Abstract
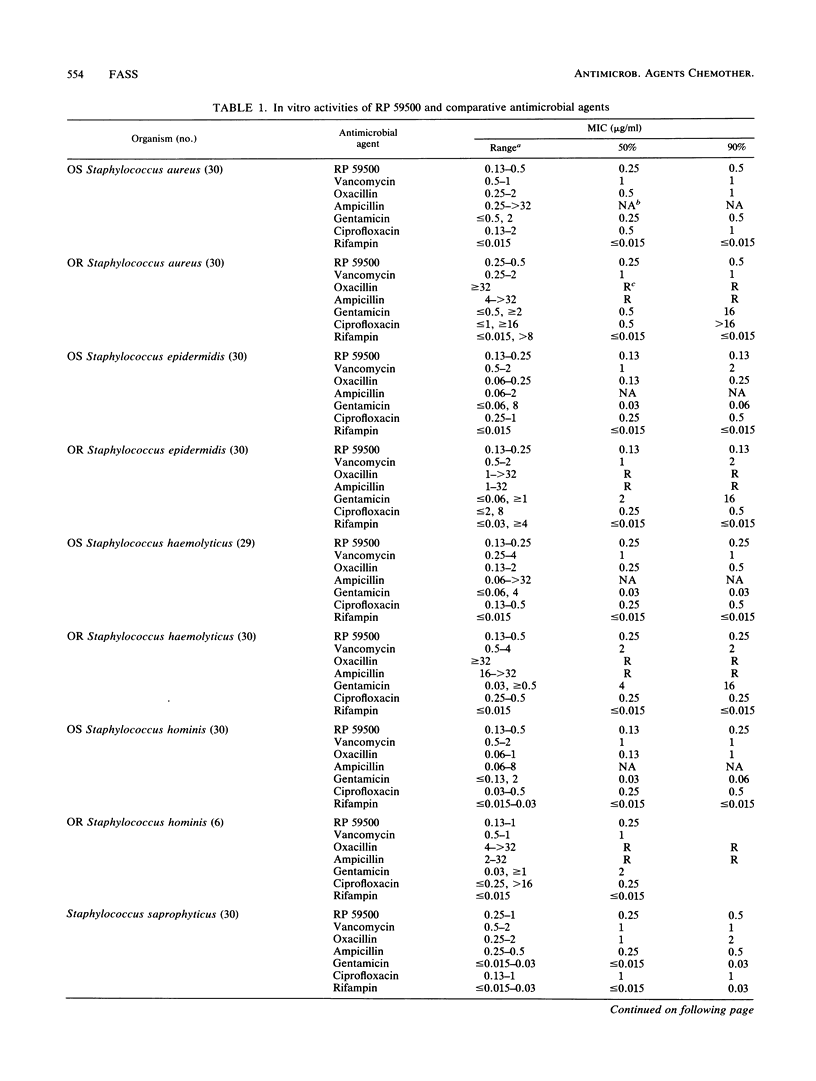
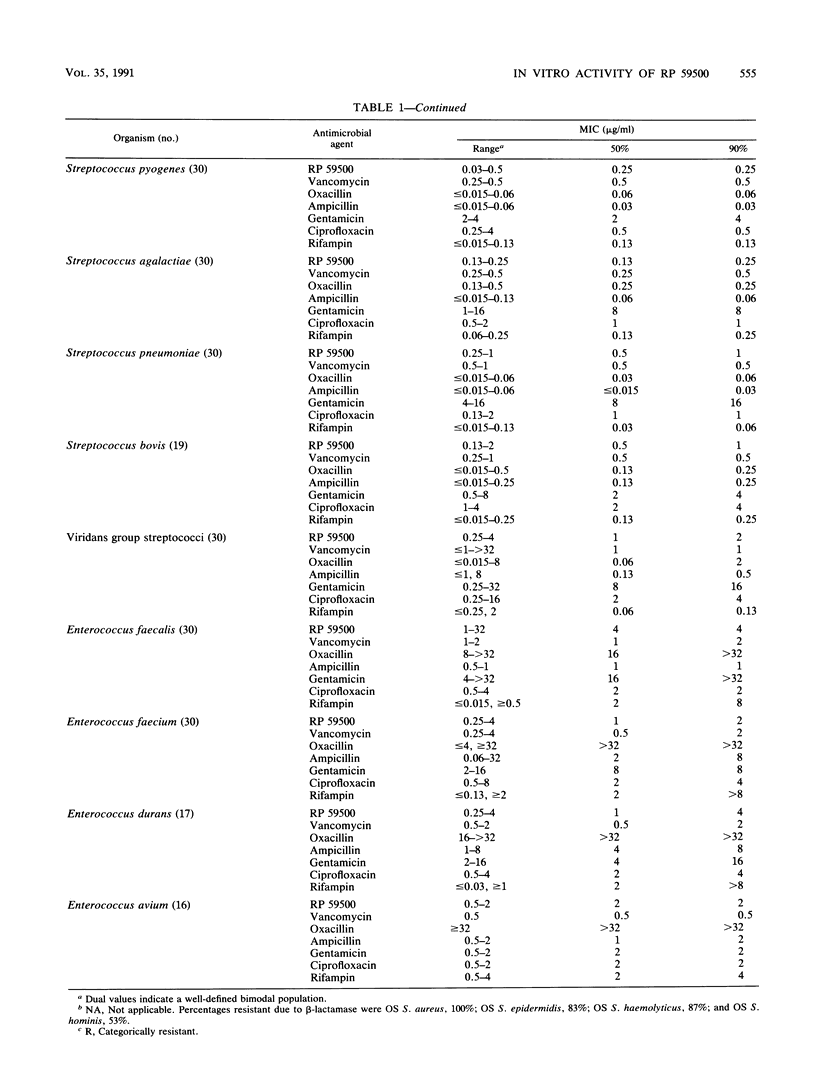
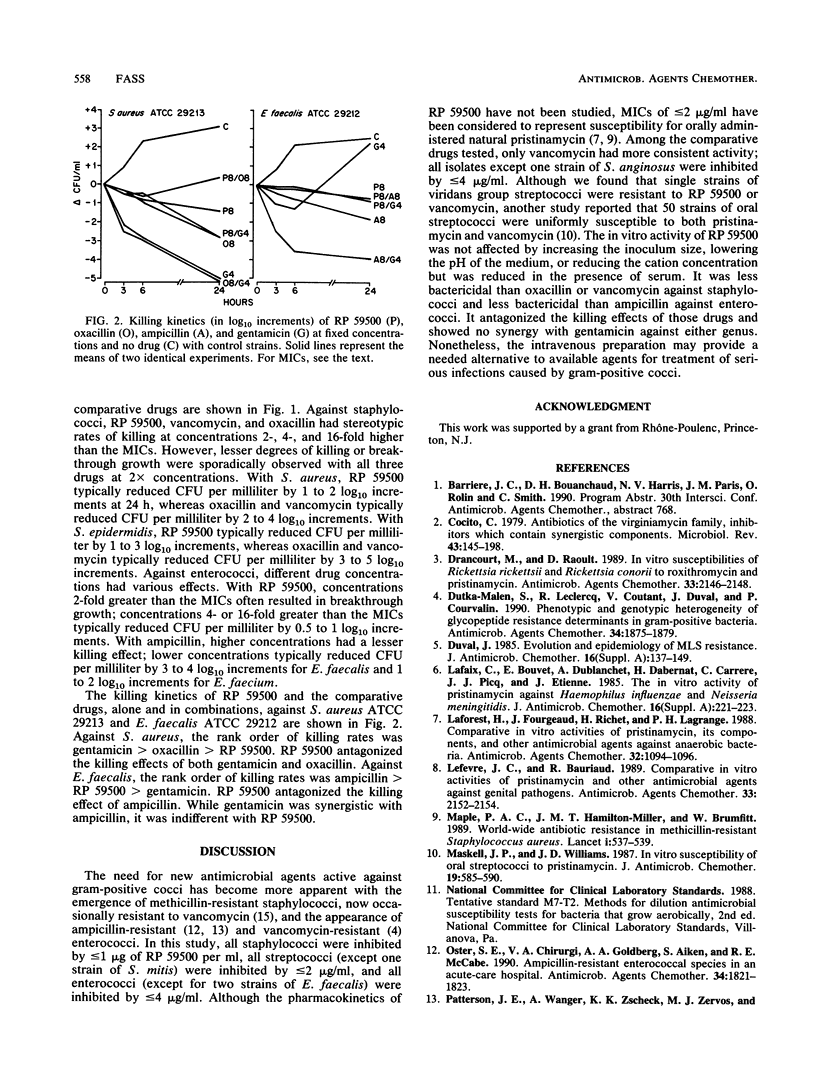
The in vitro activity of RP 59500, a semisynthetic pristinamycin, was compared with the activities of vancomycin, oxacillin, ampicillin, gentamicin, ciprofloxacin, and rifampin against five Staphylococcus species, five Streptococcus species, and four Enterococcus species. For staphylococci, MICs were 0.13 to 1 microgram/ml and the MICs for 90% of the strains tested (MIC90s) were 0.13 to 0.5 microgram/ml; there were no differences between oxacillin-susceptible and -resistant strains. For streptococci, MICs were 0.03 to 4 micrograms/ml and MIC90s were 0.25 to 2 micrograms/ml; viridans group streptococci were the least susceptible streptococci. For enterococci, MICs were 0.25 to 32 micrograms/ml and MIC90s were 2 to 4 micrograms/ml; Enterococcus faecalis was the least susceptible. Vancomycin was the only comparative drug with consistent activity against all species of gram-positive cocci. With RP 59500, raising the inoculum 100-fold, lowering the pH of cation-adjusted Mueller-Hinton broth to 5.5, or omitting cation supplementation had little effect on MICs, but 50% serum increased MICs 2 to 4 dilution steps. The differences between MBCs and MICs were greater for staphylococci and enterococci than for streptococci. Time-kill studies with 24 strains indicated that RP 59500 concentrations 2-, 4-, and 16-fold greater than the MICs usually killed bacteria of each species at similar rates; reductions in CFU per milliliter were less than those observed with oxacillin or vancomycin against staphylococci and less than those observed with ampicillin against enterococci. RP 59500 antagonized the bactericidal activities of oxacillin and gentamicin against Staphylococcus aureus ATCC 29213 and that of ampicillin against E. faecalis ATCC 29212. Against the latter, combination with gentamicin was indifferent. RP 59500 has a broad spectrum of in vitro activity against gram-positive cocci; combining it with other drugs is not advantageous.