Abstract
Temafloxacin (A-63004) is a new quinolone antibacterial agent with a broad spectrum of activity against gram-positive and gram-negative aerobes and anaerobes. The pharmacokinetics and metabolism of temafloxacin were determined in healthy volunteers after administration of single oral doses of 100, 200, 400, 600, 800, and 1,000 mg. The corresponding peak concentrations in plasma (mean +/- standard deviation) were 0.98 +/- 0.26, 1.61 +/- 0.57, 2.43 +/- 0.56, 3.87 +/- 0.64, 4.54 +/- 1.03, and 6.67 +/- 0.74 micrograms/ml. The times that elapsed to attain peak levels ranged from 1.25 to 3.5 h. Statistical analyses of parameters related to the extent of absorption and the linearity of the dispositional pharmacokinetics detected no dose-related trends. Study-wide, total clearance (223 ml/min) and renal clearance (125 ml/min) showed low intersubject variability, with coefficients of variation near 20%. The terminal-phase rate constant of 0.090 +/- 0.008 h-1 corresponds to a half-life of 7.7 h. Temafloxacin was excreted mainly in the urine, with 57 +/- 11% of the dose appearing in the urine unchanged. Conjugated temafloxacin, oxidative metabolites, and conjugates thereof were minor components in urine, collectively accounting for 5 to 8% of the dose. Since intravenously dosed dogs eliminated 50% of the dose by nonrenal processes, urinary recoveries approaching two-thirds of the dose in humans were consistent with high, if not quantitative, absorption. Reported adverse events were generally mild, were randomly distributed between temafloxacin- and placebo-treated subjects, and were not dose related.
Full text
PDF
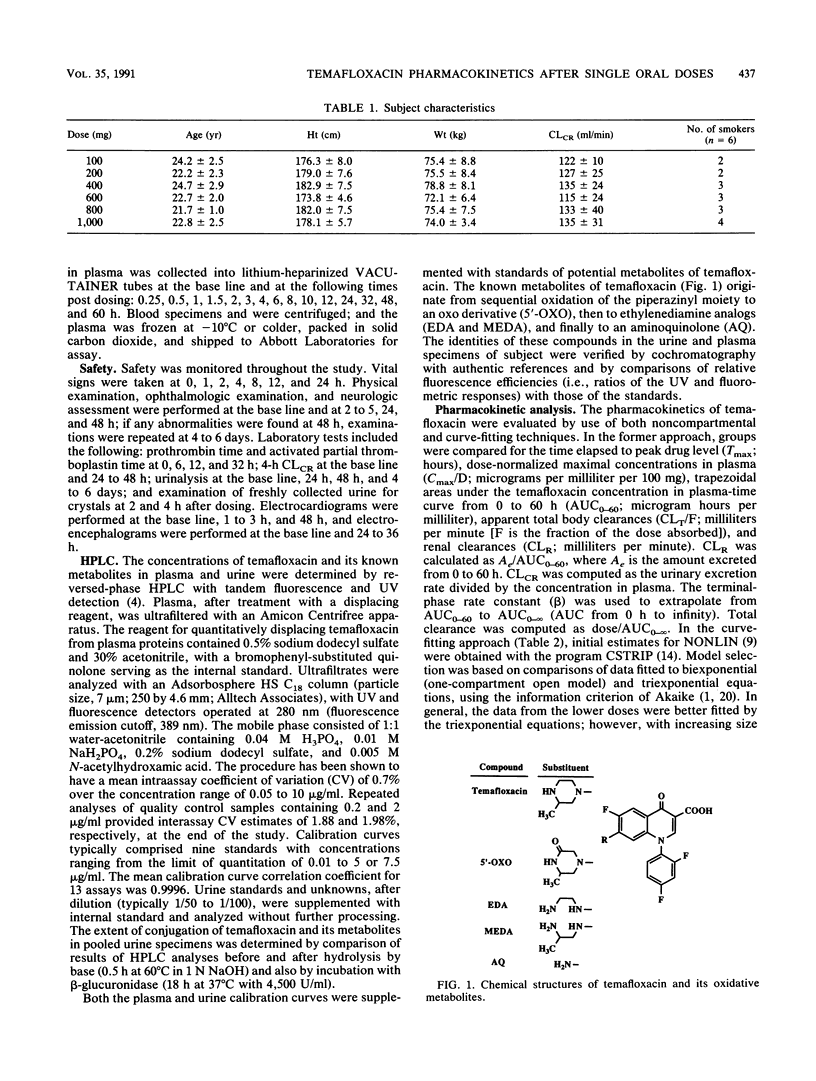
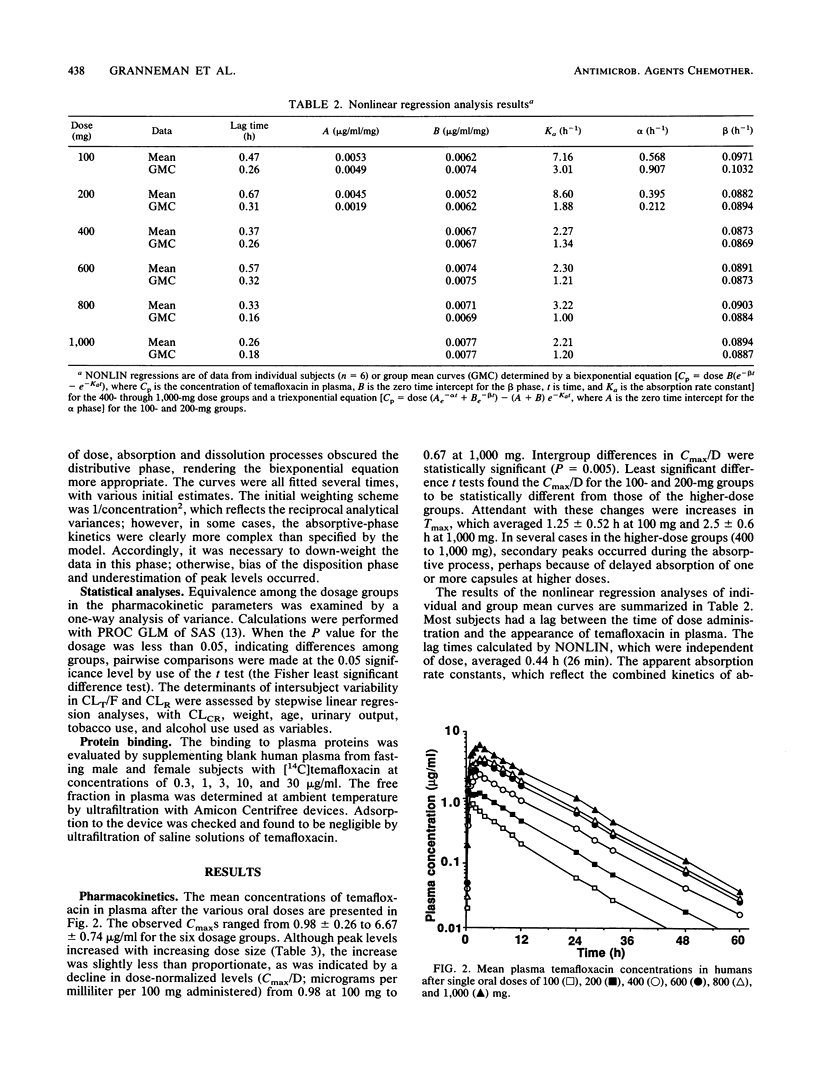
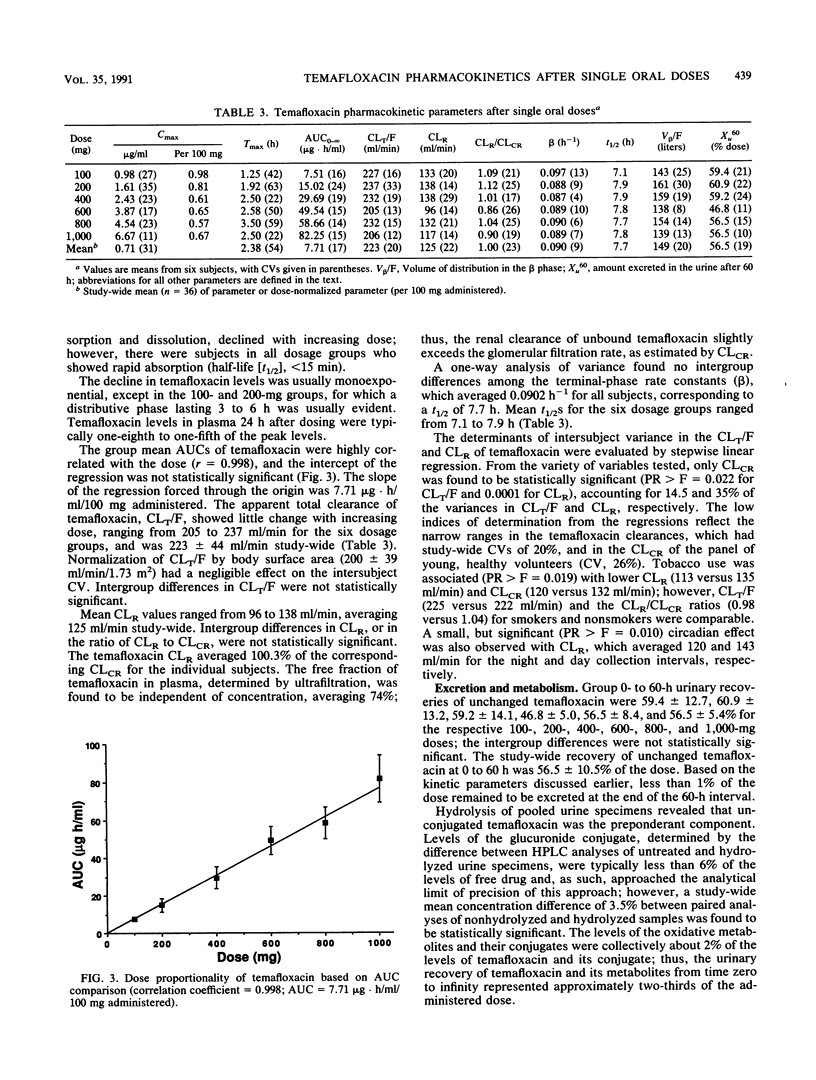


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barry A. L., Jones R. N. In-vitro activities of temafloxacin, tosufloxacin (A-61827) and five other fluoroquinolone agents. J Antimicrob Chemother. 1989 Apr;23(4):527–535. doi: 10.1093/jac/23.4.527. [DOI] [PubMed] [Google Scholar]
