Abstract
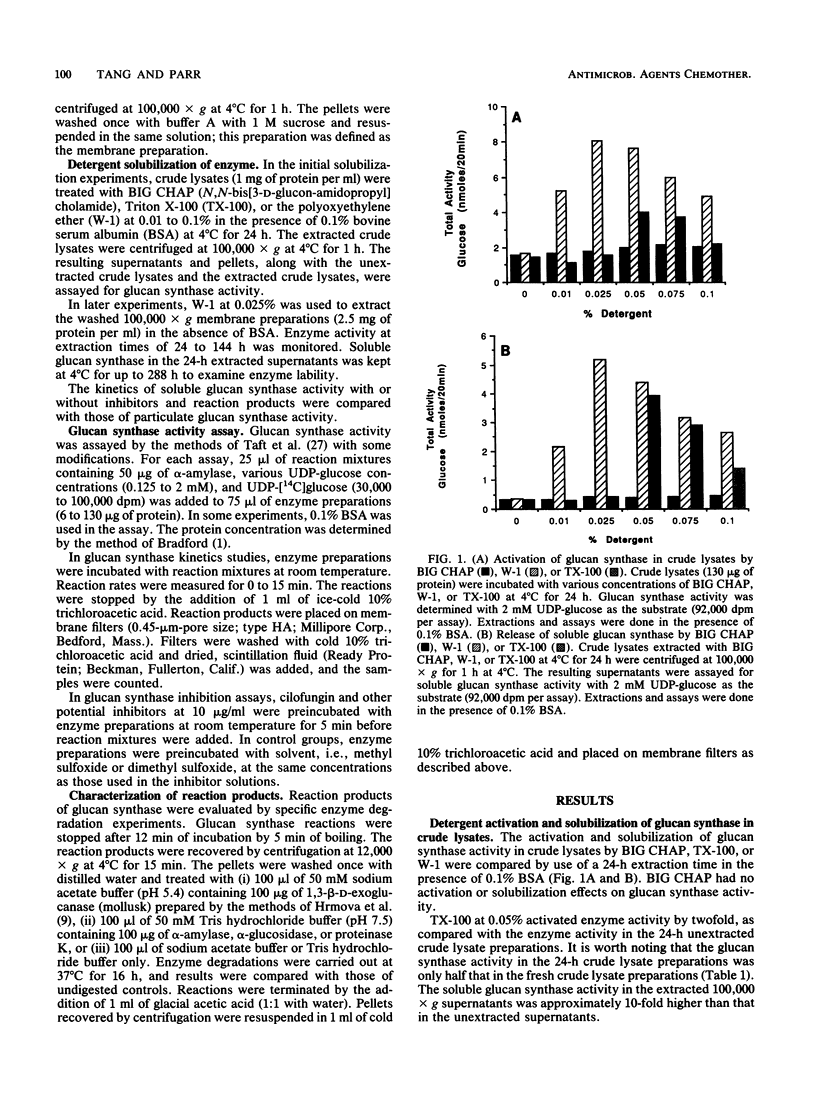
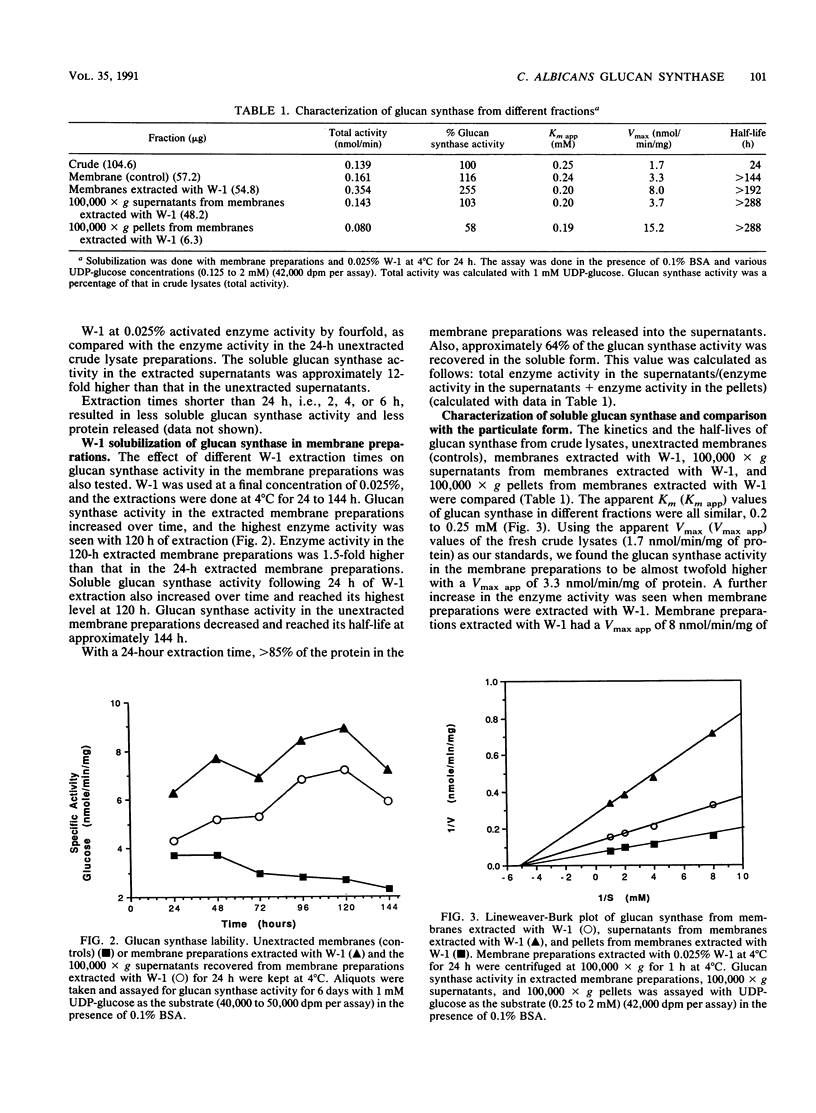
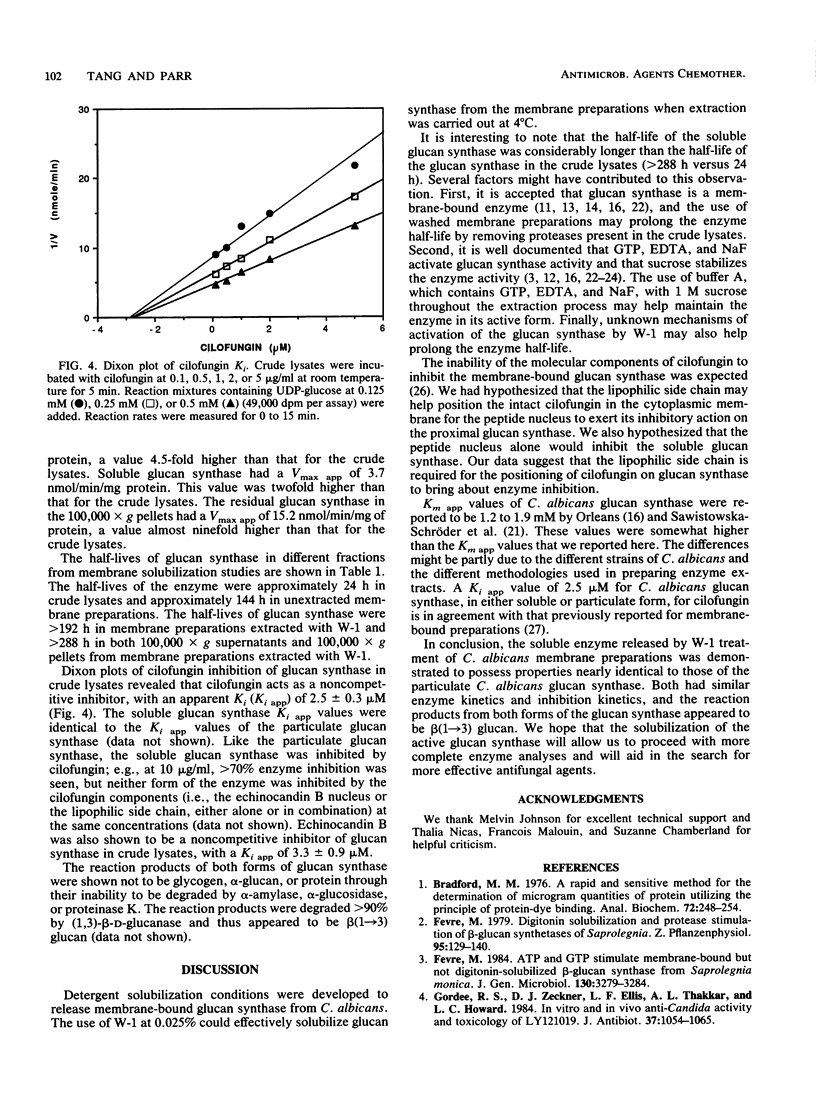
(1,3)-beta-D-Glucan synthase of Candida albicans was rendered soluble by treatment of membrane preparations with the polyoxyethylene ether detergent W-1. Extraction with 0.025% W-1 at 4 degrees C for 24 h effectively solubilized and activated the enzyme. Under these conditions, greater than 85% of the protein in membrane preparations was released, and about 64% of the glucan synthase activity could be recovered in the soluble form. Soluble enzyme activity was stable for more than 12 days at 4 degrees C. Also, glucan synthase activity in the extracted membrane preparations could be activated to achieve more than twice the enzyme activity in the original, unextracted membrane preparations. The soluble glucan synthase had characteristics similar to those of the membrane-bound enzyme. Soluble glucan synthase had an apparent Km of 2.0 mM, and particulate glucan synthase had an apparent Km of 2.5 mM. Kinetics of cilofungin inhibition for both enzyme preparations were noncompetitive, with an apparent Ki of 2.5 microM; both preparations could be inhibited by cilofungin but not by its peptide nucleus or side chain, either alone or in combination. The reaction products from both forms of the enzyme were sensitive to (1,3)-beta-D-glucanase degradation but not to alpha-amylase, alpha-glucosidase, or proteinase K degradation and thus were shown to be beta(1----3) glucan.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Gordee R. S., Zeckner D. J., Ellis L. F., Thakkar A. L., Howard L. C. In vitro and in vivo anti-Candida activity and toxicology of LY121019. J Antibiot (Tokyo) 1984 Sep;37(9):1054–1065. doi: 10.7164/antibiotics.37.1054. [DOI] [PubMed] [Google Scholar]
