Abstract
Cytochrome c release and the mitochondrial permeability transition (PT), including loss of the transmembrane potential (Δψ), play an important role in apoptosis. Using isolated mitochondria, we found that recombinant Bax and Bak, proapoptotic members of the Bcl-2 family, induced mitochondrial Δψ loss, swelling, and cytochrome c release. All of these changes were dependent on Ca2+ and were prevented by cyclosporin A (CsA) and bongkrekic acid, both of which close the PT pores (megachannels), indicating that Bax- and Bak-induced mitochondrial changes were mediated through the opening of these pores. Bax-induced mitochondrial changes were inhibited by recombinant Bcl-xL and transgene-derived Bcl-2, antiapoptotic members of the Bcl-2 family, as well as by oligomycin, suggesting a possible regulatory effect of F0F1-ATPase on Bax-induced mitochondrial changes. Proapoptotic Bax- and Bak-BH3 (Bcl-2 homology) peptides, but not a mutant BH3 peptide nor a mutant Bak lacking BH3, induced the mitochondrial changes, indicating an essential role of the BH3 region. A coimmunoprecipitation study revealed that Bax and Bak interacted with the voltage-dependent anion channel, which is a component of PT pores. Taken together, these findings suggest that proapoptotic Bcl-2 family proteins, including Bax and Bak, induce the mitochondrial PT and cytochrome c release by interacting with the PT pores.
Apoptosis is an evolutionarily conserved cell suicide mechanism that plays a crucial role in various biological events, including development, maintenance of homeostasis, and removal of unwanted cells (1). Apoptotic signals are activated by various stimuli and converge toward a common death pathway, for which Bcl-2 family proteins act as regulators (2) and caspase family proteases act as signal transducers (3).
Recent evidence has shown that the mitochondria play a crucial role in apoptosis (4, 5) by releasing apoptogenic factors such as cytochrome c (6–8) and apoptosis-inducing factor (AIF) (9) from the intermembrane space into the cytoplasm. Cytochrome c release activates caspase-9, in concert with the cytosolic factors dATP (or ATP) and Apaf-1, and subsequently activates caspase-3 (10). AIF also has been reported to activate caspase-3 as well as induce apoptotic changes in the nucleus (9, 11). Antiapoptotic Bcl-2 and Bcl-xL inhibit the apoptosis-associated mitochondrial release of both cytochrome c and AIF (7–9), although the basis for these actions is still unknown. The only biochemical activity known to be associated with Bcl-2 family proteins, including Bcl-2, Bcl-xL, and Bax, is the formation of ion channels in synthetic lipid membranes (12–15), but it is still to be determined whether this activity directly regulates apoptosis.
Apoptosis-associated release of AIF but not cytochrome c depends on loss of the mitochondrial transmembrane potential (Δψ) (6–9). Mitochondria are compartmentalized by two membranes; the outer membrane is permeable to all molecules <6,000 Da, while the inner membrane is impermeable to all but a limited number of metabolites and ions. The limited permeability of the inner membrane allows the existence of a matrix that is distinct from the cytoplasm and also is essential for generation of the Δψ and the pH gradient across the membrane. Permeabilization of the inner membrane allows solutes to efflux from the matrix, disrupting the Δψ and the pH gradient, changes that characterize the permeability transition (PT) (16, 17). The apoptotic mitochondrial PT seems to be mediated by opening of the PT pore complex (or megachannel), which is proposed to consist of several proteins, including adenine nucleotide translocator (ANT), the voltage-dependent anion channel (VDAC, also termed mitochondrial porin), and the peripheral benzodiazepine receptor (PBR) (17–19), because some forms of apoptosis have been shown to be suppressed by PT inhibitors such as bongkrekic acid (BK), which directly targets ANT, or by cyclosporin A (CsA), which regulates the PT pore complex (20–23).
Although the apoptotic signal-transduction pathway downstream from the mitochondria is relatively clear, the precise mechanism by which apoptotic signals are transmitted to the mitochondria has not yet been elucidated. It was reported recently that proapoptotic Bax is localized in the cytoplasm and translocates to the mitochondria at the early stage of apoptosis (24, 25), suggesting an important role of Bax in apoptotic signal transduction via the mitochondria. Thus, we hypothesized Bax and its relative, Bak, directly transmit death signals to mitochondria, which then undergo PT and/or cytochrome c release. Indeed, we have shown previously that recombinant Bak protein is capable of inducing Δψ loss in isolated mitochondria (23), and it was also shown that recombinant Bax induces cytochrome c release from isolated mitochondria (26). In the present study, we showed that Bax and Bak induce these mitochondrial changes by directly interacting with the PT pores.
MATERIALS AND METHODS
Chemicals.
Anti-cytochrome c mAb (7H8.2C12) was a kind gift from E. Margoliash (University of Illinois). Anti-Xpress antibody was purchased from Invitrogen. Anti-VDAC (porin) mAb (31HL) was from Calbiochem. Anti-Bak polyclonal antibody (G23) and anti-Bax polyclonal antibody (N20) were from Santa Cruz Biotechnology. Lipofectamine was purchased from Life Technologies (Gaithersburg, MD). Dimethyl 3,3′-dithiobis(propionimidate)⋅2HCl (DTBP) was obtained from Pierce. Bongkrekic acid was kindly provided by H. Terada and Y. Shinohara (Tokushima University, Japan) and also by M. Klingenberg (University of Munich, Germany). Diisopropylcarbodiimide/1-hydroxybenzotriazole-activated fluorenylmethoxycarbonyl-protected amino acids were obtained from Genzyme. Other chemicals were obtained from Wako Biochemicals (Osaka).
Preparation of Isolated Mitochondria and Cytochrome c-Depleted Mitochondria.