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cocito C. Antibiotics of the virginiamycin family, inhibitors which contain synergistic components. Microbiol Rev. 1979 Jun;43(2):145–192. doi: 10.1128/mr.43.2.145-192.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drancourt M., Raoult D. In vitro susceptibilities of Rickettsia rickettsii and Rickettsia conorii to roxithromycin and pristinamycin. Antimicrob Agents Chemother. 1989 Dec;33(12):2146–2148. doi: 10.1128/aac.33.12.2146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutka-Malen S., Leclercq R., Coutant V., Duval J., Courvalin P. Phenotypic and genotypic heterogeneity of glycopeptide resistance determinants in gram-positive bacteria. Antimicrob Agents Chemother. 1990 Oct;34(10):1875–1879. doi: 10.1128/aac.34.10.1875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duval J. Evolution and epidemiology of MLS resistance. J Antimicrob Chemother. 1985 Jul;16 (Suppl A):137–149. doi: 10.1093/jac/16.suppl_a.137. [DOI] [PubMed] [Google Scholar]
- Lafaix C., Bouvet E., Dublanchet A., Dabernat H., Carrere C., Picq J. J., Etienne J. The in-vitro activity of pristinamycin against Haemophilus influenzae and Neisseria meningitidis. J Antimicrob Chemother. 1985 Jul;16 (Suppl A):221–223. doi: 10.1093/jac/16.suppl_a.221. [DOI] [PubMed] [Google Scholar]
- Laforest H., Fourgeaud M., Richet H., Lagrange P. H. Comparative in vitro activities of pristinamycin, its components, and other antimicrobial agents against anaerobic bacteria. Antimicrob Agents Chemother. 1988 Jul;32(7):1094–1096. doi: 10.1128/aac.32.7.1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefevre J. C., Bauriaud R. Comparative in vitro activities of pristinamycin and other antimicrobial agents against genital pathogens. Antimicrob Agents Chemother. 