Abstract
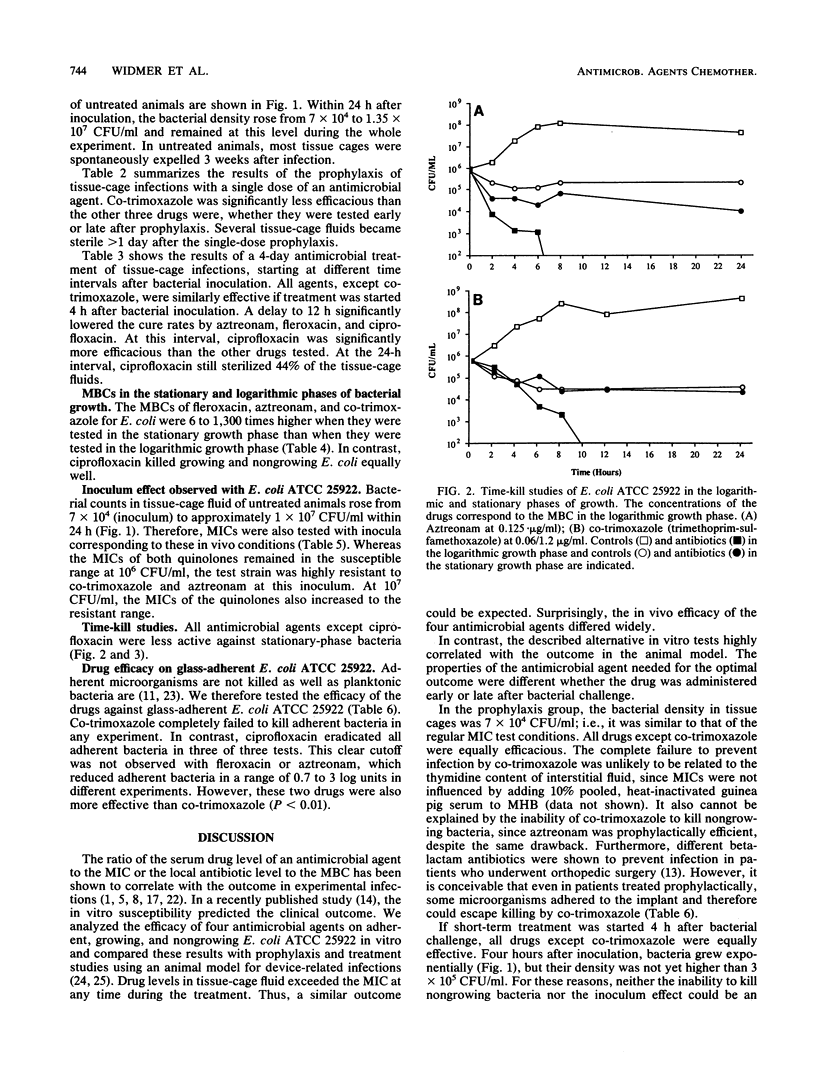
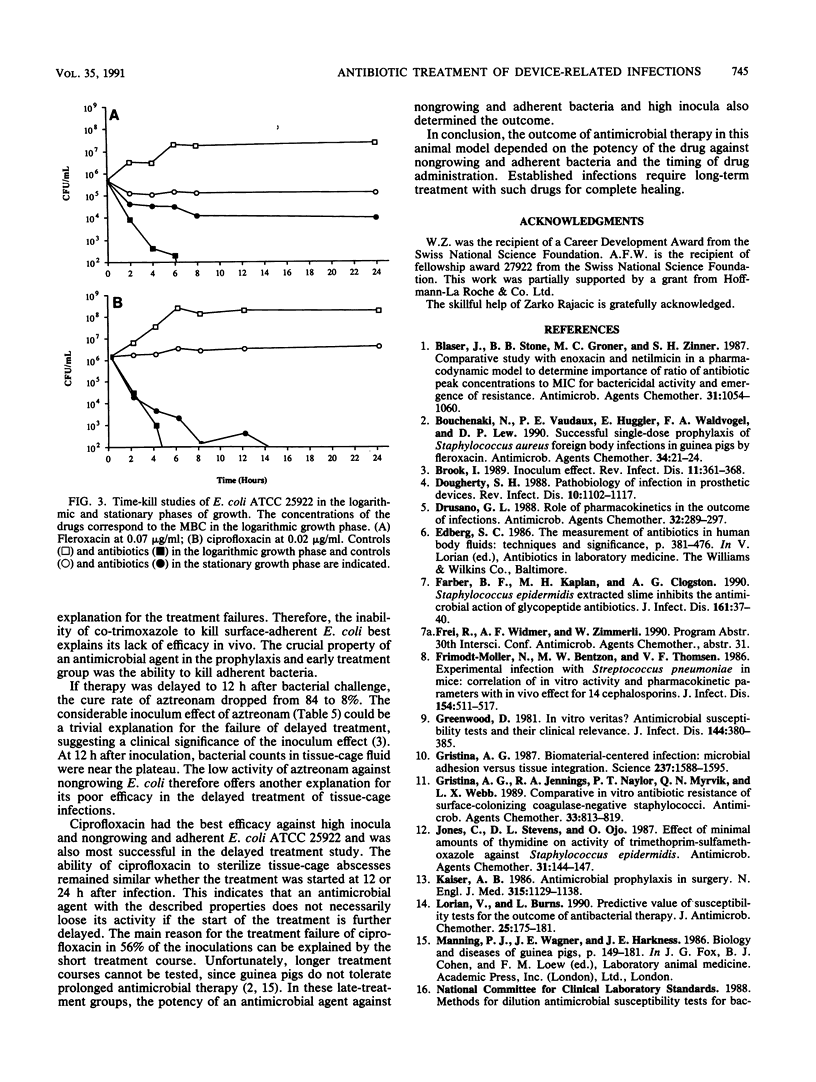
Antimicrobial therapy of device-related infections often fails, despite the in vitro susceptibility of the infecting strain. Therefore, alternative laboratory-based in vitro tests are required to predict the outcome. Fleroxacin, ciprofloxacin, aztreonam, and co-trimoxazole were tested against Escherichia coli ATCC 25922 in vitro and in the tissue-cage animal model. The importance of early treatment was evaluated by starting the drugs either 30 min before or 4, 12, and 24 h after bacterial challenge. Results were compared with the in vitro drug efficacy against nongrowing and adherent Escherichia coli ATCC 25922. The alternative in vitro tests correlated highly with the outcome in the tissue-cage animal model. In the prophylaxis group (drug given 30 min before bacterial challenge), co-trimoxazole was less efficacious than the other three drugs (P less than 0.001). In delayed treatment, ciprofloxacin showed the highest cure rate. It was also more potent than the other drugs against nongrowing and adherent E. coli ATCC 25922. The efficacies of aztreonan, fleroxacin, and ciprofloxacin dropped significantly (P less than 0.01) when the time interval between bacterial challenge and the start of treatment was delayed to greater than 4 h. These data emphasize (i) the need for proper timing of prophylaxis in patients undergoing implant surgery, and (ii) the possibility of successful treatment of established device-related infections with drugs which kill not only growing but also nongrowing and adherent bacteria.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blaser J., Stone B. B., Groner M. C., Zinner S. H. Comparative study with enoxacin and netilmicin in a pharmacodynamic model to determine importance of ratio of antibiotic peak concentration to MIC for bactericidal activity and emergence of resistance. Antimicrob Agents Chemother. 1987 Jul;31(7):1054–1060. doi: 10.1128/aac.31.7.1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouchenaki N., Vaudaux P. E., Huggler E., Waldvogel F. A., Lew D. P. Successful single-dose prophylaxis of Staphylococcus aureus foreign body infections in guinea pigs by fleroxacin. Antimicrob Agents Chemother. 1990 Jan;34(1):21–24. doi: 10.1128/aac.34.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brook I. Inoculum effect. Rev Infect Dis. 1989 May-Jun;11(3):361–368. doi: 10.1093/clinids/11.3.361. [DOI] [PubMed] [Google Scholar]