- Hall G. S., Myles C., Pratt K. J., Washington J. A. Cilofungin (LY121019), an antifungal agent with specific activity against Candida albicans and Candida tropicalis. Antimicrob Agents Chemother. 1988 Sep;32(9):1331–1335. doi: 10.1128/aac.32.9.1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson L. H., Stevens D. A. Evaluation of cilofungin, a lipopeptide antifungal agent, in vitro against fungi isolated from clinical specimens. Antimicrob Agents Chemother. 1989 Aug;33(8):1391–1392. doi: 10.1128/aac.33.8.1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobbs M., Perfect J., Durack D. Evaluation of in vitro antifungal activity of LY121019. Eur J Clin Microbiol Infect Dis. 1988 Feb;7(1):77–80. doi: 10.1007/BF01962182. [DOI] [PubMed] [Google Scholar]
- Kang M. S., Cabib E. Regulation of fungal cell wall growth: a guanine nucleotide-binding, proteinaceous component required for activity of (1----3)-beta-D-glucan synthase. Proc Natl Acad Sci U S A. 1986 Aug;83(16):5808–5812. doi: 10.1073/pnas.83.16.5808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leal F., Ruiz-Herrera J., Villanueva J. R., Larriba G. An examination of factors affecting the instability of Saccharomyces cerevisiae glucan synthetase in cell free extracts. Arch Microbiol. 1984 Mar;137(3):209–214. doi: 10.1007/BF00414545. [DOI] [PubMed] [Google Scholar]