1989 Dec;33(12):2152–2154. doi: 10.1128/aac.33.12.2152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maple P. A., Hamilton-Miller J. M., Brumfitt W. World-wide antibiotic resistance in methicillin-resistant Staphylococcus aureus. Lancet. 1989 Mar 11;1(8637):537–540. doi: 10.1016/s0140-6736(89)90076-7. [DOI] [PubMed] [Google Scholar]
- Maskell J. P., Williams J. D. In-vitro susceptibility of oral streptococci to pristinamycin. J Antimicrob Chemother. 1987 May;19(5):585–590. doi: 10.1093/jac/19.5.585. [DOI] [PubMed] [Google Scholar]
- Oster S. E., Chirurgi V. A., Goldberg A. A., Aiken S., McCabe R. E. Ampicillin-resistant enterococcal species in an acute-care hospital. Antimicrob Agents Chemother. 1990 Sep;34(9):1821–1823. doi: 10.1128/aac.34.9.1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson J. E., Wanger A., Zscheck K. K., Zervos M. J., Murray B. E. Molecular epidemiology of beta-lactamase-producing enterococci. Antimicrob Agents Chemother. 1990 Feb;34(2):302–305. doi: 10.1128/aac.34.2.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson R. D., Steigbigel R. T., Davis H. T., Chapman S. W. Method of reliable determination of minimal lethal antibiotic concentrations. Antimicrob Agents Chemother. 1980 Nov;18(5):699–708. doi: 10.1128/aac.18.5.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwalbe R. S., Stapleton J. T., Gilligan P. H. Emergence of vancomycin resistance in coagulase-negative staphylococci. N Engl J Med. 1987 Apr 9;316(15):927–931. doi: 10.1056/NEJM198704093161507. [DOI] [PubMed] [Google Scholar]
- Weber P., Dubois S., Boussougant Y. In-vitro activity of pristinamycin and its components against gram-negative anaerobic bacilli and Gardnerella vaginalis. J Antimicrob Chemother. 1989 Jun;23(6):825–830. doi: 10.1093/jac/23.6.825. [DOI] [PubMed] [Google Scholar]