- Crump B., Wise R., Dent J. Pharmacokinetics and tissue penetration of ciprofloxacin. Antimicrob Agents Chemother. 1983 Nov;24(5):784–786. doi: 10.1128/aac.24.5.784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granneman G. R., Sennello L. T. High-performance liquid chromatographic procedures for the determination of difloxacin and its metabolites in biological matrices. J Chromatogr. 1987 Jan 23;413:199–206. doi: 10.1016/0378-4347(87)80227-x. [DOI] [PubMed] [Google Scholar]
- Granneman G. R., Snyder K. M., Shu V. S. Difloxacin metabolism and pharmacokinetics in humans after single oral doses. Antimicrob Agents Chemother. 1986 Nov;30(5):689–693. doi: 10.1128/aac.30.5.689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy D. J., Swanson R. N., Hensey D. M., Ramer N. R., Bower R. R., Hanson C. W., Chu D. T., Fernandes P. B. Comparative antibacterial activities of temafloxacin hydrochloride (A-62254) and two reference fluoroquinolones. Antimicrob Agents Chemother. 1987 Nov;31(11):1768–1774. doi: 10.1128/aac.31.11.1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leroy A., Fillastre J. P., Humbert G. Lomefloxacin pharmacokinetics in subjects with normal and impaired renal function. Antimicrob Agents Chemother. 1990 Jan;34(1):17–20. doi: 10.1128/aac.34.1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lode H., Höffken G., Olschewski P., Sievers B., Kirch A., Borner K., Koeppe P. Pharmacokinetics of ofloxacin after parenteral and oral administration. Antimicrob Agents Chemother. 1987 Sep;31(9):1338–1342. doi: 10.1128/aac.31.9.1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montay G., Goueffon Y., Roquet F. Absorption, distribution, metabolic fate, and elimination of pefloxacin mesylate in mice, rats, dogs, monkeys, and humans. Antimicrob Agents Chemother. 1984 Apr;25(4):463–472. doi: 10.1128/aac.25.4.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinberg A., Smolensky M. H. Circadian changes of drug disposition in man. Clin Pharmacokinet. 1982 Sep-Oct;7(5):401–420. doi: 10.2165/00003088-198207050-00002. [DOI] [PubMed] [Google Scholar]