- López-Romero E., Ruiz-Herrera J. Biosynthesis of beta-glucans by cell-free extracts from Saccharomyces cerevisiae. Biochim Biophys Acta. 1977 Dec 22;500(2):372–384. doi: 10.1016/0304-4165(77)90028-9. [DOI] [PubMed] [Google Scholar]
- López-Romero E., Ruiz-Herrera J. Properties of beta-glucan synthetase from Saccharomyces cerevisiae. Antonie Van Leeuwenhoek. 1978;44(3-4):329–339. doi: 10.1007/BF00394310. [DOI] [PubMed] [Google Scholar]
- Miragall F., Rico H., Sentandreu R. Changes in the plasma membrane of regenerating protoplasts of Candida albicans as revealed by freeze-fracture electron microscopy. J Gen Microbiol. 1986 Oct;132(10):2845–2853. doi: 10.1099/00221287-132-10-2845. [DOI] [PubMed] [Google Scholar]
- Orlean P. A. (1,3)-beta-D-Glucan synthase from budding and filamentous cultures of the dimorphic fungus Candida albicans. Eur J Biochem. 1982 Oct;127(2):397–403. doi: 10.1111/j.1432-1033.1982.tb06885.x. [DOI] [PubMed] [Google Scholar]
- Pfaller M., Gordee R., Gerarden T., Yu M., Wenzel R. Fungicidal activity of cilofungin (LY121019) alone and in combination with anticapsin or other antifungal agents. Eur J Clin Microbiol Infect Dis. 1989 Jun;8(6):564–567. doi: 10.1007/BF01967483. [DOI] [PubMed] [Google Scholar]
- San-Blas G., San-Blas F. Effect of detergents on membrane-associated glucan synthetase from Paracoccidioides brasiliensis. J Bacteriol. 1982 Nov;152(2):563–566. doi: 10.1128/jb.152.2.563-566.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawistowska-Schröder E. T., Kerridge D., Perry H. Echinocandin inhibition of 1,3-beta-D-glucan synthase from Candida albicans. FEBS Lett. 1984 Jul 23;173(1):134–138. doi: 10.1016/0014-5793(84)81032-7. [DOI] [PubMed] [Google Scholar]