- Dougherty S. H. Pathobiology of infection in prosthetic devices. Rev Infect Dis. 1988 Nov-Dec;10(6):1102–1117. doi: 10.1093/clinids/10.6.1102. [DOI] [PubMed] [Google Scholar]
- Drusano G. L. Role of pharmacokinetics in the outcome of infections. Antimicrob Agents Chemother. 1988 Mar;32(3):289–297. doi: 10.1128/aac.32.3.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farber B. F., Kaplan M. H., Clogston A. G. Staphylococcus epidermidis extracted slime inhibits the antimicrobial action of glycopeptide antibiotics. J Infect Dis. 1990 Jan;161(1):37–40. doi: 10.1093/infdis/161.1.37. [DOI] [PubMed] [Google Scholar]
- Frimodt-Møller N., Bentzon M. W., Thomsen V. F. Experimental infection with Streptococcus pneumoniae in mice: correlation of in vitro activity and pharmacokinetic parameters with in vivo effect for 14 cephalosporins. J Infect Dis. 1986 Sep;154(3):511–517. doi: 10.1093/infdis/154.3.511. [DOI] [PubMed] [Google Scholar]
- Greenwood D. In vitro veritas? Antimicrobial susceptibility tests and their clinical relevance. J Infect Dis. 1981 Oct;144(4):380–385. doi: 10.1093/infdis/144.4.380. [DOI] [PubMed] [Google Scholar]
- Gristina A. G. Biomaterial-centered infection: microbial adhesion versus tissue integration. Science. 1987 Sep 25;237(4822):1588–1595. doi: 10.1126/science.3629258. [DOI] [PubMed] [Google Scholar]
- Gristina A. G., Jennings R. A., Naylor P. T., Myrvik Q. N., Webb L. X. Comparative in vitro antibiotic resistance of surface-colonizing coagulase-negative staphylococci. Antimicrob Agents Chemother. 1989 Jun;33(6):813–816. doi: 10.1128/aac.33.6.813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones C., Stevens D. L., Ojo O. Effect of minimal amounts of thymidine on activity of trimethoprim-sulfamethoxazole against Staphylococcus epidermidis. Antimicrob Agents Chemother. 1987 Feb;31(2):144–147. doi: 10.1128/aac.31.2.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser A. B. Antimicrobial prophylaxis in surgery. N Engl J Med. 1986 Oct 30;315(18):1129–1138. doi: 10.1056/NEJM198610303151805. [DOI] [PubMed] [Google Scholar]
