Abstract
A common expression of neuroadaptations induced by repeated exposure to addictive drugs is a persistent sensitized behavioral response to their stimulant properties. Neuroplasticity underlying drug-induced sensitization has been proposed to explain compulsive drug pursuit and consumption characteristic of addiction. The hypothalamic-pituitary-adrenal (HPA) axis-activating neuropeptide, corticotropin-releasing factor (CRF), may be the keystone in drug-induced neuroadaptation. Corticosterone-activated glucocorticoid receptors (GRs) mediate the development of sensitization to ethanol (EtOH), implicating the HPA axis in this process. EtOH-induced increases in corticosterone require CRF activation of CRF1 receptors. We posited that CRF1 signaling pathways are crucial for EtOH-induced sensitization. We demonstrate that mice lacking CRF1 receptors do not show psychomotor sensitization to EtOH, a phenomenon that was also absent in CRF1 + 2 receptor double-knockout mice. Deletion of CRF2 receptors alone did not prevent sensitization. A blunted endocrine response to EtOH was found only in the genotypes showing no sensitization. The CRF1 receptor antagonist CP-154,526 attenuated the acquisition and prevented the expression of EtOH-induced psychomotor sensitization. Because CRF1 receptors are also activated by urocortin-1 (Ucn1), we tested Ucn1 knockout mice for EtOH sensitization and found normal sensitization in this genotype. Finally, we show that the GR antagonist mifepristone does not block the expression of EtOH sensitization. CRF and CRF1 receptors, therefore, are involved in the neurobiological adaptations that underlie the development and expression of psychomotor sensitization to EtOH. A CRF/CRF1-mediated mechanism involving the HPA axis is proposed for acquisition, whereas an extrahypothalamic CRF/CRF1 participation is suggested for expression of sensitization to EtOH.
Keywords: addiction; CP-154,526; HPA axis; knockout mice; psychomotor sensitization
Despite diverse molecular mechanisms of action, all addictive drugs share the ability to trigger persistent neuroadaptations subsequent to their repeated administration (1–3). Common sequelae of these neuroplastic changes can be seen in behavior, and include negative affective symptoms associated with drug withdrawal (4, 5), robust changes in motivated behavior predicted by associative learning about drug-related environmental cues that are resistant to extinction (2, 6, 7), and protracted periods of sensitization (8–11). Sensitization occurs to behavioral stimulating effects of addictive drugs (8–10) and to symptoms of hyperexcitability associated with repeated bouts of drug withdrawal (12, 13). Repeated administration of drugs sensitizes neural circuits that assign biological significance to drugs and drug-related cues, a consequence underlying increased motivational effects (10, 14–16). Therefore, it has been suggested that neuroplasticity associated with drug-induced sensitization contributes to the transition from controlled consumption to the compulsive patterns of drug-seeking and -taking that characterize addiction (2, 10).
The neuropeptide corticotropin-releasing factor (CRF) plays a key role in drug-induced neuroplasticity. All abused drugs, including ethanol (EtOH), have effects that mirror stressors in their activation of the hypothalamic-pituitary-adrenal (HPA) axis, via central mechanisms (17–20) that depend on the stimulation of CRF-containing neurons of the paraventricular nucleus of the hypothalamus. CRF is a primary activator of the HPA axis and an essential mediator of behavioral and autonomic outcomes of stress. It causes release of adrenocorticotropic hormone (ACTH) from the pituitary (21, 22), which in turn induces the secretion of glucocorticoids such as corticosterone (CORT) from the adrenal gland (23, 24). CORT, through glucocorticoid receptors (GR), can bidirectionally modulate further CRF activity via hypothalamic negative feedback (which decreases CRF activity), and extrahypothalamic positive feedforward regulation (which increases CRF activity) of structures such as the amygdala and the bed nucleus of the stria terminalis (23, 25). Extrahypothalamic CRF is involved in the negative emotional states and excessive drug consumption characteristic of the postdependent phenotype (4, 26, 27). Endocrine-independent effects of CRF have also been suggested for the expression of psychomotor sensitization to psychostimulants (28). However, CRF-initiated HPA axis activity appears to be critical in the acquisition of drug-induced sensitization, because the blockade of ACTH or CORT actions prevents development of sensitization to psychostimulants and opiates (29–34). Indeed, it has been suggested that activation of the HPA axis is a common pathway by which abused drugs induce sensitization-associated neuroplasticity (35, 36). The long-term adaptations in the mesolimbic dopamine (DA) system characteristic of sensitized animals (10), such as cocaine- and morphine-sensitized DA release in the nucleus accumbens (NAcb), also depend on the activation of this neuroendocrine axis (37, 38). Thus, by facilitating both endocrine-dependent and independent actions, CRF may be a principle element, perhaps even the keystone, in stress-associated mechanisms of addiction.
The acquisition of sensitization to EtOH is mediated by glucocorticoids; the GR antagonist RU-38486 (also known as mifepristone) blocks psychomotor sensitization to EtOH (9, 39). A history of stress, moreover, results in a sensitized locomotor response to a subsequent EtOH challenge, and this effect is prevented by mifepristone (39). Chronic EtOH induces a sensitized response to the activating effects of centrally administered CRF (40). Therefore, those mechanisms that initiate EtOH-induced activation of the HPA axis, such as increased CRF, may be necessary for induction of the neuroplasticity underlying EtOH sensitization. Two known G protein positively coupled receptors, CRF1 and CRF2, have been described. CRF has tenfold greater affinity for CRF1 than for CRF2 (23). The predominant expression of CRF1 receptors on pituitary corticotropes (41) and the blunted endocrine response to EtOH and stress seen in mice lacking CRF1 receptors (42–45) suggest that CRF1 receptor manipulations might mediate neuroadaptive changes associated with the acquisition of sensitization. CRF2 receptors, although not implicated in the initiation of HPA axis activation, are proposed to participate in the maintenance and recovery of HPA axis responses after stress (42, 46).
The current studies were directed toward identification of upstream mechanisms in the HPA axis involved in the neuroadaptations supporting acquisition of EtOH-induced psychomotor sensitization. A possible endocrine-independent role of CRF in the expression of EtOH sensitization was also investigated. Genetically engineered mice lacking CRF1 or CRF2 receptors, and double-knockout (KO) mice for both CRF1 + 2 receptors were tested. Plasma CORT levels were measured in these genotypes. CP-154,526, the brain-penetrating, nonpeptide CRF1 receptor antagonist (47), was used to verify the involvement of CRF1 receptors that was suggested by results from the mutant mice and also to further investigate the specific role of CRF1 receptors in the acquisition and expression of EtOH sensitization. Recent advances in the knowledge of the functions of urocortin-1 (Ucn1), the only Ucn that acts at CRF1 receptors (23), have revealed a role for this peptide in explaining EtOH sensitivity and consumption (48). However, the involvement of Ucn1 in drug-induced sensitization has never been studied. Ucn1 KO mice were used here to evaluate EtOH sensitization. Finally, we used the GR antagonist mifepristone to investigate whether the expression of EtOH sensitization is HPA axis-dependent.
