Abstract
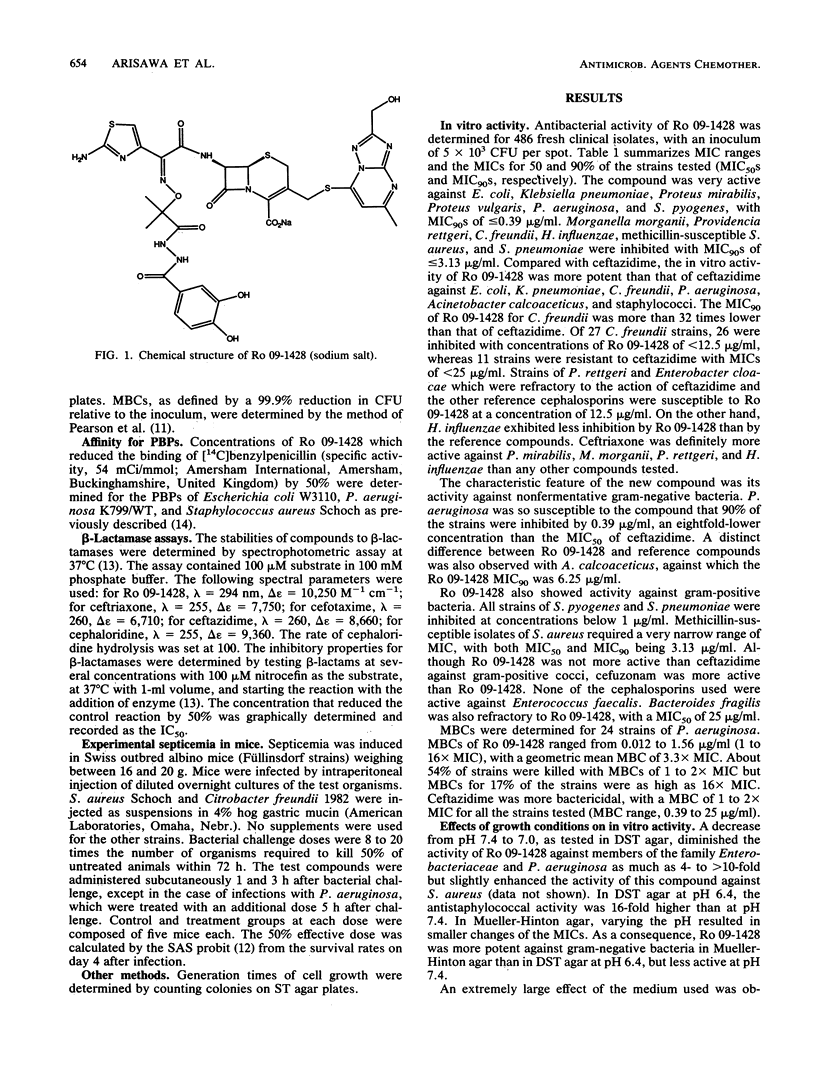
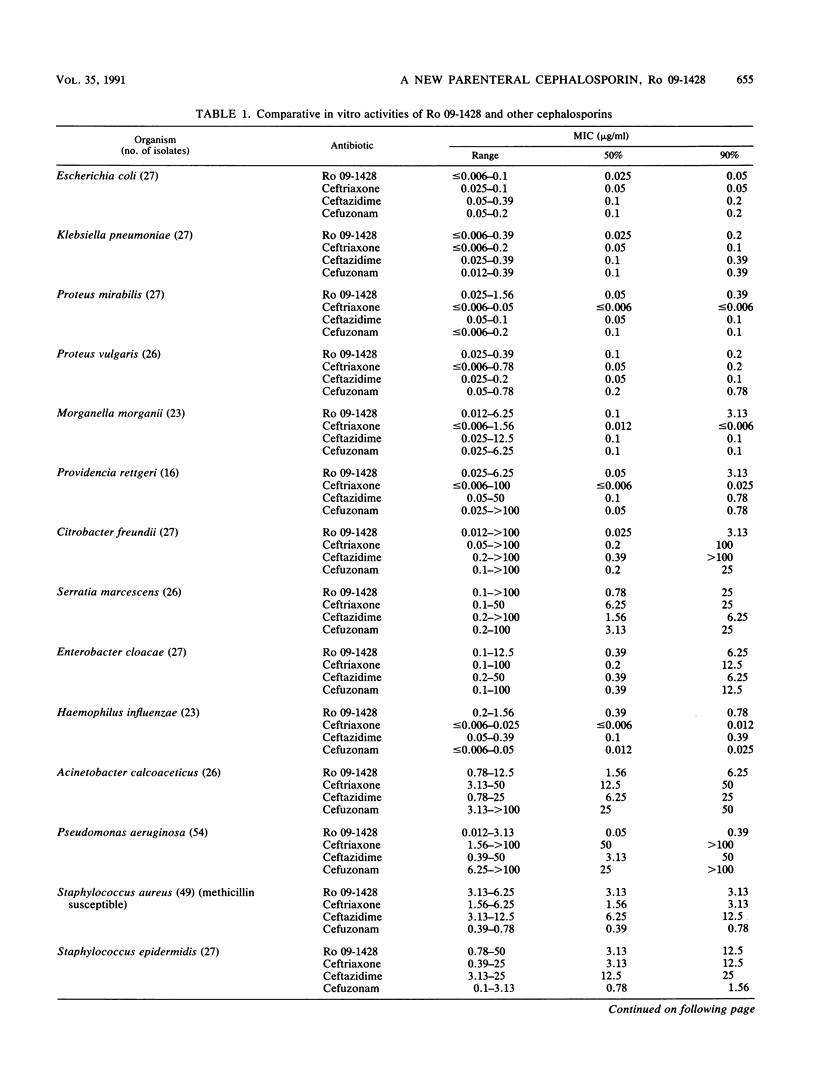
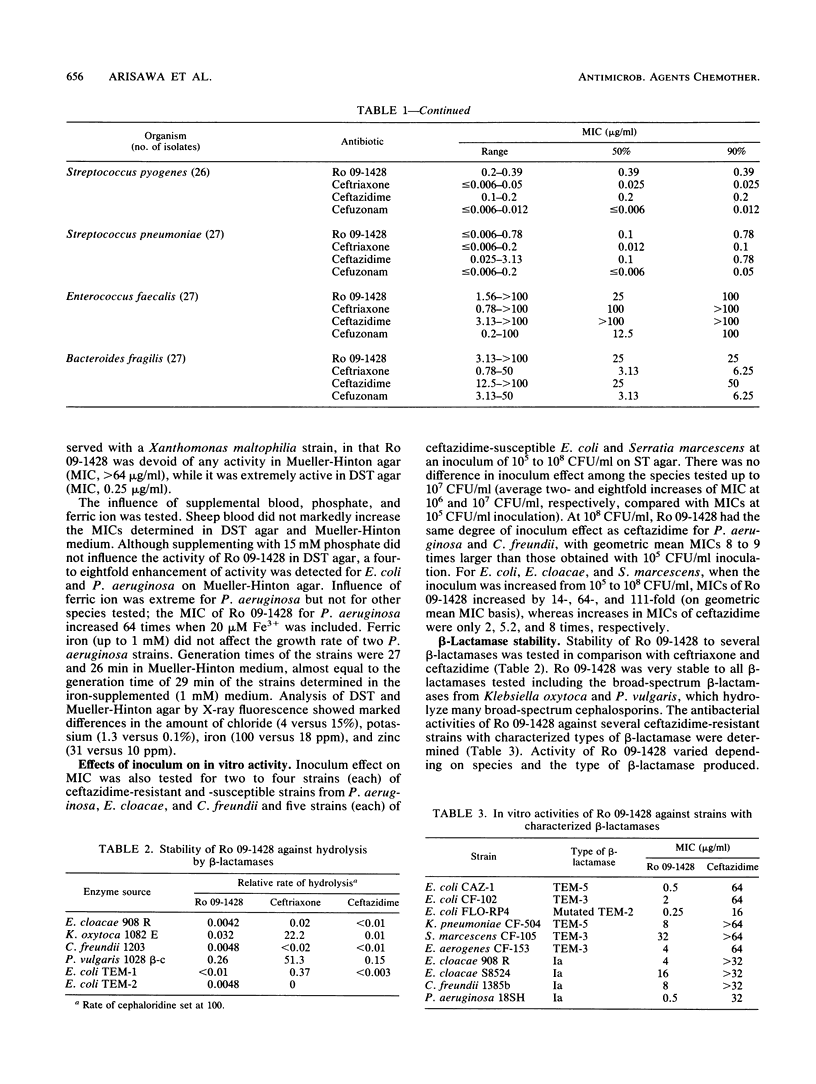
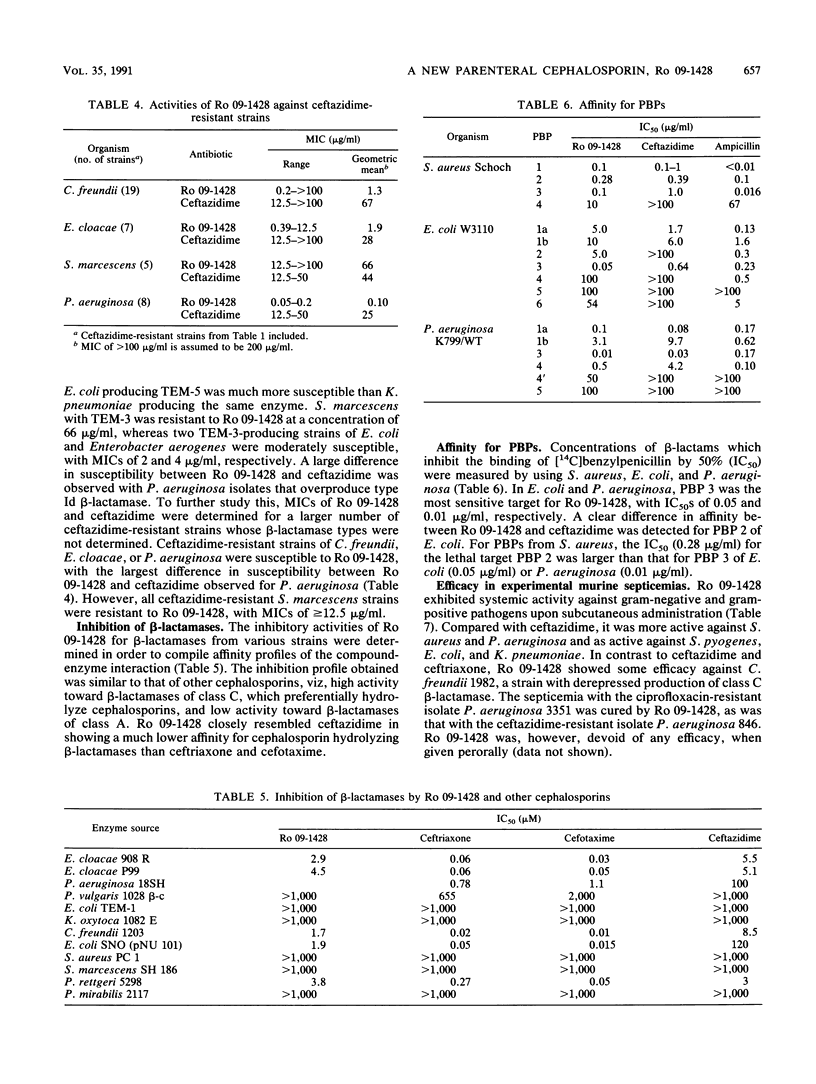
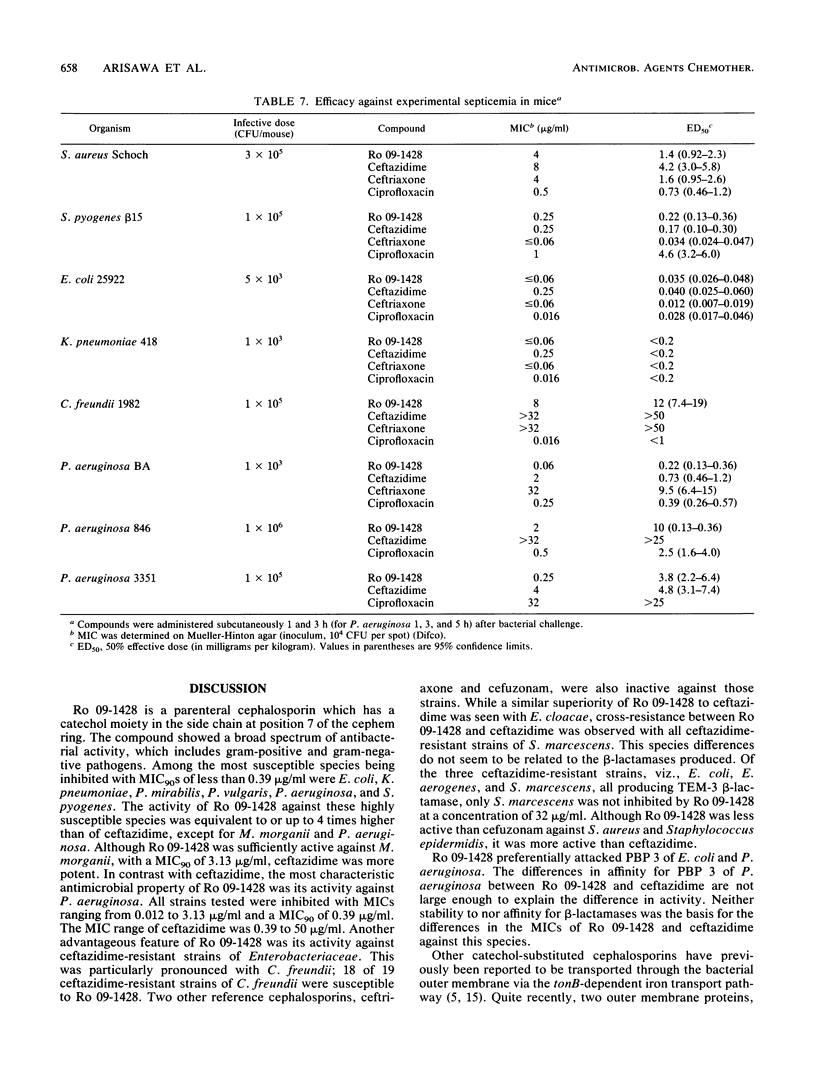
Ro 09-1428, a new parenteral cephalosporin with a catechol moiety attached at position 7 of the cephalosporin ring, showed high in vitro activity against Escherichia coli, Klebsiella pneumoniae, Proteus mirabilis, Proteus vulgaris, and Streptococcus pyogenes, with MICs for 90% of strains tested (MIC90s) of less than or equal to 0.39 micrograms/ml. Morganella morganii, Providencia rettgeri, Citrobacter freundii, Haemophilus influenzae, Staphylococcus aureus, and Streptococcus pneumoniae were inhibited with MIC90s of less than or equal to 3.13 micrograms/ml. Serratia marcescens was less susceptible to Ro 09-1428, with a MIC90 of 25 micrograms/ml. The most distinctive feature of Ro 09-1428 was its potent activity against Pseudomonas aeruginosa and Acinetobacter calcoaceticus, with MIC90s of 0.39 and 6.25 micrograms/ml, respectively. Most of the ceftazidime-resistant strains of P. aeruginosa, E. cloacae, and C. freundii were inhibited by Ro 09-1428, while those of S. marcescens were resistant at a concentration of 12.5 micrograms/ml. Ro 