Livers of male Donryu rats were homogenized with a glass–Teflon Potter homogenizer. Mitochondria were isolated in 0.3 M mannitol/10 mM potassium Hepes, pH 7.4/0.2 mM EDTA/0.1% fatty acid-free BSA, as described previously (23). The mitochondria were washed twice with and resuspended in the same medium without EDTA (MT-1 medium). Cytochrome c-depleted mitochondria were prepared as described elsewhere (27). Briefly, isolated mitochondria were suspended at 2 mg/ml in a hypotonic buffer consisting of 10 mM KCl and 2 mM Hepes and were placed on ice for 20 min. Then, the mitochondria were washed twice with 150 mM KCl and 2 mM Hepes, and were suspended in MT-1 medium. Mitochondria also were prepared from the livers of the hepatic Bcl-2 transgenic mice as described previously (23).
Protein Purification.
Human BakΔC lacking the C-terminal 21 amino acid residues, its Bcl-2 homology 3 (BH3) deletion mutant (BakΔGDΔC) that additionally lacks amino acid residues 82–94, full-length human Bcl-xL, and two Bcl-xL BH1 mutants, mt1 (F131V, D133A) and mt7 (VNW at 135, 136, and 137 to AIL) (28), were expressed as glutathione S-transferase (GST)-fusion proteins in Escherichia coli strain DH5α and purified on a glutathione-Sepharose column. BakΔC, BakΔGDΔC, Bcl-xL, and Bcl-xL mutants were released from GST by cleavage with thrombin. Human Bax was expressed as a His-tagged protein in E. coli strain XL1-Blue using the Xpress System (Invitrogen) and was purified on a Ni-NTA (nitrilotriacetate) agarose (Qiagen) column according to the supplier’s protocol. All purified proteins finally were suspended in the same control buffer composed of 20 mM Tris⋅HCl, pH 7.4/2 mM MgCl2/1 mM DTT. Mock control proteins were prepared using GST- and His-tagged proteins from empty vectors.
Synthesis of BH3 Peptides.
Peptides were synthesized on a Model 396 Multiple Peptide Synthesizer (Advanced ChemTech) using diisopropylcarbodiimide/1-hydroxybenzotriazole-activated fluorenylmethoxycarbonyl-protected amino acids. The purity of each peptide was determined to be >90% by MALDI-TOF (matrix-assisted laser desorption ionization–time-of-flight) mass spectrometry.
Measurement of Mitochondrial Biochemical Parameters.
Isolated mitochondria (1 mg of protein per ml) were incubated at 25°C in MT-1 medium plus 0.5 mM potassium phosphate/40 μM CaCl2/4.2 mM potassium succinate to energize mitochondria (MT-2 medium), except when experiments were done under Ca2+-free conditions. Δψ was assessed by measuring the Δψ-dependent uptake of rhodamine 123 (Rh123) by using a spectrophotometer (Hitachi F-4500) with excitation at 505 nm and emission at 534 nm after addition of 10 μM Rh123 to a mitochondrial suspension. Mitochondrial swelling was monitored at 0.1 mg/ml by the decrease of 90° light scatter at 520 nm, which was determined by using a spectrophotometer (Hitachi F-4500) as described elsewhere (29). Mitochondrial aspartate aminotransferase (mAST) activity was determined by a spectrophotometric procedure employing a modification of the German Society for Clinical Chemistry method, as described previously (30). In the experiments done under Ca2+-free conditions, mitochondria were incubated in MT-1 medium plus 0.2 mM EGTA/5 mM potassium phosphate/1 mM MgCl2/4.2 mM potassium succinate.
Plasmid Construction.
Human bak cDNA was isolated as an EcoRI fragment and subcloned into the pUC-CAGGS expression vector (31). Mouse bax cDNA was also isolated as a EcoRI fragment and inserted into the pUC-CAGGS expression vector. Human vdac1 cDNA was obtained as an XhoI fragment by PCR and was subcloned into pUC-CAGGS.
Cell Culture and DNA Transfection.
Cos7 cells were grown in DMEM supplemented with 10% fetal bovine serum (FBS). Transfection was performed by using Lipofectamine (GIBCO) according to the supplier’s protocol. Cos7 cells, plated in 10-cm dishes at a density of 1 × 106 cells per ml, were transfected with mouse bax DNA (3 μg) or human bak DNA (2 μg) together with human vdac1 DNA (2 μg).
Immunoprecipitation and Western Blot Analysis.
For immunoprecipitation with isolated mitochondria, mitochondria were pelleted, washed twice with MT-1, and resuspended in lysis buffer (10 mM Hepes, pH 7.4/142.5 mM KCl/5 mM MgCl2/1 mM EGTA/0.5% Nonidet P-40) in the presence of proteinase inhibitors (0.1 mM p-amidinophenylmethanesulfonyl fluoride, 10 μg/ml aprotinin, 1 μg/ml chymostatin, 1 μg/ml leupeptin, 1 μg/ml antipain, 1 μg/ml pepstatin), followed by sonication. Immunoprecipitation was performed by using an anti-Bax polyclonal antibody or an anti-VDAC mAb. For immunoprecipitation experiments with whole cells, we used DTBP as a protein cross-linker as described previously (32). Transfected Cos7 cells were washed twice with PBS and incubated with 2 mM DTBP in PBS for 30 min at room temperature. After washing three times with PBS, cells were lysed and sonicated in the same way as the mitochondria, except that 10 mM Hepes was changed to 10 mM Tris in the lysis buffer. Cell lysates were used for immunoprecipitation of Bax and Bak with anti-Bax and anti-Bak polyclonal antibodies, respectively. VDAC was detected by Western blotting using anti-VDAC mAb. For detection of cytochrome c release and mitochondrial integration of rBakΔC, mitochondria were spun and the pellet was resuspended in RIPA buffer (50 mM Tris⋅HCl/150 mM NaCl/1% Nonidet P-40/0.5% deoxycholate/0.1% SDS). The supernatant and the resuspended mitochondria were subjected to Western blotting by using anti-cytochrome c and anti-Bak antibodies.
RESULTS
Bax and Bak Induce the PT.