Results
Psychomotor Sensitization to EtOH Is Absent in CRF1 Receptor KO Mice.
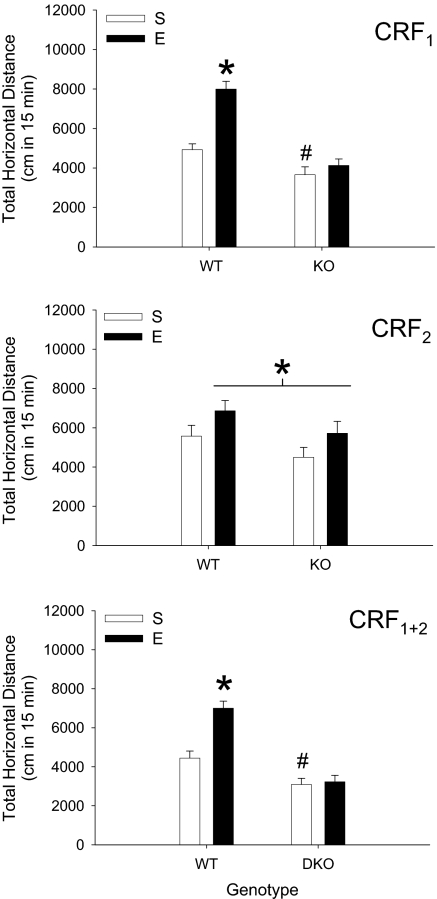
Significant sensitization to EtOH was seen in wild-type (WT) but not CRF1 KO mice (Fig. 1Top). Follow-up analysis of a significant EtOH pretreatment dose × genotype interaction (F1,53 = 13.2; P < 0.01) confirmed the presence of sensitization in WT but not CRF1 KO mice. EtOH-induced acute stimulation was also lower in CRF1 KO compared with WT mice. Time course analyses (supporting information (SI) Fig. S1 Top Left) revealed an EtOH pretreatment dose × genotype × time interaction (F2,106 = 14.9; P < 0.01) that was associated with the sensitized response to EtOH in WT mice being present during only the last 10 min of the test. Blood ethanol concentration (BEC; Table S1) and levels of locomotion after saline treatment (Fig. S1 Top Right) were not different among groups.
Fig. 1.
EtOH-induced sensitization is absent in CRF1 and CRF1 + 2 receptor KO, but not CRF2 KO mice. Total 15-min locomotor response (mean ± SEM) to 1.5 g/kg EtOH in KO and WT mice pretreated for 10 days with saline (S) or 2.5 g/kg EtOH (E). CRF1 (Top; n = 13–15 per group): *, different from saline-pretreated WT and EtOH-pretreated KO mice [simple main effect (SME) P < 0.01]; #, different from saline-pretreated WT mice (SME P < 0.05). CRF2 (Middle; n = 9–15 per group): *, main effect of EtOH pretreatment; no statistically significant difference in the magnitude of EtOH sensitization was found between genotypes. CRF1 + 2 (Bottom; n = 12–15 per group): *, different from saline-pretreated WT and EtOH-pretreated double-KO mice (SME P < 0.01); #, different from saline-pretreated WT mice (SME P < 0.05).
CRF2 Receptor Deletion Does Not Affect Psychomotor Sensitization to EtOH.
Previous EtOH exposure (Fig. 1 Middle) resulted in significant sensitization (main effect of EtOH pretreatment dose; F1,44 = 4.9; P < 0.05). There was no effect of the CRF2 mutation, although a relatively higher proportion of C57BL/6J background alleles (see Animals) may have limited the degree of sensitization (9). Time course analyses (Fig. S1 Middle Left) showed a significant main effect of time (F2,88 = 110.5; P < 0.01), but no interactions. There were no effects of deletion of CRF2 receptors on BEC or locomotion after saline (Table S1 and Fig. S1 Middle Right).
CRF1 + 2 Receptor Double-KO Mice Do Not Show Sensitization to EtOH.
Sensitization to EtOH was found in WT but not in CRF1 + 2 receptor KO mice (Fig. 1 Bottom). Follow-up analysis of a significant EtOH pretreatment dose × genotype interaction (F1,51 = 11.8; P < 0.01) confirmed the presence of sensitization in WT but not double-KO mice. Acute stimulation to EtOH was also reduced in CRF1 + 2 KO mice. Activity data varied across time (Fig. S1 Bottom Left; F2,102 = 209.5; P < 0.01), but there was no interaction of time with EtOH pretreatment or genotype. BEC (Table S1) and the response to saline (Fig. S1 Bottom Right were not different among groups.
The effect of EtOH on Plasma CORT Levels Is Absent in CRF1 and CRF1 + 2 but Not in CRF2 KO Mice.
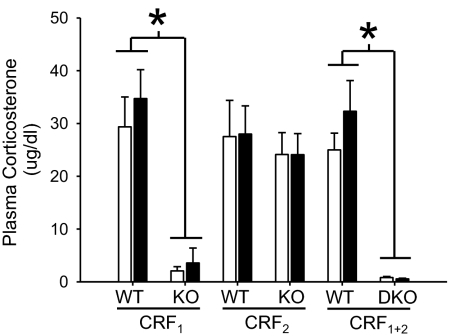
Plasma CORT values (Fig. 2) were profoundly attenuated in CRF1 and CRF1 + 2 KO mice, whereas levels equivalent to those in WT mice were found for CRF2 KO mice. A significant effect of genotype was found for data from CRF1 vs. WT (F1,52 = 45.2; P < 0.01) and double-KO vs. WT (F1,50 = 56.8; P < 0.01) mice. No effect of EtOH pretreatment dose or interaction between genotype and pretreatment was found for any of the three pairs of genotypes; thus, prior exposure to EtOH did not alter the CORT response to EtOH (measured 15 min after EtOH treatment).
Fig. 2.
Plasma CORT after EtOH administration is blunted in CRF1 and CRF1 + 2 KO, but not in CRF2 KO mice. Shown is the concentration of CORT (mean ± SEM) in plasma samples taken 15 min after 1.5 g/kg EtOH. Open and filled bars represent animals pretreated with saline or EtOH, respectively, on days 1–10. *, main effect of genotype. Group sizes are as listed in the legend to Fig. 1.