09-1428 was more active than ceftazidime against staphylococci. PBP 3 was the most sensitive target in E. coli and P. aeruginosa. The response to ferric iron in growth medium suggests that Ro 09-1428 may be taken up by transport mechanisms similar to those of other catechol cephalosporins. In accordance with its in vitro activity, Ro 09-1428 activity was equal to or greater than ceftazidime activity in efficacy against experimental septicemias in mice. The results indicate that Ro 09-1428 is a broad-spectrum cephalosporin with advantages over ceftazidime in its activity against P. aeruginosa, staphylococci, and ceftazidime-resistant strains of C. freundii and E. cloacae.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Curtis N. A., Eisenstadt R. L., East S. J., Cornford R. J., Walker L. A., White A. J. Iron-regulated outer membrane proteins of Escherichia coli K-12 and mechanism of action of catechol-substituted cephalosporins. Antimicrob Agents Chemother. 1988 Dec;32(12):1879–1886. doi: 10.1128/aac.32.12.1879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrington F. P., Knott S. J., O'Hanlon P. J., Southgate R. Structure-activity relationships in a series of piperazine-2,3-dione containing penicillins and cephalosporins. II. Derivatives substituted at N(4) of the piperazine ring. J Antibiot (Tokyo) 1989 Aug;42(8):1241–1247. doi: 10.7164/antibiotics.42.1241. [DOI] [PubMed] [Google Scholar]
