Abstract
A gene coding for an aminoglycoside 6'-N-acetyltransferase that was able to modify amikacin was cloned from a plasmid isolated from a clinical strain of Enterobacter cloacae. Sequencing of a 955-bp segment which mediates the modifying activity revealed a single open reading frame of 432 nucleotides that predicted a polypeptide of 144 amino acid residues with a molecular weight of 16,021. Putative ribosomal binding sites and -10 and -35 sequences were located at the 5' end of the gene. The size of the polypeptide was confirmed through minicell analysis of the expression products of plasmids containing the sequence. The use of the gene as a molecular probe revealed its specificity toward strains harboring genes coding for related enzymes. This probe is therefore useful for epidemiological studies.
Full text
PDF


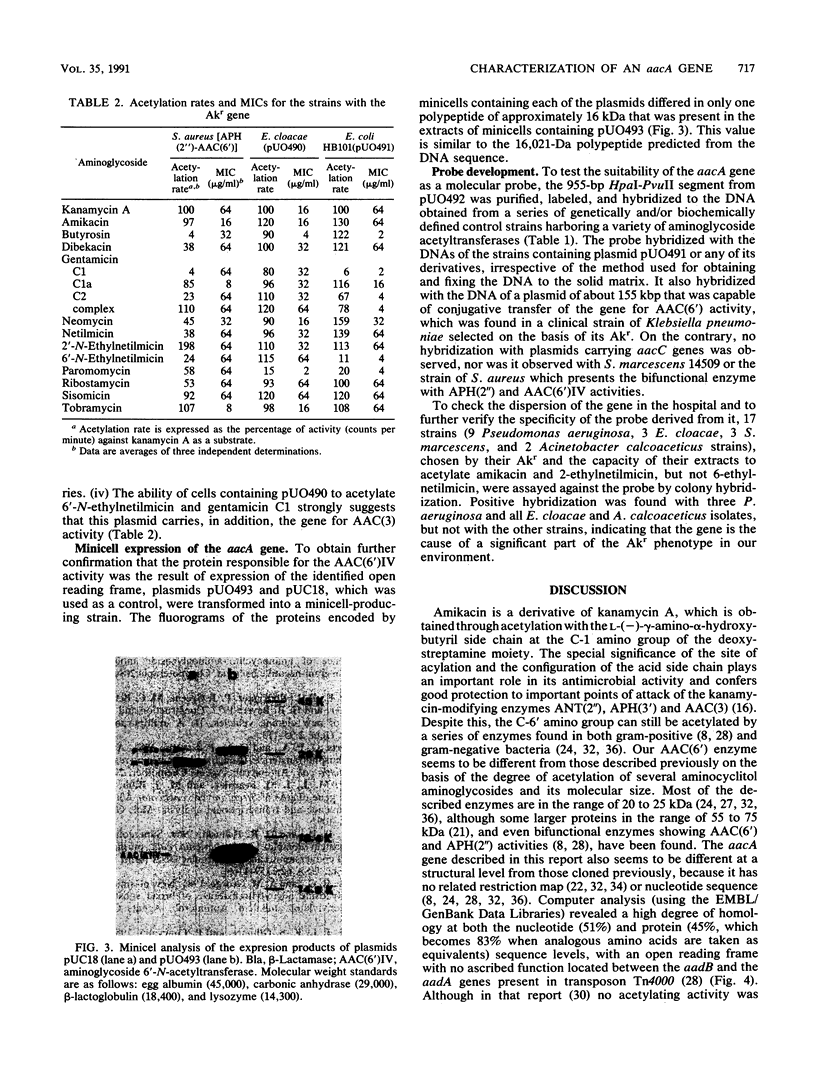


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolivar F., Rodriguez R. L., Greene P. J., Betlach M. C., Heyneker H. L., Boyer H. W., Crosa J. H., Falkow S. Construction and characterization of new cloning vehicles. II. A multipurpose cloning system. Gene. 1977;2(2):95–113. [PubMed] [Google Scholar]
- Bongaerts G. P., Kaptijn G. M. Aminoglycoside phosphotransferase-II-mediated amikacin resistance in Escherichia coli. Antimicrob Agents Chemother. 1981 Sep;20(3):344–350. doi: 10.1128/aac.20.3.344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buttner M J, Fearnley I M, Bibb M J. The agarase gene (dagA) of Streptomyces coelicolor A3(2): nucleotide sequence and transcriptional analysis. Mol Gen Genet. 1987 Aug;209(1):101–109. doi: 10.1007/BF00329843. [DOI] [PubMed] [Google Scholar]
- Clewell D. B., Helinski D. R. Supercoiled circular DNA-protein complex in Escherichia coli: purification and induced conversion to an opern circular DNA form. Proc Natl Acad Sci U S A. 1969 Apr;62(4):1159–1166. doi: 10.1073/pnas.62.4.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coombe R. G., George A. M. New plasmid-mediated aminoglycoside adenylyltransferase of broad substrate range that adenylylates amikacin. Antimicrob Agents Chemother. 1981 Jul;20(1):75–80. doi: 10.1128/aac.20.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferretti J. J., Gilmore K. S., Courvalin P. Nucleotide sequence analysis of the gene specifying the bifunctional 6'-aminoglycoside acetyltransferase 2"-aminoglycoside phosphotransferase enzyme in Streptococcus faecalis and identification and cloning of gene regions specifying the two activities. J Bacteriol. 1986 Aug;167(2):631–638. doi: 10.1128/jb.167.2.631-638.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaynes R., Groisman E., Nelson E., Casadaban M., Lerner S. A. Isolation, characterization, and cloning of a plasmid-borne gene encoding a phosphotransferase that confers high-level amikacin resistance in enteric bacilli. Antimicrob Agents Chemother. 1988 Sep;32(9):1379–1384. doi: 10.1128/aac.32.9.1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas M. J., Dowding J. E. Aminoglycoside-modifying enzymes. Methods Enzymol. 1975;43:611–628. doi: 10.1016/0076-6879(75)43124-x. [DOI] [PubMed] [Google Scholar]