CP-154,526 Attenuates the Acquisition of Psychomotor Sensitization to EtOH.
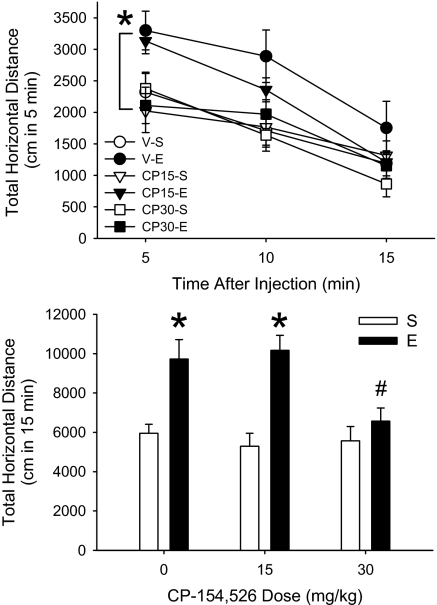
CP-154,526 dose- and time-dependently attenuated the acquisition of sensitization to EtOH in DBA/2J (D2) mice. There was a significant interaction (Fig. 3Upper) of time, EtOH pretreatment dose and CP-154,526 dose (F4,140 = 3.8; P < 0.01). The sensitized response to EtOH was blocked during the first 5 min of the test in animals pretreated with 30 mg/kg CP-154,526. There was also a trend (P = 0.07) toward an effect of CP-154,526 on sensitization when data accumulated for the 15-min period were examined (Fig. S2 Upper Left). There were no group differences in BEC (Table S2) or locomotor behavior after saline (Fig. S2 Upper Right).
Fig. 3.
The CRF1 receptor antagonist, CP-154,526, inhibits the acquisition (Upper) and blocks the expression (Lower) of EtOH sensitization. Shown in the Upper is the time course for the locomotor response (mean ± SEM) to 1.5 g/kg EtOH in D2 mice treated for 10 days with saline (S) or 1.5 g/kg EtOH (E), 30 min after vehicle (V), 15 or 30 mg/kg CP-154,526 (CP15 and CP30, respectively); n = 10–15 per group. * indicates that CP30-E is different from V-E and CP15-E at the 5-min time point (SME P < 0.01). Shown in Lower is total distance traveled in 15 min (mean ± SEM) after 1.5 g/kg EtOH in D2 mice treated for 10 days with saline (S) or 1.5 g/kg EtOH (E), 30 min after vehicle (V) and then challenged with E, 30 min after V, 15 or 30 mg/kg CP-154,526 (CP15 and CP30 respectively); n = 12–14 per group. *, different from respective saline pretreated groups (SME P < 0.01); #, different from animals pretreated with E on days 1–10 and tested with V-E on day 11 (Newman–Keuls, P < 0.05).
CP-154,526 blocks the Expression of Psychomotor Sensitization to EtOH.
The expression of sensitization was prevented in D2 mice by 30 mg/kg CP-154,526 (Fig. 3 Lower). A significant interaction of EtOH pretreatment dose (days 1–10) and CP-154,526 test day dose (day 11) (F2,73 = 3.6; P < 0.05) was associated with the expression of sensitization in mice pretreated with vehicle or 15 mg/kg CP-154,526, but not in mice treated with 30 mg/kg CP-154,526. There were no differences in acute response to EtOH. Behavior changed across time (F2,146 = 54.3; P < 0.01), but time did not interact with the other variables (Fig. S2 Lower Left). BEC did not differ among groups (Table S2) and levels of behavior after saline were comparable (Fig. S2 Lower Right).
Deletion of Ucn1 Does Not Prevent Psychomotor Sensitization to EtOH.
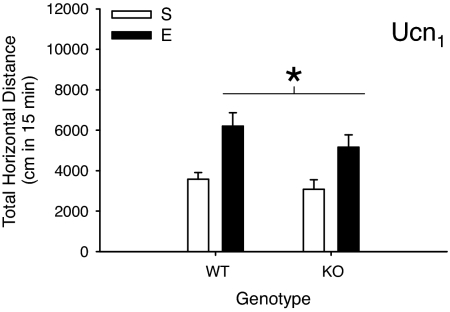
EtOH induced sensitization in both Ucn1 WT and KO mice (Fig. 4). There was a main effect of EtOH pretreatment dose (F1,33 = 18.7; P < 0.01), but no effect of genotype nor interaction. There was an EtOH pretreatment × time interaction (F2,66 = 8.5; P < 0.01); sensitization was most robust during the last 10 min of the 15-min test (Fig. S3 Left). BEC (Table S1) was comparable among groups, as was locomotion after saline (Fig. S3 Right); when time was included in the analysis of locomotion after saline (data not shown), a time × genotype interaction (F2,66 = 3.6; P < 0.05) indicated less activity in KO mice after saline during the first 5 min of the test, but not the last 10 min (when EtOH sensitization was most robust on test day 11).
Fig. 4.
Absence of an effect of Ucn1 deletion on EtOH sensitization. Shown are means ± SEM for total 15 min of the locomotor stimulant response to 1.5 g/kg EtOH in Ucn1 WT and KO mice pretreated with saline (S) or 2.5 g/kg EtOH (E) for 10 days. No differences between genotypes were found; both genotypes sensitized to EtOH. n = 9–10 per group. *, main effect of EtOH pretreatment; no statistically significant difference in the magnitude of EtOH sensitization was found between genotypes.
Mifepristone Does Not Block the Expression of Psychomotor Sensitization to EtOH.
Both vehicle and mifepristone-pretreated D2 mice expressed sensitization to EtOH. Statistical results and summarized data are presented in Fig. S4 and Table S3.
Discussion
CRF and CRF1 receptors are critically involved in the neuroadaptations supporting the acquisition and expression of EtOH-induced psychomotor sensitization. Six findings presented here support this conclusion and do not provide support for the involvement of CRF2 receptors or the peptide Ucn1: (i) CRF1 receptor KO and double-KO CRF1 + 2 mice did not show sensitization to the psychomotor effects of EtOH, (ii) CRF2 KO mice were capable of sensitization to the stimulant effects of EtOH, (iii) a blunted endocrine response to EtOH was found in CRF1 and CRF1 + 2, but not in CRF2 KO mice, (iv) the CRF1 receptor antagonist CP-154,526 attenuated the acquisition and prevented the expression of EtOH-induced sensitization, (v) Ucn1 KO mice showed normal sensitization to EtOH, and (vi) the GR antagonist mifepristone (see Fig. S4) did not affect the expression of psychomotor sensitization to EtOH.