- Katsu K., Kitoh K., Inoue M., Mitsuhashi S. In vitro antibacterial activity of E-0702, a new semisynthetic cephalosporin. Antimicrob Agents Chemother. 1982 Aug;22(2):181–185. doi: 10.1128/aac.22.2.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mochida K., Ono Y., Yamasaki M., Shiraki C., Hirata T., Sato K., Okachi R. Aminothiazolylglycyl derivatives of carbacephem antibiotics. II. Synthesis and antibacterial activity of novel aminothiazolyl cephem compounds with hydroxypyridone moiety. J Antibiot (Tokyo) 1987 Feb;40(2):182–189. doi: 10.7164/antibiotics.40.182. [DOI] [PubMed] [Google Scholar]
- Mochida K., Shiraki C., Yamasaki M., Hirata T., Sato K., Okachi R. Aminothiazolylglycyl derivatives of carbacephems. I. Synthesis and antibacterial activity of novel carbacephems with substituted aminothiazolyl groups. J Antibiot (Tokyo) 1987 Jan;40(1):14–21. doi: 10.7164/antibiotics.40.14. [DOI] [PubMed] [Google Scholar]
- Mochizuki H., Oikawa Y., Yamada H., Kusakabe S., Shiihara T., Murakami K., Kato K., Ishiguro J., Kosuzume H. Antibacterial and pharmacokinetic properties of M14659, a new injectable semisynthetic cephalosporin. J Antibiot (Tokyo) 1988 Mar;41(3):377–391. doi: 10.7164/antibiotics.41.377. [DOI] [PubMed] [Google Scholar]
