14158–14164. doi: 10.1074/jbc.M700611200. [DOI] [PubMed] [Google Scholar]
- 18.Adkins MW, Tyler JK. Transcriptional activators are dispensable for transcription in the absence of Spt6-mediated chromatin reassembly of promoter regions. Mol Cell. 2006;21:405–416. doi: 10.1016/j.molcel.2005.12.010. [DOI] [PubMed] [Google Scholar]
- 19.Luger K, Mader AW, Richmond RK, Sargent DF, Richmond TJ. Crystal structure of the nucleosome core particle at 2.8 A resolution. Nature. 1997;389:251–260. doi: 10.1038/38444. [DOI] [PubMed] [Google Scholar]
- 20.Schneider J, Bajwa P, Johnson FC, Bhaumik SR, Shilatifard A. Rtt109 is required for proper H3K56 acetylation: A chromatin mark associated with the elongating RNA polymerase II. J Biol Chem. 2006;281:37270–37274. doi: 10.1074/jbc.C600265200. [DOI] [PubMed] [Google Scholar]
- 21.Rufiange A, Jacques PE, Bhat W, Robert F, Nourani A. Genome-wide replication-independent histone H3 exchange occurs predominantly at promoters and implicates H3 K56 acetylation and Asf1. Mol Cell. 2007;27:393–405. doi: 10.1016/j.molcel.2007.07.011. [DOI] [PubMed] [Google Scholar]
- 22.Xu F, Zhang Q, Zhang K, Xie W, Grunstein M. Sir2 deacetylates histone H3 lysine 56 to regulate telomeric heterochromatin structure in yeast. Mol Cell. 2007;27:890–900. doi: 10.1016/j.molcel.2007.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Linger J, Tyler JK. Global replication-independent histone H4 exchange in budding yeast. Eukaryot Cell. 2006;5:1780–1787. doi: 10.1128/EC.00202-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dion MF, et al. Dynamics of replication-independent histone turnover in budding yeast. Science. 2007;315:1405–1408. doi: 10.1126/science.1134053. [DOI] [PubMed] [Google Scholar]
- 25.Jamai A, Imoberdorf RM, Strubin M. Continuous histone H2B and transcription-dependent histone H3 exchange in yeast cells outside of replication. Mol Cell. 2007;25:345–355. doi: 10.1016/j.molcel.2007.01.019. [DOI] [PubMed] [Google Scholar]
- 26.Ferreira H, Somers J, Webster R, Flaus A, Owen-Hughes T. Histone tails and the H3 alphaN helix regulate nucleosome mobility and stability. Mol Cell Biol. 2007;27:4037–4048. doi: 10.1128/MCB.02229-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.