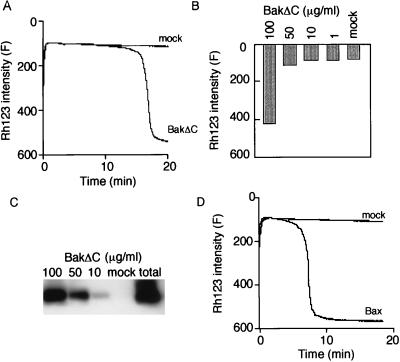
To elucidate the direct effect of Bax and Bak on mitochondrial bioenergetics, isolated rat liver mitochondria were incubated with recombinant human Bax (rBax) or human Bak lacking the carboxyl-terminal hydrophobic domain (rBakΔC), and the mitochondrial Δψ was measured with Rh123 as described in Materials and Methods. Addition of rBakΔC induced Δψ loss in a concentration-dependent manner, whereas addition of mock proteins did not affect Δψ (Fig. 1 A and B), confirming our previous observations using a GST-BakΔC fusion protein (23). rBakΔC was integrated into mitochondria under these conditions (Fig. 1C). Similar results were obtained with rBax (Fig. 1D and data not shown).
Figure 1.
Δψ loss induced by rBakΔC and rBax in isolated mitochondria. (A and B) Δψ loss induced by rBakΔC. Isolated mitochondria (1 mg/ml) were incubated with 100 μg/ml (A) or the indicated concentration (B) of rBakΔC, and Δψ was measured by using Rh123 uptake over 20 min (A) or at 20 min (B). (C) Uptake of rBakΔC into mitochondria. After the same experiments as in B were performed, mitochondria (30 μg) were centrifuged, washed, and subjected to Western blot analysis using anti-Bak antibody. The total amount of rBakΔC protein in 100 μg/ml is indicated as total. (D) Δψ loss induced by rBax. Isolated mitochondria (1 mg/ml) were incubated with 100 μg/ml rBax, and Δψ was measured by using Rh123 uptake over 20 min.
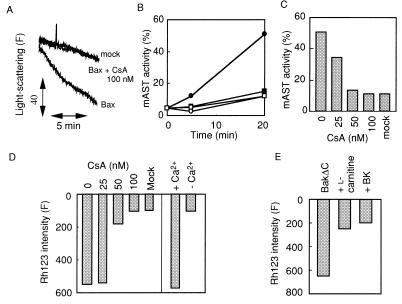
As shown in Fig. 2 A and B, addition of rBax induced both mitochondrial swelling assessed by light scatter and release of a matrix enzyme, mAST. rBax-induced swelling, release of mAST, and Δψ loss were inhibited by CsA in a concentration-dependent manner, with almost complete inhibition at 100 nM (Fig. 2 A–D). The calculated Ki value (5.3 nM) was similar to that for PT inhibition (30). Similar results also were obtained with rBakΔC (data not shown). rBax induced mitochondrial Δψ loss, mAST release, and swelling in a Ca2+-dependent manner (Fig. 2 B and D), which is one of the characteristics of the PT. Other PT inhibitors, BK (an inhibitor of ANT), and l-carnitine (a transporter of acyl groups to the matrix), also inhibited rBakΔC-induced Δψ loss (Fig. 2E). Taken together, these data indicate that both rBax and rBakΔC induce the PT in isolated mitochondria via the PT pores.
Figure 2.
Induction of the PT by rBax and rBakΔC. (A) Prevention of rBax-induced mitochondrial swelling by CsA. Isolated mitochondria (0.1 mg/ml) were incubated with rBax (10 μg/ml) or an equivalent concentration of mock protein in the presence or absence of CsA at 100 nM, and light scatter was monitored as described in Materials and Methods. (B and C) Prevention of rBax-induced release of mAST by CsA or Ca2+ chelation. Mitochondria (1 mg/ml) were incubated with rBax (100 μg/ml) or an equivalent concentration of mock protein in the presence or absence of CsA at 100 nM (B) or at the indicated concentrations (C). Mitochondria were also incubated with rBax (100 μg/ml) after Ca2+ chelation in B. mAST activity was measured over 20 min (B) or at 20 min (C) as described in Materials and Methods. •, rBax; ○, mock protein; ■; rBax + CsA (100 nM); □, rBax with Ca2+ chelation. (D) Prevention of rBax-induced Δψ loss by CsA or Ca2+ chelation. Mitochondria (1 mg/ml) were incubated with rBax (100 μg/ml) or an equivalent concentration of mock protein in the presence or absence of CsA at the indicated concentrations. Mitochondria also were incubated with rBax (100 μg/ml) after Ca2+ chelation. Δψ was measured by using Rh123 at 20 min. (E) Inhibition of rBakΔC-induced Δψ loss by BK and l-carnitine. Mitochondria (1 mg/ml) were incubated with rBakΔC (100 μg/ml) in the presence or absence of 10 mM BK or 5 mM l-carnitine. Δψ was measured by using Rh123 at 20 min.
BH3 Is Essential for PT Induction.
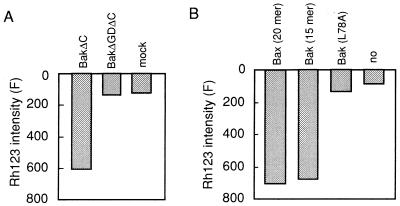
Because it has been reported that the BH3 regions of Bak and Bax are required for their proapoptotic function as well as the ability to bind with Bcl-2 and Bcl-xL (33, 34), we examined the effect on the PT of a BH3 deletion mutant, rBakΔGDΔC, lacking amino acid residues 82–94 as well as the C-terminal hydrophobic domain. We also examined the effect of oligopeptides corresponding to the BH3 region of Bak or Bax. As shown in Fig. 3A, rBakΔC induced Δψ loss, whereas rBakΔGDΔC had no effect on mitochondrial Δψ. Furthermore, Δψ loss was induced by BH3 peptides derived from Bak (amino acid residues 73–87) and Bax (amino acid residues 55–74), both of which are proapoptotic (35). A mutant BH3 peptide L78A (Leu-78 → Ala) lacking proapoptotic ability (35) showed no reduction of Δψ (Fig. 3B). These results indicated that BH3 is required for the rBak- and rBax-induced PT and that it is sufficient to induce the PT.