Extensive research has implicated CRF neurotransmission in the regulation of endocrine responses to stress and in stress- and drug-related behavioral outcomes (23, 27, 49, 50). Dysregulation of CRF-mediated systems underlies stress-related psychiatric disorders and perhaps alcoholism (51, 52). Given the comorbidity of drug addiction with affective disorders, CRF systems have been suggested as potential common targets for their treatment (50, 53). Regarding alcoholism, human and rodent data support a genetically determined relationship between central CRF function and EtOH consumption (54–57). EtOH-induced long-term neuroadaptations responsible for increased EtOH intake and stress-evoked relapse-like behavior in EtOH-dependent animals withdrawn from EtOH have also been linked to CRF function (54, 58, 59), and repeated EtOH exposure sensitizes the response to the activating effects of a central CRF administration (40). Together, these data provide convergent evidence that the neuroadaptive processes underlying chronic EtOH effects involve CRF neurotransmission. Although we did not test mice lacking CRF itself, the CRF1 + 2 KO mouse is a model of absent CRF signaling, and these mice did not exhibit a neuroadaptive behavioral response to EtOH, also supporting the importance of CRF in this role.
CRF initiates HPA axis activation (21, 23). Immunoneutralization of endogenous CRF abolishes EtOH-induced ACTH and CORT release (60), an effect that was found to be CRF1 receptor-mediated (43). EtOH increases the expression of CRF heteronuclear RNA (18) and CRF1, but not CRF2 receptor mRNA expression (61). Our behavioral data demonstrate a role for CRF1 but not CRF2 receptors in EtOH sensitization. The CRF1 antagonist CP-154,526 reduces ACTH and CORT responses to stress (62) and here we demonstrate attenuation of acquisition of sensitization to EtOH with 30 mg/kg of this compound. Another study using procedures similar to ours (71) revealed this antagonist's lack of effect in preventing acquisition of sensitization to EtOH; however, this study explored doses only up to 20 mg/kg, which may not be adequate for full CRF1 receptor occupancy (see SI Text for additional information regarding this point). In addition to these results, we found that deletion of Ucn1, which does not alter HPA axis function (23), failed to prevent sensitization to EtOH. Hence, a possible mechanism of CRF1 receptor involvement in neuroplasticity underlying EtOH sensitization may be through CRF1 receptor modulation of EtOH-induced HPA axis activation. Previous evidence from our laboratory strongly supports this hypothesis for both EtOH- and stress-induced sensitization to EtOH, wherein the inactivation of the actions of CORT at GR prevented acquisition of sensitization (39). EtOH and stress, therefore, seem to require CRF1-mediated CORT activation of GR to produce those changes supporting the initiation of EtOH sensitization. Although DA-independent mechanisms cannot be discarded, it has been suggested that GR found on the cell bodies of ventral tegmental area DA neurons (63) may mediate the HPA axis activation-induced increase in NAcb DA suggested to be necessary for stress and abused drugs to achieve sensitization (10).
A number of chronic effects of EtOH and/or stress, such as dependence- and stress-induced increases in EtOH consumption and seeking that involve CRF1 receptors, are not influenced by HPA axis hormones (45, 59, 64, 65). Substantial evidence suggests that neuroendocrine-independent effects of CRF underlie dependence- and withdrawal-related outcomes for EtOH and other abused drugs (26, 66, 67). Spontaneous withdrawal from benzodiazepines, for instance, has been shown to be mediated by CRF and CRF1 receptors (68, 69). These data appear to be especially interesting in view of the shared behavioral effects of EtOH and benzodiazepines and the common effects of these drugs on CRF systems (12, 68, 69). A role of extrahypothalamic CRF in the expression of drug-induced psychomotor sensitization has also been proposed (28). Consistent with this idea, our present data on the effect of CP-154,526 on the expression of EtOH sensitization, together with the results that we obtained with the GR antagonist, indicate an involvement of extrahypothalamic CRF1 receptors. CRF can influence behavior per se through its actions on structures outside of the hypothalamus (26, 67). In fact, microinfusions of CRF into the NAcb have been found to induce robust increases in locomotor activity (70). These data are in line with what has been recently proposed by Fee et al. (71). Like us, these authors found a blockade of the expression of EtOH sensitization with CP-154,526 and suggested that CRF1 receptors located in the NAcb might mediate expression of sensitization to EtOH as proposed for other abused drugs (8).
For various endocrine-independent effects of chronic EtOH, more profound effects of CRF1 receptor manipulations have been described in postdependent animals and in animals that were selectively bred for elevated EtOH preference (54, 58, 59). CRF1 receptor antagonists can reduce levels of intake of postdependent animals to the levels observed in nondependent ones (54, 58, 59). Similarly, we found that 30 mg/kg CP-154,526 reduced the expression of the sensitized locomotor response to EtOH so that the level of activation was similar to that observed in mice that had received EtOH for the first time. This indicates that the mice retained a normal acute stimulant response (see also ref. 71) and, thus, that this dose of the antagonist had no effect on general locomotion. CRF1 receptor manipulations may be specifically effective in blocking the behavioral expression of different forms of neuroadaptation caused by repeated EtOH challenges, forced EtOH dependency, or genetically driven excessive EtOH consumption, as compared with acute effects, or levels of EtOH intake in nondependent or moderate drinkers.
In summary, combined genetic and pharmacological evidence strongly supports a role for CRF and CRF1 receptors in mediating EtOH-induced psychomotor sensitization, a process that, although not associated with dependence, is suggested to be relevant to the early (acquisition) and recurring (relapse) stages of addiction. In particular, CRF1 receptor-mediated activation of the HPA axis, involving CORT and GR, appears to be a possible mechanism underlying the acquisition of EtOH sensitization. Extrahypothalamic CRF signaling via CRF1 receptors might be responsible for the expression of those neural changes once already formed. Together with current theories proposing a critical role of CRF and CRF1 receptors in excessive EtOH intake seen after stress or dependency, the present data extend the contributions of this system to other signs of EtOH-induced neuroadaptations such as psychomotor sensitization. Especially important appear to be the present data showing that CRF1 receptors participate in the expression of sensitization, because these changes are suggested to underlie increased salience and biological significance attributed to drugs and drug-related cues after repeated drug exposure. CRF1 receptor manipulations, therefore, either by blocking the negative emotional symptoms associated with EtOH withdrawal, sensitivity to stress, or the hypermotivation and compulsion toward drug-taking induced by sensitization may be ideal candidates for future therapeutic applications in preventing EtOH relapse.