- Shematek E. M., Braatz J. A., Cabib E. Biosynthesis of the yeast cell wall. I. Preparation and properties of beta-(1 leads to 3)glucan synthetase. J Biol Chem. 1980 Feb 10;255(3):888–894. [PubMed] [Google Scholar]
- Shematek E. M., Cabib E. Biosynthesis of the yeast cell wall. II. Regulation of beta-(1 leads to 3)glucan synthetase by ATP and GTP. J Biol Chem. 1980 Feb 10;255(3):895–902. [PubMed] [Google Scholar]
- Szaniszlo P. J., Kang M. S., Cabib E. Stimulation of beta(1----3)glucan synthetase of various fungi by nucleoside triphosphates: generalized regulatory mechanism for cell wall biosynthesis. J Bacteriol. 1985 Mar;161(3):1188–1194. doi: 10.1128/jb.161.3.1188-1194.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taft C. S., Selitrennikoff C. P. Cilofungin inhibition of (1-3)-beta-glucan synthase: the lipophilic side chain is essential for inhibition of enzyme activity. J Antibiot (Tokyo) 1990 Apr;43(4):433–437. doi: 10.7164/antibiotics.43.433. [DOI] [PubMed] [Google Scholar]
- Taft C. S., Selitrennikoff C. P. LY121019 inhibits Neurospora crassa growth and (1-3)-beta-D-glucan synthase. J Antibiot (Tokyo) 1988 May;41(5):697–701. doi: 10.7164/antibiotics.41.697. [DOI] [PubMed] [Google Scholar]
- Taft C. S., Stark T., Selitrennikoff C. P. Cilofungin (LY121019) inhibits Candida albicans (1-3)-beta-D-glucan synthase activity. Antimicrob Agents Chemother. 1988 Dec;32(12):1901–1903. doi: 10.1128/aac.32.12.1901. [DOI] [PMC free article] [PubMed] [Google Scholar]