Figure 3.
Requirement for the BH3 domain in rBakΔC-induced PT. (A) Lack of Δψ loss induction by rBakΔGDΔC. Mitochondria (1 mg/ml) were incubated with 100 μg/ml of rBakΔC, rBakΔGDΔC, or mock protein and Δψ was measured using Rh123 at 20 min. (B) Δψ loss induction by BH3 peptides. Mitochondria (1 mg/ml) were incubated in the presence of the following peptides (10 μM), and Δψ was measured at 20 min using Rh123: Bak (amino acid residues 73–87), mutant BakL78A (amino acid residues 73–87), and Bax (amino acid residues 55–74).
Bax Is Coimmunoprecipitated with VDAC.
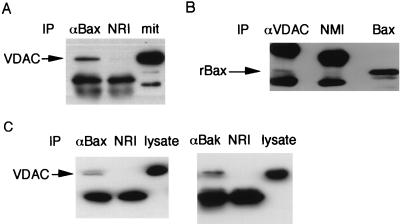
Induction of the CsA- and BK-sensitive and Ca2+-dependent PT by rBax and rBakΔC raised the possibility that Bax and Bak could interact directly with the PT pore complex, which has been proposed to consist of ANT, VDAC, and PBR (17–19). Using immunoprecipitation experiments, we examined the possible interaction of Bax with the PT pore complex, particularly with VDAC localized at the mitochondrial outer membrane side of the complex, assuming that Bax acts from outside the mitochondria. As shown in Figs. 4 A and B, VDAC was coimmunoprecipitated with rBax. We also examined whether Bax and Bak interacted with VDAC in mammalian cells. As shown in Fig. 4C, VDAC was coimmunoprecipitated with overexpressed Bax or Bak in Cos7 cells. These data indicate that Bax and Bak interact directly or indirectly with VDAC and suggest that Bax and Bak induce the PT through a direct interaction with the PT pore complex.
Figure 4.
Interaction of Bax and Bak with VDAC. (A and B) Interaction of rBax with VDAC in mitochondria. Mitochondria (1 mg/ml) were incubated with rBax (100 μg/ml). After induction of the PT, the mitochondria were centrifuged and washed twice. The mitochondrial lysates were subjected to immunoprecipitation (IP) with anti-human Bax antibody (αBax) or normal rabbit IgG (NRI) in A and with anti-human VDAC antibody (αVDAC) or normal mouse IgG (NMI) in B. The immune complexes were analyzed by Western blotting using anti-human VDAC antibody (A) and anti-Xpress antibody (B). An aliquot of mitochondria (mit) in A and rBax in B were used as the controls. (C) Interaction of Bax or Bak with VDAC in mammalian cells. Cos7 cells were cotransfected with pUC-CAGGS-mouse bax or pUC-CAGGS-human bak and pUC-CAGGS-human vdac1, and were treated with DTBP as described in Materials and Methods. Cell lysates were immunoprecipitated with anti-Bax (αBax) or anti-Bak (αBak) polyclonal antibody and normal rabbit IgG (NRI). Then the immune complexes were analyzed by Western blotting using anti-human VDAC antibody.
Bax and Bak Induce Cytochrome c Release Through the PT.
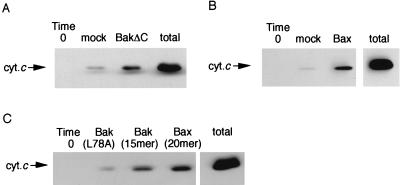
Cytochrome c release from mitochondria has been reported to be a key event in apoptosis induced by various stimuli (7, 8), including overexpression of Bax (36, 37). Therefore, we next examined the effect of rBakΔC and rBax on cytochrome c release by using isolated mitochondria. As shown in Fig. 5 A and B, both rBakΔC and rBax induced cytochrome c release, confirming the previous observation (26). The slight increase of cytochrome c release after addition of mock protein was probably a result of detergent contamination during purification. A significant amount of cytochrome c also was released when BH3 peptides, but not the mutant peptide, L78A, were added (Fig. 5C). These results indicated that Bak, Bax, and BH3 peptides have the ability to induce cytochrome c release.
Figure 5.
Induction of cytochrome c release by rBax and rBakΔC. Mitochondria (1 mg/ml) were incubated with 100 μg/ml of rBax (A), 100 μg/ml of rBakΔC (B), or 10 μM BH3 peptides (C). Mock proteins were used as controls in A and B. At 20 min, samples were centrifuged and aliquots (20 μl) of the supernatants were subjected to Western blot analysis for cytochrome c (cyt.c). “Total” represents an equivalent aliquot of mitochondria.
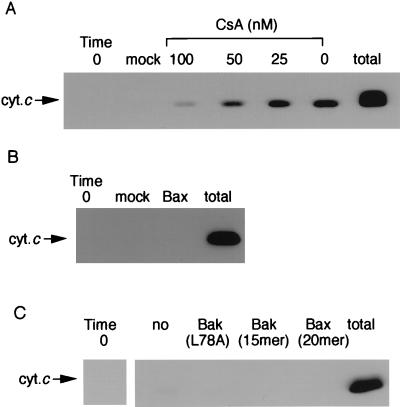
To investigate the relationship between PT induction and cytochrome c release, the effect of CsA and Ca2+ chelation on cytochrome c release was examined. Addition of CsA reduced rBax-induced cytochrome c release in a concentration-dependent manner (Fig. 6A), similar to that for the inhibitory effect on the rBax-induced PT (Fig. 2 C and D), and cytochrome c release was not observed under Ca2+-free conditions, even in the presence of rBax or BH3 peptides (Fig. 6 B and C). These results suggest that rBax- and rBak-induced cytochrome c release was largely dependent on the PT in isolated mitochondria.
Figure 6.