- Mochizuki H., Yamada H., Oikawa Y., Murakami K., Ishiguro J., Kosuzume H., Aizawa N., Mochida E. Bactericidal activity of M14659 enhanced in low-iron environments. Antimicrob Agents Chemother. 1988 Nov;32(11):1648–1654. doi: 10.1128/aac.32.11.1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa S., Sanada M., Matsuda K., Hashizume T., Asahi Y., Ushijima R., Ohtake N., Tanaka N. In vitro and in vivo antibacterial activities of BO-1341, a new antipseudomonal cephalosporin. Antimicrob Agents Chemother. 1989 Sep;33(9):1423–1427. doi: 10.1128/aac.33.9.1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa S., Sanada M., Matsuda K., Hazumi N., Tanaka N. Biological activity of BO-1236, a new antipseudomonal cephalosporin. Antimicrob Agents Chemother. 1987 Jul;31(7):1100–1105. doi: 10.1128/aac.31.7.1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikaido H., Rosenberg E. Y. Cir and Fiu proteins in the outer membrane of Escherichia coli catalyze transport of monomeric catechols: study with beta-lactam antibiotics containing catechol and analogous groups. J Bacteriol. 1990 Mar;172(3):1361–1367. doi: 10.1128/jb.172.3.1361-1367.