Materials and Methods
Animals.
Mutant and WT mice of a mixed C57BL/6J × 129SV/J background were backcrossed onto the C57BL/6J strain for 5, 8, 6, and 7 generations, respectively, to produce the CRF1, CRF2, CRF1 + 2, and Ucn1 mice used in these studies. All offspring were generated by heterozygous mating. Creation of these mice by gene-targeted inactivation in embryonic stem cells has been described (45, 46, 72, 73). Sex-balanced groups of males and females (60 to 119 days old) were used. Female DBA/2J (D2) inbred strain mice (63–70 days old; The Jackson Laboratory) were used for all pharmacological studies, as they had been used in our previous work of susceptibility to EtOH- and stress-induced sensitization and HPA axis involvement (39, 74). Mice were housed two to five per cage in standard, acrylic mouse cages with corncob bedding, with food and water available ad libitum. All procedures were approved by the Portland Department of Veterans Affairs and Oregon Health and Science University animal care and use committee and observed the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals.
Drugs.
EtOH (100%; Pharmco Products) was diluted to a 20% vol/vol solution in 0.9% NaCl and injected in a volume to administer a dose of 1.5 or 2.5 g/kg. CP-154,526 (a generous gift from Pfizer) was dissolved in 5% emulphor (Cremophor EL, Sigma Chemicals) in 0.9% NaCl, at concentrations of 1.5 or 3 mg/ml, and injected at a volume of 10 ml/kg. Mifepristone (Sigma) was prepared in 20% (wt/vol) 2-hydroxypropyl-β-cyclodextrin (Sigma) at concentrations of 1 or 2 mg/ml and injected at a volume of 10 ml/kg. All injections were administered i.p.
Behavioral Procedure.
Experimental methods and EtOH doses matched those previously established for inducing and measuring EtOH sensitization (39). For studies using KO and WT mice, the animals were treated with saline or 2.5 g/kg EtOH, once daily for 10 consecutive days. On day 11, all mice were challenged with 1.5 g/kg EtOH immediately before placement in the automated activity monitors, where locomotor activity (SI Text) was measured for 15 min. On day 12, a 15-min activity test was performed after saline treatment. To assess effects of CP-154,526 on the acquisition of sensitization to EtOH, D2 mice were pretreated on days 1–10 with vehicle, 15 or 30 mg/kg CP-154,526, 30 min before EtOH (0 or 1.5 g/kg). Locomotor activity was measured for 15 min after 1.5 g/kg EtOH or saline on days 11 and 12, respectively, preceded by vehicle treatment (30 min before). This mirrored the injection conditions during the pretreatment phase (days 1–10). To assess effects of CP-154,526 or mifepristone on the expression of sensitization to EtOH, D2 mice were treated for 10 days with vehicle–saline or vehicle–1.5 g/kg EtOH (injections spaced 30 min apart). These two treatment groups were then subdivided into three groups each for day 11 treatment and locomotor activity testing for 15 min, when mice were pretreated with vehicle, 15 or 30 mg/kg CP-154,526, or vehicle, 10 or 20 mg/kg mifepristone, 30 min before 1.5 g/kg EtOH. On day 12, a 15-min activity test was performed after vehicle then saline treatment separated by 30 min. On day 11, two 20-μl tail-blood samples were taken for determination of BEC and for plasma CORT levels in CRF receptor genetic models (see SI Text for methods).
Statistical Analysis.
Horizontal distance traveled during the 15-min test, or in 5-min segments, served as the dependent measure of locomotor activity. Interactions with time were studied by using three-way ANOVA with time (5-min periods), EtOH pretreatment dose and genotype as the main factors. Two-way ANOVA (EtOH pretreatment dose and genotype) was used in the absence of time interactions. For the antagonist studies, in addition to time, CP-154,526 pretreatment dose and EtOH treatment dose, both referring to injections given on days 1–10, were included in the analysis of the acquisition study. EtOH treatment dose (days 1–10) and CP-154,526 or mifepristone pretreatment dose (day 11) were included in addition to time, in the analysis of expression data. Significant interactions were examined for simple main effects (SME), and the Newman–Keuls test was used for mean comparisons. Statistica 6.1 software (StatSoft) was used.
Supplementary Material
Acknowledgments.
This work was supported by Department of Veterans Affairs, National Institutes of Health, and National Institute on Alcohol Abuse and Alcoholism Grants R01AA13331, P60AA010760, R01MH65689, R01AA013738, and UO1016647. We thank Dr. Wylie Vale for providing Ucn1 KO mice, Dr. Helen Kamens, Sarah Holstein, Lauren Brown, Ronjon Datta, and Dawn Cote for technical assistance, and Dr. Suzanne Mitchell for the use of test equipment.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
See Commentary on page 8809.
This article contains supporting information online at www.pnas.org/cgi/content/full/0710181105/DCSupplemental.