Inhibition of rBax and rBakΔC-induced cytochrome c release by inhibiting the PT. (A) Prevention of rBax-induced cytochrome c release by CsA. Mitochondria (1 mg/ml) were incubated with rBax (100 μg/ml) in the presence of CsA at the indicated concentrations. At 20 min, samples were centrifuged and aliquots (20 μl) of the supernatants were analyzed by Western blotting using anti-cytochrome c antibody. (B and C) Prevention of rBax- and BH3-peptide-induced cytochrome c release by depletion of Ca2+. Mitochondria were incubated with 100 μg/mg of rBax, mock proteins, or BH3 peptides derived from Bak or Bax (10 μM) under Ca2+-depleted conditions. At 20 min, samples were obtained as described in A and the amount of cytochrome c (cyt.c) released was analyzed by Western blotting.
Bax-Induced Δψ Loss Is Independent of Cytochrome c Release.
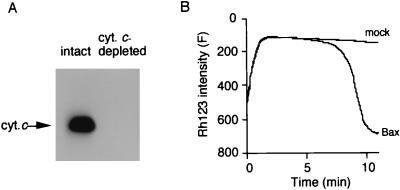
Since cytochrome c is an essential component of the electron transfer system that generates Δψ, we tested the possibility that rBax-induced Δψ loss was a consequence of cytochrome c release by using mitochondria with a disrupted outer membrane. By washing mitochondria with a hypotonic buffer, the outer membrane was disrupted and cytochrome c was removed (Fig. 7A). These mitochondria had no Δψ (data not shown), but it could be restored by adding cytochrome c (Fig. 7B). When cytochrome c-depleted mitochondria were incubated with either mock protein or rBax in the presence of 1 μM cytochrome c, rBax but not the mock protein still induced Δψ loss (Fig. 7B), indicating that the rBax-induced Δψ loss was not a consequence of cytochrome c release.
Figure 7.
No requirement of cytochrome c release for rBax-induced Δψ loss. (A) Cytochrome c depletion from mitochondria. Cytochrome c was depleted by washing mitochondria with a hypotonic buffer as described in Materials and Methods. Intact mitochondria (intact) and cytochrome c-depleted mitochondria (cyt.c-depleted) (30 μg each) were analyzed for cytochrome c (cyt.c) by Western blotting. (B) rBax-induced Δψ loss in cytochrome c-depleted mitochondria with exogenous cytochrome c. Cytochrome c-depleted mitochondria (1 mg/ml) were incubated with rBax (50 μg/ml) or mock protein in the presence of exogenous cytochrome c (1 mM), and Δψ was measured using Rh123.
Bcl-2 and Bcl-xL Prevent both PT and Cytochrome c Release Induced by Bax.
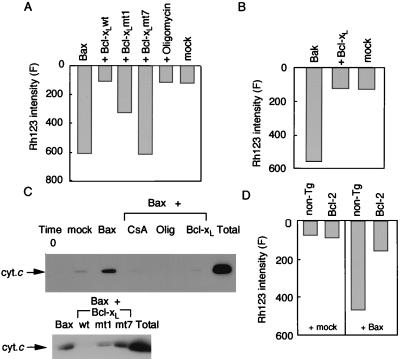
Bcl-2 and Bcl-xL, antiapoptotic members of the Bcl-2 family, have been reported to prevent induction of the PT by various stimuli (38, 39) and also to antagonize some of the proapoptotic Bcl-2 family members (40–42). Accordingly, we examined the effects of recombinant Bcl-xL (rBcl-xL) and its mutants on the PT induced by rBax and rBakΔC. Incubation of mitochondria with rBax or rBakΔC together with rBcl-xL did not cause Δψ loss (Fig. 8 A and B), mAST release, mitochondrial swelling (data not shown), or cytochrome c release (Fig. 8 C Upper), indicating that Bcl-xL could prevent induction of the PT and cytochrome c release by rBakΔC and rBax. We also studied two non-Bax-binding Bcl-xL mutants (mt1 and mt7), which previously were shown to have no and partial antiapoptotic activity, respectively (28). Consistent with their reported antiapoptotic activity, Bcl-xL mt7 and mt1, respectively, had no and a partial protective effect on Bax-induced cytochrome c release and Δψ loss (Fig. 8 A and C Lower). Bcl-2 also exerted a protective effect on rBax-induced Δψ loss (Fig. 8D) and cytochrome c release (data not shown).
Figure 8.
Prevention of rBax-induced Δψ loss and cytochrome c release by rBcl-xL, transgene-derived Bcl-2, and oligomycin. (A) Prevention of rBax-induced Δψ loss by rBcl-xL and oligomycin. Mitochondria (1 mg/ml) were incubated with mock protein or rBax at 50 μg/ml in the presence or absence of rBcl-xL (wt) and its mutants (mt1 and mt7) (20 μg/ml) or oligomycin (10 μM), and Δψ was measured using Rh123 at 20 min. (B) Prevention of rBakΔC-induced Δψ loss by rBcl-xL. Mitochondria (1 mg/ml) were incubated with rBakΔC (100 μg/ml) in the presence or absence of rBcl-xL (20 μg/ml), and Δψ was measured using Rh123 at 30 min. (C) Prevention of rBax-induced cytochrome c release by rBcl-xL and oligomycin. Mitochondria (1 mg/ml) were incubated with rBax (50 μg/ml) in the presence or absence of Bcl-xL (wt) and its mutants (mt 1 and mt 7) (20 μg/ml), CsA (100 nM) or oligomycin (10 μM). After 12 min, samples were centrifuged and aliquots of the supernatants (20 μl each) were subjected to Western blot analysis for cytochrome c (cyt.c). (D) Prevention of rBax-induced Δψ loss by transgene-derived Bcl-2. Mitochondria were isolated from the livers of hepatic bcl-2 transgenic mice (Bcl-2) and nontransgenic littermates (non-Tg). Mitochondria (1 mg/ml) were incubated with mock protein or rBax at 50 μg/ml, and Δψ was measured at 30 min.