- Hanahan D. Studies on transformation of Escherichia coli with plasmids. J Mol Biol. 1983 Jun 5;166(4):557–580. doi: 10.1016/s0022-2836(83)80284-8. [DOI] [PubMed] [Google Scholar]
- Havekes L. M., Lugtenberg B. J., Hoekstra W. P. Conjugation deficient E. coli K12 F- mutants with heptose-less lipopolysaccharide. Mol Gen Genet. 1976 Jul 5;146(1):43–50. doi: 10.1007/BF00267981. [DOI] [PubMed] [Google Scholar]
- John J. F., Jr, McNeill W. F., Price K. E., Kresel P. A. Evidence for a chromosomal site specifying amikacin resistance in multiresistant Serratia marcescens. Antimicrob Agents Chemother. 1982 Apr;21(4):587–591. doi: 10.1128/aac.21.4.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawabe H., Kondo S., Umezawa H., Mitsuhashi S. R factor-mediated aminoglycoside antibiotic resistance in Pseudomonas aeruginosa: a new aminoglycoside 6'-N-acetyltransferase. Antimicrob Agents Chemother. 1975 May;7(5):494–499. doi: 10.1128/aac.7.5.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert T., Gerbaud G., Courvalin P. Transferable amikacin resistance in Acinetobacter spp. due to a new type of 3'-aminoglycoside phosphotransferase. Antimicrob Agents Chemother. 1988 Jan;32(1):15–19. doi: 10.1128/aac.32.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendoza M. C., Blanco M. G., Javier Mendez F., Hardisson C. Evolution de la résistance aux aminosides chez des souches hospitalières de Serratia. Pathol Biol (Paris) 1984 Sep;32(7):750–754. [PubMed] [Google Scholar]
- Meyer J. F., Nies B. A., Wiedemann B. Amikacin resistance mediated by multiresistance transposon Tn2424. J Bacteriol. 1983 Aug;155(2):755–760. doi: 10.1128/jb.155.2.755-760.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer J. F., Wiedemann B. Characterization of aminoglycoside 6'-N-acetyltransferases [AAC(6')] from gram-negative bacteria and Streptomyces kanamyceticus. J Antimicrob Chemother. 1985 Mar;15(3):271–282. doi: 10.1093/jac/15.3.271. [DOI] [PubMed] [Google Scholar]
- Nobuta K., Tolmasky M. E., Crosa L. M., Crosa J. H. Sequencing and expression of the 6'-N-acetyltransferase gene of transposon Tn1331 from Klebsiella pneumoniae. J Bacteriol. 1988 Aug;170(8):3769–3773. doi: 10.1128/jb.170.8.3769-3773.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price K. E. Aminoglycoside research 1975-1985: prospects for development of improved agents. Antimicrob Agents Chemother. 1986 Apr;29(4):543–548. doi: 10.1128/aac.29.4.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price K. E., Kresel P. A., Farchione L. A., Siskin S. B., Karpow S. A. Epidemiological studies of aminoglycoside resistance in the U.S.A. J Antimicrob Chemother. 1981 Jul;8 (Suppl A):89–105. doi: 10.1093/jac/8.suppl_a.89. [DOI] [PubMed] [Google Scholar]