References
- 1.Hyman SE, Malenka RC, Nestler EJ. Neural mechanisms of addiction: The role of reward-related learning and memory. Annu Rev Neurosci. 2006;29:565–598. doi: 10.1146/annurev.neuro.29.051605.113009. [DOI] [PubMed] [Google Scholar]
- 2.Kalivas PW, Volkow N, Seamans J. Unmanageable motivation in addiction: A pathology in prefrontal-accumbens glutamate transmission. Neuron. 2005;45:647–650. doi: 10.1016/j.neuron.2005.02.005. [DOI] [PubMed] [Google Scholar]
- 3.Nestler EJ. Is there a common molecular pathway for addiction? Nat Neurosci. 2005;8:1445–1449. doi: 10.1038/nn1578. [DOI] [PubMed] [Google Scholar]
- 4.Koob GF, Le Moal Plasticity of reward neurocircuitry and the ‘dark side’ of drug addiction. Nat Neurosci. 2005;8:1442–1444. doi: 10.1038/nn1105-1442. [DOI] [PubMed] [Google Scholar]
- 5.Weiss F, et al. Compulsive drug-seeking behavior and relapse. Neuroadaptation, stress, and conditioning factors. Ann N Y Acad Sci. 2001;937:1–26. doi: 10.1111/j.1749-6632.2001.tb03556.x. [DOI] [PubMed] [Google Scholar]
- 6.Bardo MT, Bevins RA. Conditioned place preference: What does it add to our preclinical understanding of drug reward? Psychopharmacology (Berl) 2000;153:31–43. doi: 10.1007/s002130000569. [DOI] [PubMed] [Google Scholar]
- 7.Kenny PJ. Brain reward systems and compulsive drug use. Trends Pharmacol Sci. 2007;28:135–141. doi: 10.1016/j.tips.2007.01.008. [DOI] [PubMed] [Google Scholar]
- 8.Kalivas PW, Stewart J. Dopamine transmission in the initiation and expression of drug- and stress-induced sensitization of motor activity. Brain Res Brain Res Rev. 1991;16:223–244. doi: 10.1016/0165-0173(91)90007-u. [DOI] [PubMed] [Google Scholar]
- 9.Phillips TJ, Roberts AJ, Lessov CN. Behavioral sensitization to ethanol: Genetics and the effects of stress. Pharmacol Biochem Behav. 1997;57:487–493. doi: 10.1016/s0091-3057(96)00448-0. [DOI] [PubMed] [Google Scholar]
- 10.Robinson TE, Berridge KC. The neural basis of drug craving: An incentive sensitization theory of addiction. Brain Res Rev. 1993;18:247–291. doi: 10.1016/0165-0173(93)90013-p. [DOI] [PubMed] [Google Scholar]
- 11.Robinson TE, Berridge KC. Addiction. Annu Rev Psychol. 2003;54:25–53. doi: 10.1146/annurev.psych.54.101601.145237. [DOI] [PubMed] [Google Scholar]
- 12.Breese GR, Overstreet DH, Knapp DJ, Navarro M. Prior multiple ethanol withdrawals enhance stress-induced anxiety-like behavior: Inhibition by CRF1- and benzodiazepine-receptor antagonists and a 5-HT1a-receptor agonist. Neuropsychopharmacology. 2005;30:1662–1669. doi: 10.1038/sj.npp.1300706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Veatch LM, Becker HC. Lorazepam and MK-801 effects on behavioral and electrographic indices of alcohol withdrawal sensitization. Brain Res. 2005;1065:92–106. doi: 10.1016/j.brainres.2005.10.047. [DOI] [PubMed] [Google Scholar]
- 14.Lett BT. Repeated exposures intensify rather than diminish the rewarding effects of amphetamine, morphine, and cocaine. Psychopharmacology (Berl) 1989;98:357–362. doi: 10.1007/BF00451687. [DOI] [PubMed] [Google Scholar]
- 15.Piazza PV, Deminière J-M, Le Moal M, Simon H. Factors that predict individual vulnerability to amphetamine self-administration. Science. 1989;245:1511–1513. doi: 10.1126/science.2781295. [DOI] [PubMed] [Google Scholar]
- 16.Vezina P. Sensitization of midbrain dopamine neuron reactivity and the self-administration of psychomotor stimulant drugs. Neurosci Biobehav Rev. 2004;27:827–839. doi: 10.1016/j.neubiorev.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 17.Laorden ML, Castells MT, Milanes MV. Effects of morphine and morphine withdrawal on brainstem neurons innervating hypothalamic nuclei that control the pituitary-adrenocortical axis in rats. Br J Pharmacol. 2002;136:67–75. doi: 10.1038/sj.bjp.0704684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rivier C. Alcohol stimulates ACTH secretion in the rat: Mechanisms of action and interactions with other stimuli. Alcohol Clin Exp Res. 1996;20:240–254. doi: 10.1111/j.1530-0277.1996.tb01636.x. [DOI] [PubMed] [Google Scholar]
- 19.Vanderschuren LJ, et al. A single exposure to amphetamine is sufficient to induce long-term behavioral, neuroendocrine, and neurochemical sensitization in rats. J Neurosci. 1999;19:9579–9586. doi: 10.1523/JNEUROSCI.19-21-09579.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhou Y, et al. Effects of acute “binge” cocaine on preprodynorphin, preproenkephalin, proopiomelanocortin, and corticotropin-releasing hormone receptor mRNA levels in the striatum and hypothalamic-pituitary-adrenal axis of mu-opioid receptor knockout mice. Synapse. 2002;45:220–229. doi: 10.1002/syn.10101. [DOI] [PubMed] [Google Scholar]
- 21.Bilezikjian LM, Vale WW. Regulation of ACTH secretion from corticotrophs: The interaction of vasopressin and CRF. Ann N Y Acad Sci. 1987;512:85–96. doi: 10.1111/j.1749-6632.1987.tb24952.x. [DOI] [PubMed] [Google Scholar]
- 22.Ogilvie K, Lee S, Weiss B, Rivier C. Mechanisms mediating the influence of alcohol on the hypothalamic-pituitary-adrenal axis responses to immune and nonimmune signals. Alcohol Clin Exp Res. 1998;22(5) Suppl:243S–247S. doi: 10.1097/00000374-199805001-00005. [DOI] [PubMed] [Google Scholar]
- 23.Bale TL, Vale WW. CRF and CRF receptors: Role in stress responsivity and other behaviors. Annu Rev Pharmacol Toxicol. 2004;44:525–557. doi: 10.1146/annurev.pharmtox.44.101802.121410. [DOI] [PubMed] [Google Scholar]
- 24.Brown MR, Rivier C, Vale W. Central nervous system regulation of adrenocorticotropin secretion: Role of somatostatins. Endocrinology. 1984;114:1546–1549. doi: 10.1210/endo-114-5-1546. [DOI] [PubMed] [Google Scholar]
- 25.Shepard JD, Schulkin J, Myers DA. Chronically elevated corticosterone in the amygdala increases corticotropin releasing factor mRNA in the dorsolateral bed nucleus of stria terminalis following duress. Behav Brain Res. 2006;174:193–196. doi: 10.1016/j.bbr.2006.07.019. [DOI] [PubMed] [Google Scholar]