Oligomycin Prevents Bax-Induced PT and Cytochrome c Release.
It was reported recently that Bax-induced apoptosis required F0F1-ATPase in yeast and that oligomycin, a specific F0F1-ATPase inhibitor, rescued Bax-induced cell death in both yeast and mammalian cells (43). Accordingly, we assessed the effect of oligomycin on mitochondrial changes induced by rBax. As shown in Fig. 8 A and C, 10 μM oligomycin, which completely blocked F0F1-ATPase activity (data not shown), completely inhibited both rBax-induced mitochondrial Δψ loss and cytochrome c release, suggesting an important role of F0F1-ATPase in the Bax-induced PT.
DISCUSSION
Mitochondrial changes have been shown to be involved in the early phase of apoptosis (4, 5). Two events, the PT and cytochrome c release, are induced by various apoptogenic reagents, followed by activation of caspases. In the present study, we used isolated mitochondria to demonstrate that rBax and rBakΔC induce both the PT and cytochrome c release through interaction with the PT complex.
Mechanism of Bax- and Bak-Induced PT.
We showed that exogenous rBax and rBakΔC induced the PT in isolated mitochondria, with this induction being inhibited by CsA, BK, and Ca2+ depletion, indicating that Bax/Bak is able to act directly on mitochondria to open the PT pores. Although the possibility was not excluded that Bax/Bak functions independently of the PT pores but induces the PT, for example, by acting as an ion channel (14, 15), the ability of Bax/Bak to interact with VDAC strongly suggests that Bax/Bak induces opening of the PT pores through direct interaction.
Since a Bak BH3 deletion mutant (ΔGD) could not induce the PT, and BH3 peptides of Bak and Bax induced it, the BH3 region seemed to play an essential role in PT induction. Interestingly, the PT-inducing ability of different peptides was correlated closely with their proapoptotic effect, suggesting Bax- and Bak-mediated apoptosis occurs through the PT. Since the PT-inducing activities of Bax, Bak, and BH3 peptides also were well correlated with their binding to antiapoptotic members of the Bcl-2 family, these peptides might interact with and antagonize antiapoptotic Bcl-2 family members in the mitochondria or may interact with a non-Bcl-2 mitochondrial protein possessing a BH3-binding structure similar to that of Bcl-2. Alternatively, Bax and Bak might form homodimers with preexisting proteins in the mitochondria to exert their activity.
We showed that exogenous rBcl-xL and transgene-derived Bcl-2 inhibited the rBax-induced PT. However, it was not determined whether this inhibition was caused by heterodimerization of Bcl-xL or Bcl-2 with Bax on the mitochondrial membrane or was a result of independent activity of Bcl-xL or Bcl-2.
Mechanism of Bax- and Bak-Induced Cytochrome c Release.
Little cytochrome c release was induced by rBax and rBakΔC in the absence of the PT, while release was massively enhanced by the PT. Therefore, the PT seemed to be crucial for cytochrome c release, consistent with a previous report suggesting that mitochondrial swelling is required for the release of cytochrome c (44). Although the precise mechanism underlying enhancement of cytochrome c release by the PT is uncertain, outer mitochondrial membrane rupture due to swelling may be involved (44), or cytochrome c might be released through as yet unidentified channels that are dependent on the PT. Alternatively, Bax may bind to the PT pore complex and induce a conformational change to open the pore, through which cytochrome c is released. Since cytochrome c needs to pass only through the mitochondrial outer membrane, VDAC might serve as a pore for its release, an idea that might be supported by the previous observation that cytochrome c interacts with VDAC (45). However, the estimated diameter of the VDAC pore (2–3 nm) does not seem to be sufficient for folded cytochrome c, so it might pass through the pore in an unfolded form. The outer membrane portion of the PT pore seems to consist of not only VDAC but also other molecules, any of which might form a pore large enough for cytochrome c to pass through. Since cytochrome c could be released with maintenance of Δψ in apoptotic cells (7, 8, 46), there might be another mechanism of apoptosis-associated cytochrome c release that may be independent of Bax/Bak.
We showed that rBcl-xL and transgene-derived Bcl-2 inhibit rBax/BakΔC-induced cytochrome c release, consistent with previous observations that overexpression of Bcl-2 prevents apoptosis-associated cytochrome c release in cells (7, 8). However, a recent study showed that Bcl-2 inhibits Bax-induced cell death while not preventing Bax-induced cytochrome c release (37). Given that Bcl-xL/Bcl-2 have multiple functions, the discrepancy might be due to the amount of Bcl-xL/Bcl-2, i.e., inhibition of PT and cytochrome c release may require a large amount of these proteins and a small amount may not prevent PT/cytochrome c release but may block cell death further downstream.
Oligomycin Inhibits Bax-Induced PT and Cytochrome c Release.
Recently, F0F1-ATPase was reported to be required for Bax-induced cell death, based on the observations that a yeast mutant lacking ATP4 (an F0F1-ATPase component) became resistant to Bax-induced cell death and oligomycin rescued both yeast and mammalian cells from Bax-induced cell death (43). Consistent with the above, oligomycin inhibited the Bax-induced PT and cytochrome c release in isolated mitochondria. Although the possibility was not excluded that Bax targets F0F1-ATPase to exerts its activity, the lack of evidence for an interaction between Bax and F0F1-ATPase (43) and the lack of effect of Bax on F0F1-ATPase activity in isolated mitochondria (data not shown) suggest that F0F1-ATPase is not a direct target of Bax. Oligomycin inhibits F0F1-ATPase and therefore modulates the ADP/ATP ratio in a manner that probably promotes PT pore closure, as suggested previously (47).