- Radika K., Northrop D. B. Purification of two forms of kanamycin acetyltransferase from Escherichia coli. Arch Biochem Biophys. 1984 Aug 15;233(1):272–285. doi: 10.1016/0003-9861(84)90626-x. [DOI] [PubMed] [Google Scholar]
- Rouch D. A., Byrne M. E., Kong Y. C., Skurray R. A. The aacA-aphD gentamicin and kanamycin resistance determinant of Tn4001 from Staphylococcus aureus: expression and nucleotide sequence analysis. J Gen Microbiol. 1987 Nov;133(11):3039–3052. doi: 10.1099/00221287-133-11-3039. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt F. R., Nücken E. J., Henschke R. B. Nucleotide sequence analysis of 2''-aminoglycoside nucleotidyl-transferase ANT(2'') from Tn4000: its relationship with AAD(3'') and impact on Tn21 evolution. Mol Microbiol. 1988 Nov;2(6):709–717. doi: 10.1111/j.1365-2958.1988.tb00081.x. [DOI] [PubMed] [Google Scholar]
- Shimizu K., Kumada T., Hsieh W. C., Chung H. Y., Chong Y., Hare R. S., Miller G. H., Sabatelli F. J., Howard J. Comparison of aminoglycoside resistance patterns in Japan, Formosa, and Korea, Chile, and the United States. Antimicrob Agents Chemother. 1985 Aug;28(2):282–288. doi: 10.1128/aac.28.2.282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tenover F. C., Filpula D., Phillips K. L., Plorde J. J. Cloning and sequencing of a gene encoding an aminoglycoside 6'-N-acetyltransferase from an R factor of Citrobacter diversus. J Bacteriol. 1988 Jan;170(1):471–473. doi: 10.1128/jb.170.1.471-473.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tenover F. C., Phillips K. L., Gilbert T., Lockhart P., O'Hara P. J., Plorde J. J. Development of a DNA probe from the deoxyribonucleotide sequence of a 3-N-aminoglycoside acetyltransferase [AAC(3)-I] resistance gene. Antimicrob Agents Chemother. 1989 Apr;33(4):551–559. doi: 10.1128/aac.33.4.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolmasky M. E., Crosa J. H. Tn1331, a novel multiresistance transposon encoding resistance to amikacin and ampicillin in Klebsiella pneumoniae. Antimicrob Agents Chemother. 1987 Dec;31(12):1955–1960. doi: 10.1128/aac.31.12.1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolmasky M. E., Roberts M., Woloj M., Crosa J. H. Molecular cloning of amikacin resistance determinants from a Klebsiella pneumoniae plasmid. Antimicrob Agents Chemother. 1986 Aug;30(2):315–320. doi: 10.1128/aac.30.2.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran van Nhieu G., Collatz E. Primary structure of an aminoglycoside 6'-N-acetyltransferase AAC(6')-4, fused in vivo with the signal peptide of the Tn3-encoded beta-lactamase. J Bacteriol. 1987 Dec;169(12):5708–5714. doi: 10.1128/jb.169.12.5708-5714.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villar C. J., Hardisson C., Suárez J. E. Cloning and molecular epidemiology of plasmid-determined fosfomycin resistance. Antimicrob Agents Chemother. 1986 Feb;29(2):309–314. doi: 10.1128/aac.29.2.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]