- 26.Heilig M, Koob GF. A key role for corticotropin-releasing factor in alcohol dependence. Trends Neurosci. 2007;30:399–406. doi: 10.1016/j.tins.2007.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kiefer F, Wiedemann K. Neuroendocrine pathways of addictive behaviour. Addict Biol. 2004;9:205–212. doi: 10.1080/13556210412331292532. [DOI] [PubMed] [Google Scholar]
- 28.Schmidt ED, Tilders FJ, Binnekade R, Schoffelmeer AN, De Vries TJ. Stressor- or drug-induced sensitization of the corticosterone response is not critically involved in the long-term expression of behavioural sensitization to amphetamine. J Neurosci. 1999;92:343–352. doi: 10.1016/s0306-4522(98)00725-8. [DOI] [PubMed] [Google Scholar]
- 29.Cador M, Cole BJ, Koob GF, Stinus L, Le Moal M. Central administration of corticotropin releasing factor induces long-term sensitization to D-amphetamine. Brain Res. 1993;606:181–186. doi: 10.1016/0006-8993(93)90982-s. [DOI] [PubMed] [Google Scholar]
- 30.Cole BJ, et al. Central administration of a CRF antagonist blocks the development of stress-induced behavioral sensitization. Brain Res. 1990;512:343–346. doi: 10.1016/0006-8993(90)90646-S. [DOI] [PubMed] [Google Scholar]
- 31.Deroche V, et al. Stress induced sensitization to amphetamine and morphine psychomotor effects depend on stress-induced corticosterone secretion. Brain Res. 1992;598:343–348. doi: 10.1016/0006-8993(92)90205-n. [DOI] [PubMed] [Google Scholar]
- 32.Erb S, Brown ZJ. A role for corticotropin-releasing factor in the long-term expression of behavioral sensitization to cocaine. Behav Brain Res. 2006;172:360–364. doi: 10.1016/j.bbr.2006.05.024. [DOI] [PubMed] [Google Scholar]
- 33.Goeders NE. Stress and cocaine addiction. J Pharmacol Exp Ther. 2002;301:785–789. doi: 10.1124/jpet.301.3.785. [DOI] [PubMed] [Google Scholar]
- 34.Xu GP, Van Bockstaele E, Reyes B, Bethea T, Valentino RJ. Chronic morphine sensitizes the brain norepinephrine system to corticotropin-releasing factor and stress. J Neurosci. 2004;24:8193–8197. doi: 10.1523/JNEUROSCI.1657-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Koob GF, Cador M. Psychomotor stimulant sensitization: The corticotropin-releasing factor-steroid connection. Commentary on Wise RA, Leeb K “Psychomotor-stimulant sensitization: A unitary phenomenon?”. Behav Pharmacol. 1993;4:351–354. [PubMed] [Google Scholar]
- 36.Wise RA, Leeb K. Psychomotor-stimulant sensitization: A unitary phenomenon? Behav Pharmacol. 1993;4:339–349. [PubMed] [Google Scholar]
- 37.Lu L, Liu Z, Huang M, Zhang Z. Dopamine-dependent responses to cocaine depend on corticotropin-releasing factor receptor subtypes. J Neurochem. 2003;84:1378–1386. doi: 10.1046/j.1471-4159.2003.01635.x. [DOI] [PubMed] [Google Scholar]
- 38.Piazza PV, et al. Suppression of glucocorticoid secretion and antipsychotic drugs have similar effects on the mesolimbic dopaminergic transmission. Proc Natl Acad Sci USA. 1996;93:15445–15450. doi: 10.1073/pnas.93.26.15445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Roberts AJ, Lessov CN, Phillips TJ. Critical role for glucocorticoid receptors in stress- and ethanol-induced locomotor sensitization. J Pharmacol Exp Ther. 1995;275:790–797. [PubMed] [Google Scholar]
- 40.Ehlers CL, Chaplin RI. Chronic ethanol exposure potentiates the locomotor-activating effects of corticotropin-releasing factor (CRF) in rats. Regul Pept. 1987;19:345–353. doi: 10.1016/0167-0115(87)90176-5. [DOI] [PubMed] [Google Scholar]
- 41.Chalmers DT, Lovenberg TW, De Souza EB. Localization of novel corticotropin-releasing factor receptor (CRF2) mRNA expression to specific subcortical nuclei in rat brain: comparison with CRF1 receptor mRNA expression. J Neurosci. 1995;15:6340–6350. doi: 10.1523/JNEUROSCI.15-10-06340.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Coste SC, Murray SE, Stenzel-Poore MP. Animal models of CRH excess and CRH receptor deficiency display altered adaptations to stress. Peptides. 2001;22:733–741. doi: 10.1016/s0196-9781(01)00386-2. [DOI] [PubMed] [Google Scholar]
- 43.Lee S, Smith GW, Vale W, Lee KF, Rivier C. Mice that lack corticotropin-releasing factor (CRF) receptors type 1 show a blunted ACTH response to acute alcohol despite up-regulated constitutive hypothalamic CRF gene expression. Alcohol Clin Exp Res. 2001;25:427–433. [PubMed] [Google Scholar]
- 44.Smith GW, et al. Corticotropin releasing factor receptor 1-deficient mice display decreased anxiety, impaired stress response, and aberrant neuroendocrine development. Neuron. 1998;20:1093–1102. doi: 10.1016/s0896-6273(00)80491-2. [DOI] [PubMed] [Google Scholar]
- 45.Timpl P, et al. Impaired stress response and reduced anxiety in mice lacking a functional corticotropin-releasing hormone receptor 1. Nat Genet. 1998;19:162–166. doi: 10.1038/520. [DOI] [PubMed] [Google Scholar]
- 46.Coste SC, et al. Abnormal adaptations to stress and impaired cardiovascular function in mice lacking corticotropin-releasing hormone receptor-2. Nat Genet. 2000;24:403–409. doi: 10.1038/74255. [DOI] [PubMed] [Google Scholar]
- 47.Schulz DW, et al. CP-154,526: A potent and selective nonpeptide antagonist of corticotropin releasing factor receptors. Proc Natl Acad Sci USA. 1996;93:10477–10482. doi: 10.1073/pnas.93.19.10477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ryabinin AE, Weitemier AZ. The urocortin 1 neurocircuit: Ethanol-sensitivity and potential involvement in alcohol consumption. Brain Res Rev. 2006;52:368–380. doi: 10.1016/j.brainresrev.2006.04.007. [DOI] [PubMed] [Google Scholar]
- 49.Koob GF. The neurobiology of addiction: A neuroadaptational view relevant for diagnosis. Addiction. 2006;101(Suppl 1):23–30. doi: 10.1111/j.1360-0443.2006.01586.x. [DOI] [PubMed] [Google Scholar]
- 50.Heinrichs SC, Menzaghi F, Merlo Pich E, Britton KT, Koob GF. The role of CRF in behavioral aspects of stress. Ann N Y Acad Sci. 1995;771:92–104. doi: 10.1111/j.1749-6632.1995.tb44673.x. [DOI] [PubMed] [Google Scholar]