Acknowledgments
We are grateful to Drs. A. Mignon and V. Lacronique for hepatic bcl-2 transgenic mice, Dr. E. Margoliash for providing anti-cytochrome c mAb (7H8.2C12), Drs. H. Terada and Y. Shinohara and Dr. M. Klingenberg for providing BK, Dr. S. J. Korsmeyer for mouse bax cDNA, Dr. S. Ohta for human bax cDNA, Dr. M. Forte for human vdac1 cDNA, and Dr. J. M. Hardwick for Bcl-xL mutant cDNAs. We also thank Dr. Y. Eguchi for his advice in preparing the recombinant Bax protein. This study was supported in part by a grant for Scientific Research on Priority Areas, by a grant for Center of Excellence Research, and by a grant for Scientific Research from the Ministry of Education, Science, Sports and Culture of Japan.
ABBREVIATIONS
- PT
permeability transition
- CsA
cyclosporin A
- AIF
apoptosis-inducing factor
- ANT
adenine nucleotide translocator
- VDAC
voltage-dependent anion channel
- PBR
peripheral benzodiazepine receptor
- BK
bongkrekic acid
- GST
glutathione S-transferase
- mAST
mitochondrial aspartate aminotransferase
- BH3
Bcl-2 homology 3
- DTBP
dimethyl 3,3′-dithiobis(propionimidate)⋅2HCl
- Rh123
rhodamine 123
Note Added in Proof
Bax has recently been shown to bind to ANT (48).
Footnotes
This paper was submitted directly (Track II) to the Proceedings Office.
References
- 1.Steller H. Science. 1995;267:1445–1449. doi: 10.1126/science.7878463. [DOI] [PubMed] [Google Scholar]
- 2.Cory S. Annu Rev Immunol. 1995;13:513–543. doi: 10.1146/annurev.iy.13.040195.002501. [DOI] [PubMed] [Google Scholar]
- 3.Salvesen G S, Dixit V M. Cell. 1997;91:443–446. doi: 10.1016/s0092-8674(00)80430-4. [DOI] [PubMed] [Google Scholar]
- 4.Reed J C. Cell. 1997;91:559–562. doi: 10.1016/s0092-8674(00)80442-0. [DOI] [PubMed] [Google Scholar]
- 5.Kroemer G, Dallaporta B, Resche-Rigon M. Annu Rev Physiol. 1998;60:619–642. doi: 10.1146/annurev.physiol.60.1.619. [DOI] [PubMed] [Google Scholar]
- 6.Liu X, Kim C N, Yang J, Jemmerson R, Wang X. Cell. 1996;86:147–157. doi: 10.1016/s0092-8674(00)80085-9. [DOI] [PubMed] [Google Scholar]
- 7.Yang J, Liu X, Bhalla K, Kim C N, Ibrado A M, Cai J, Peng T I, Jones D P, Wang X. Science. 1997;275:1129–1132. doi: 10.1126/science.275.5303.1129. [DOI] [PubMed] [Google Scholar]
- 8.Kluck R M, Bossy-Wetzel E, Green D R, Newmeyer D D. Science. 1997;275:1132–1136. doi: 10.1126/science.275.5303.1132. [DOI] [PubMed] [Google Scholar]
- 9.Susin S A, Zamzami N, Castedo M, Hirsch T, Marchetti P, Macho A, Daugas E, Geuskens M, Kroemer G. J Exp Med. 1996;184:1331–1341. doi: 10.1084/jem.184.4.1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li P, Nijhawan D, Budihardjo I, Srinivasula S M, Ahmad M, Alnemri E S, Wang X. Cell. 1997;91:479–489. doi: 10.1016/s0092-8674(00)80434-1. [DOI] [PubMed] [Google Scholar]
- 11.Susin S A, Zamzami N, Castedo M, Daugas E, Wang H G, Geley S, Fassy F, Reed J C, Kroemer G. J Exp Med. 1997;186:25–37. doi: 10.1084/jem.186.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Minn A J, Velez P, Schende S L, Liang H, Muchmore S W, Fesik S W, Fill M, Thompson C B. Nature (London) 1997;385:353–357. doi: 10.1038/385353a0. [DOI] [PubMed] [Google Scholar]
- 13.Schendel S L, Xie Z, Montal M O, Matsuyama S, Montal M, Reed J C. Proc Natl Acad Sci USA. 1997;94:5113–5118. doi: 10.1073/pnas.94.10.5113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schlesinger P H, Gross A, Yin X M, Yamamoto K, Saito M, Waksman G, Korsmeyer S J. Proc Natl Acad Sci USA. 1997;94:11357–11362. doi: 10.1073/pnas.94.21.11357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Antonsson B, Conti F, Ciavatta A, Montessuit S, Lewis S, Martinou I, Bernasconi L, Bernard A, Mermod J J, Mazzei G, et al. Science. 1997;277:370–372. doi: 10.1126/science.277.5324.370. [DOI] [PubMed] [Google Scholar]
- 16.Halestrap A P. Biochim Biophys Acta. 1989;973:355–382. doi: 10.1016/s0005-2728(89)80378-0. [DOI] [PubMed] [Google Scholar]
- 17.Zoratti M, Szabo I. Biochim Biophys Acta. 1995;1241:139–176. doi: 10.1016/0304-4157(95)00003-a. [DOI] [PubMed] [Google Scholar]
- 18.McEnery M W, Snowman A M, Trifiletti R R, Snyder S H. Proc Natl Acad Sci USA. 1992;89:3170–3174. doi: 10.1073/pnas.89.8.3170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Beutner G, Ruck A, Riede B, Brdiczka D. Biochim Biophys Acta. 1998;1368:7–18. doi: 10.1016/s0005-2736(97)00175-2. [DOI] [PubMed] [Google Scholar]
- 20.Kass G E, Juedes M J, Orrenius S. Biochem Pharmacol. 1992;44:1995–2003. doi: 10.1016/0006-2952(92)90102-o. [DOI] [PubMed] [Google Scholar]