- Rolston K. V., Ho D. H., LeBlanc B., Gooch G., Bodey G. P. Comparative in vitro activity of the new difluoro-quinolone temafloxacin (A-62254) against bacterial isolates from cancer patients. Eur J Clin Microbiol Infect Dis. 1988 Oct;7(5):684–686. doi: 10.1007/BF01964255. [DOI] [PubMed] [Google Scholar]
- Sedman A. J., Wagner J. G. CSTRIP, a fortran IV computer program for obtaining initial polyexponential parameter estimates. J Pharm Sci. 1976 Jul;65(7):1006–1010. doi: 10.1002/jps.2600650713. [DOI] [PubMed] [Google Scholar]
- Swanson B. N., Boppana V. K., Vlasses P. H., Rotmensch H. H., Ferguson R. K. Norfloxacin disposition after sequentially increasing oral doses. Antimicrob Agents Chemother. 1983 Feb;23(2):284–288. doi: 10.1128/aac.23.2.284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weidekamm E., Portmann R., Suter K., Partos C., Dell D., Lücker P. W. Single- and multiple-dose pharmacokinetics of fleroxacin, a trifluorinated quinolone, in humans. Antimicrob Agents Chemother. 1987 Dec;31(12):1909–1914. doi: 10.1128/aac.31.12.1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise R., Lockley R., Dent J., Webberly M. Pharmacokinetics and tissue penetration of enoxacin. Antimicrob Agents Chemother. 1984 Jul;26(1):17–19. doi: 10.1128/aac.26.1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfson J. S., Hooper D. C. The fluoroquinolones: structures, mechanisms of action and resistance, and spectra of activity in vitro. Antimicrob Agents Chemother. 1985 Oct;28(4):581–586. doi: 10.1128/aac.28.4.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaoka K., Nakagawa T., Uno T. Application of Akaike's information criterion (AIC) in the evaluation of linear pharmacokinetic equations. J Pharmacokinet Biopharm. 1978 Apr;6(2):165–175. doi: 10.1007/BF01117450. [DOI] [PubMed] [Google Scholar]