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson R. D., Steigbigel R. T., Davis H. T., Chapman S. W. Method of reliable determination of minimal lethal antibiotic concentrations. Antimicrob Agents Chemother. 1980 Nov;18(5):699–708. doi: 10.1128/aac.18.5.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Then R. L. Interaction of Ro 17-2301 (AMA-1080) with beta-lactamases. Chemotherapy. 1984;30(6):398–407. doi: 10.1159/000238300. [DOI] [PubMed] [Google Scholar]
- Then R. L., Kohl I. Affinity of carumonam for penicillin-binding proteins. Chemotherapy. 1985;31(4):246–254. doi: 10.1159/000238343. [DOI] [PubMed] [Google Scholar]
- Watanabe N. A., Nagasu T., Katsu K., Kitoh K. E-0702, a new cephalosporin, is incorporated into Escherichia coli cells via the tonB-dependent iron transport system. Antimicrob Agents Chemother. 1987 Apr;31(4):497–504. doi: 10.1128/aac.31.4.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissberger B. A., Abruzzo G. K., Fromtling R. A., Gill C., Ponticas S., Valiant M. E., Shungu D. L., Gadebusch H. H. L-658,310, a new injectable cephalosporin. I. In vitro antibacterial properties. J Antibiot (Tokyo) 1989 May;42(5):795–806. doi: 10.7164/antibiotics.42.795. [DOI] [PubMed] [Google Scholar]