- 51.Claes SJ. Corticotropin-releasing hormone (CRH) in psychiatry: From stress to psychopathology. Ann Med. 2004;36:50–61. doi: 10.1080/07853890310017044. [DOI] [PubMed] [Google Scholar]
- 52.Sinha R. How does stress increase risk of drug abuse and relapse? Psychopharmacology (Berl) 2001;158:343–359. doi: 10.1007/s002130100917. [DOI] [PubMed] [Google Scholar]
- 53.Arborelius L, Owens MJ, Plotsky PM, Nemeroff CB. The role of corticotropin-releasing factor in depression and anxiety disorders. J Endocrinol. 1999;160:1–12. doi: 10.1677/joe.0.1600001. [DOI] [PubMed] [Google Scholar]
- 54.Hansson AC, et al. Variation at the rat Crhr1 locus and sensitivity to relapse into alcohol seeking induced by environmental stress. Proc Natl Acad Sci USA. 2006;103:15236–15241. doi: 10.1073/pnas.0604419103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Palmer AA, et al. Corticotropin-releasing factor overexpression decreases ethanol drinking and increases sensitivity to the sedative effects of ethanol. Psychopharmacology (Berl) 2004;176:386–397. doi: 10.1007/s00213-004-1896-5. [DOI] [PubMed] [Google Scholar]
- 56.Ehlers CL, et al. Corticotropin releasing factor (CRF): Studies in alcohol preferring and non-preferring rats. Psychopharmacology (Berl) 1992;106:359–364. doi: 10.1007/BF02245418. [DOI] [PubMed] [Google Scholar]
- 57.George SR, Fan T, Roldan L, Naranjo CA. Corticotropin-releasing factor is altered in brains of animals with high preference for ethanol. Alcohol Clin Exp Res. 1990;14:425–429. doi: 10.1111/j.1530-0277.1990.tb00499.x. [DOI] [PubMed] [Google Scholar]
- 58.Funk CK, Zorrilla EP, Lee MJ, Rice KC, Koob GF. Corticotropin-releasing factor 1 antagonists selectively reduce ethanol self-administration in ethanol-dependent rats. Biol Psychiatry. 2007;61:78–86. doi: 10.1016/j.biopsych.2006.03.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gehlert DR, et al. 3-(4-Chloro-2-morpholin-4-yl-thiazol-5-yl)-8-(1-ethylpropyl)-2,6-dimethyl-imidazo [1,2-b]pyridazine: A novel brain-penetrant, orally available corticotropin-releasing factor receptor 1 antagonist with efficacy in animal models of alcoholism. J Neurosci. 2007;27:2718–2726. doi: 10.1523/JNEUROSCI.4985-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rivier C, Bruhn T, Vale W. Effect of ethanol on the hypothalamic-pituitary-adrenal axis in the rat: Role of corticotropin-releasing factor (CRF) J Pharmacol Exp Ther. 1984;229:127–131. [PubMed] [Google Scholar]
- 61.Lee S, Rivier C. Alcohol increases the expression of type 1, but not type 2 alpha corticotropin-releasing factor (CRF) receptor messenger ribonucleic acid in the rat hypothalamus. Brain Res Mol Brain Res. 1997;52:78–89. doi: 10.1016/s0169-328x(97)00226-x. [DOI] [PubMed] [Google Scholar]
- 62.Arborelius L, et al. Chronic administration of the selective corticotropin-releasing factor 1 receptor antagonist CP-154,526: Behavioral, endocrine and neurochemical effects in the rat. J Pharmacol Exp Ther. 2000;294:588–597. [PubMed] [Google Scholar]
- 63.Härfstrand A, et al. Glucocorticoid receptor immunoreactivity in monoaminergic neurons of rat brain. Proc Natl Acad Sci USA. 1986;83:9779–9783. doi: 10.1073/pnas.83.24.9779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Overstreet DH, Knapp DJ, Breese GR. Modulation of multiple ethanol withdrawal-induced anxiety-like behavior by CRF and CRF1 receptors. Pharmacol Biochem Behav. 2004;77:405–413. doi: 10.1016/j.pbb.2003.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sabino V, et al. Dissociation between opioid and CRF1 antagonist sensitive drinking in Sardinian alcohol-preferring rats. Psychopharmacology (Berl) 2006;189:175–186. doi: 10.1007/s00213-006-0546-5. [DOI] [PubMed] [Google Scholar]
- 66.Heinrichs SC, Koob GF. Corticotropin-releasing factor in brain: A role in activation, arousal, and affect regulation. J Pharmacol Exp Ther. 2004;311:427–440. doi: 10.1124/jpet.103.052092. [DOI] [PubMed] [Google Scholar]
- 67.Mello NK, Mendelson JH. Cocaine's effects on neuroendocrine systems: Clinical and preclinical studies. Pharmacol Biochem Behav. 1997;57:571–599. doi: 10.1016/s0091-3057(96)00433-9. [DOI] [PubMed] [Google Scholar]
- 68.Skelton KH, Nemeroff CB, Owens MJ. Spontaneous withdrawal from the triazolobenzodiazepine alprazolam increases cortical corticotropin-releasing factor mRNA expression. J Neurosci. 2004;24:9303–9312. doi: 10.1523/JNEUROSCI.1737-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Skelton KH, Gutman DA, Thrivikraman KV, Nemeroff CB, Owens MJ. The CRF1 receptor antagonist R121919 attenuates the neuroendocrine and behavioral effects of precipitated lorazepam withdrawal. Psychopharmacology (Berl) 2007;192:385–396. doi: 10.1007/s00213-007-0713-3. [DOI] [PubMed] [Google Scholar]
- 70.Holahan MR, Kalin NH, Kelley AE. Microinfusion of corticotropin-releasing factor into the nucleus accumbens shell results in increased behavioral arousal and oral motor activity. Psychopharmacology (Berl) 1997;130:189–196. doi: 10.1007/s002130050228. [DOI] [PubMed] [Google Scholar]
- 71.Fee JR, Sparta DR, Picker MJ, Thiele TE. Corticotropin releasing factor-1 receptor antagonist, CP-154,526, blocks the expression of ethanol-induced behavioral sensitization in DBA/2J mice. Neuroscience. 2007;150:14–21. doi: 10.1016/j.neuroscience.2007.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Vetter DE, et al. Urocortin-deficient mice show hearing impairment and increased anxiety-like behavior. Nat Genet. 2002;31:363–369. doi: 10.1038/ng914. [DOI] [PubMed] [Google Scholar]
- 73.Preil J, et al. Regulation of the hypothalamic-pituitary-adrenocortical system in mice deficient for CRH receptors 1 and 2. Endocrinology. 2001;142:4946–4955. doi: 10.1210/endo.142.11.8507. [DOI] [PubMed] [Google Scholar]
- 74.Lessov CN, Palmer AA, Quick EA, Phillips TJ. Voluntary ethanol drinking in C57BL/6J and DBA/2J mice before and after sensitization to the locomotor stimulant effects of ethanol. Psychopharmacology (Berl) 2001;155:91–99. doi: 10.1007/s002130100699. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.