- Lorian V., Burns L. Predictive value of susceptibility tests for the outcome of antibacterial therapy. J Antimicrob Chemother. 1990 Jan;25(1):175–181. doi: 10.1093/jac/25.1.175. [DOI] [PubMed] [Google Scholar]
- Pangon B., Joly V., Vallois J. M., Abel L., Buré A., Brion N., Contrepois A., Carbon C. Comparative efficacy of cefotiam, cefmenoxime, and ceftriaxone in experimental endocarditis and correlation with pharmacokinetics and in vitro efficacy. Antimicrob Agents Chemother. 1987 Apr;31(4):518–522. doi: 10.1128/aac.31.4.518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugarman B. Infections and prosthetic devices. Am J Med. 1986 Jul 28;81(1A):78–84. doi: 10.1016/0002-9343(86)90517-6. [DOI] [PubMed] [Google Scholar]
- Tuomanen E., Cozens R., Tosch W., Zak O., Tomasz A. The rate of killing of Escherichia coli by beta-lactam antibiotics is strictly proportional to the rate of bacterial growth. J Gen Microbiol. 1986 May;132(5):1297–1304. doi: 10.1099/00221287-132-5-1297. [DOI] [PubMed] [Google Scholar]
- Vaudaux P. E., Zulian G., Huggler E., Waldvogel F. A. Attachment of Staphylococcus aureus to polymethylmethacrylate increases its resistance to phagocytosis in foreign body infection. Infect Immun. 1985 Nov;50(2):472–477. doi: 10.1128/iai.50.2.472-477.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogelman B., Gudmundsson S., Leggett J., Turnidge J., Ebert S., Craig W. A. Correlation of antimicrobial pharmacokinetic parameters with therapeutic efficacy in an animal model. J Infect Dis. 1988 Oct;158(4):831–847. doi: 10.1093/infdis/158.4.831. [DOI] [PubMed] [Google Scholar]
- Widmer A. F., Frei R., Rajacic Z., Zimmerli W. Correlation between in vivo and in vitro efficacy of antimicrobial agents against foreign body infections. J Infect Dis. 1990 Jul;162(1):96–102. doi: 10.1093/infdis/162.1.96. [DOI] [PubMed] [Google Scholar]
- Zimmerli W., Lew P. D., Waldvogel F. A. Pathogenesis of foreign body infection. Evidence for a local granulocyte defect. J Clin Invest. 1984 Apr;73(4):1191–1200. doi: 10.1172/JCI111305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerli W., Waldvogel F. A., Vaudaux P., Nydegger U. E. Pathogenesis of foreign body infection: description and characteristics of an animal model. J Infect Dis. 1982 Oct;146(4):487–497. doi: 10.1093/infdis/146.4.487. [DOI] [PubMed] [Google Scholar]