- 21.Marchetti P, Hirsch T, Zamzami N, Castedo M, Decaudin D, Susin S A, Masse B, Kroemer G. J Immunol. 1996;157:4830–4836. [PubMed] [Google Scholar]
- 22.Zamzami N, Marchetti P, Castedo M, Hirsch T, Susin S A, Masse B, Kroemer G. FEBS Lett. 1996;384:53–57. doi: 10.1016/0014-5793(96)00280-3. [DOI] [PubMed] [Google Scholar]
- 23.Shimizu S, Eguchi Y, Kamiike W, Funahashi Y, Mignon A, Lacronique V, Matsuda H, Tsujimoto Y. Proc Natl Acad Sci USA. 1998;95:1455–1459. doi: 10.1073/pnas.95.4.1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hsu Y T, Wolter K G, Youle R J. Proc Natl Acad Sci USA. 1997;94:3668–3672. doi: 10.1073/pnas.94.8.3668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wolter K G, Hsu Y T, Smith C L, Nechushtan A, Xi X G, Youle R J. J Cell Biol. 1997;139:1281–1292. doi: 10.1083/jcb.139.5.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jürgensmeier J M, Xie Z, Deveraux Q, Ellerby L, Bredesen D, Reed J C. Proc Natl Acad Sci USA. 1998;95:4997–5002. doi: 10.1073/pnas.95.9.4997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Turrens J F, Alexandre A, Lehninger A L. Arch Biochem Biophys. 1985;237:408–414. doi: 10.1016/0003-9861(85)90293-0. [DOI] [PubMed] [Google Scholar]
- 28.Cheng E H, Levine B, Boise L H, Thompson C B, Hardwick J M. Nature (London) 1996;379:554–556. doi: 10.1038/379554a0. [DOI] [PubMed] [Google Scholar]
- 29.Inoue T, Yoshida Y, Nishimura M, Kurosawa K, Tagawa K. Biochim Biophys Acta. 1993;1140:313–320. doi: 10.1016/0005-2728(93)90071-m. [DOI] [PubMed] [Google Scholar]
- 30.Shimizu S, Kamiike W, Hatanaka N, Nishimura M, Miyata M, Inoue T, Yoshida Y, Tagawa K, Matsuda H. Transplantation. 1994;57:144–148. doi: 10.1097/00007890-199401000-00022. [DOI] [PubMed] [Google Scholar]
- 31.Niwa H, Yamamura K, Miyazaki J. Gene. 1991;108:193–199. doi: 10.1016/0378-1119(91)90434-d. [DOI] [PubMed] [Google Scholar]
- 32.Xu Q, Reed J C. Mol Cell. 1998;1:337–346. doi: 10.1016/s1097-2765(00)80034-9. [DOI] [PubMed] [Google Scholar]
- 33.Chittenden T, Flemington C, Houghton A B, Ebb R G, Gallo G J, Elangovan B, Chinnadurai G, Lutz R J. EMBO J. 1995;14:5589–5596. doi: 10.1002/j.1460-2075.1995.tb00246.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sattler M, Liang H, Nettesheim D, Meadows R P, Harlan J E, Eberstadt M, Yoon H S, Shuker S B, Chang B S, Minn A J, et al. Science. 1997;275:983–986. doi: 10.1126/science.275.5302.983. [DOI] [PubMed] [Google Scholar]
- 35.Cosulich S C, Worrall V, Hedge P J, Green S, Clarke P R. Curr Biol. 1997;7:913–920. doi: 10.1016/s0960-9822(06)00410-6. [DOI] [PubMed] [Google Scholar]
- 36.Manon S, Chaudhuri B, Guerin M. FEBS Lett. 1997;415:29–32. doi: 10.1016/s0014-5793(97)01087-9. [DOI] [PubMed] [Google Scholar]
- 37.Rosse T, Olivier R, Monney L, Rager M, Conus S, Fellay I, Jansen B, Borner C. Nature (London) 1998;391:496–499. doi: 10.1038/35160. [DOI] [PubMed] [Google Scholar]
- 38.Zamzami N, Susin S A, Marchetti P, Hirsch T, Gomez-Monterrey I, Castedo M, Kroemer G. J Exp Med. 1996;183:1533–1544. doi: 10.1084/jem.183.4.1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shimizu S, Eguchi Y, Kamiike W, Waguri S, Uchiyama Y, Matsuda H, Tsujimoto Y. Oncogene. 1996;13:21–29. [PubMed] [Google Scholar]
- 40.Oltvai Z N, Milliman C L, Korsmeyer S J. Cell. 1993;74:609–619. doi: 10.1016/0092-8674(93)90509-o. [DOI] [PubMed] [Google Scholar]
- 41.Chittenden T, Harrington E A, O’Connor R, Flemington C, Lutz R J, Evan G I, Guild B C. Nature (London) 1995;374:733–736. doi: 10.1038/374733a0. [DOI] [PubMed] [Google Scholar]
- 42.Kitanaka C, Namiki T, Noguchi K, Mochizuki T, Kagaya S, Chi S, Hayashi A, Asai A, Tsujimoto Y, Kuchino Y. Oncogene. 1997;15:1763–1772. doi: 10.1038/sj.onc.1201349. [DOI] [PubMed] [Google Scholar]
- 43.Matsuyama S, Xu Q, Velours J, Reed J C. Mol Cell. 1998;1:327–336. doi: 10.1016/s1097-2765(00)80033-7. [DOI] [PubMed] [Google Scholar]
- 44.Vander Heiden M G, Chandel N S, Williamson E K, Schumacker P T, Thompson C B. Cell. 1997;91:627–637. doi: 10.1016/s0092-8674(00)80450-x. [DOI] [PubMed] [Google Scholar]
- 45.Mannella C A. J Struct Biol. 1998;121:207–218. doi: 10.1006/jsbi.1997.3954. [DOI] [PubMed] [Google Scholar]
- 46.Bossy-Wetzel E, Newmeyer D D, Green D R. EMBO J. 1998;17:37–49. doi: 10.1093/emboj/17.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Novgorodov S A, Gudz T I, Brierley G P, Pfeiffer D R. Arch Biochem Biophys. 1994;311:219–228. doi: 10.1006/abbi.1994.1230. [DOI] [PubMed] [Google Scholar]
- 48.Marzo I, Brenner C, Zamzami N, Jurgensmeier J M, Susin S A, Vieira H L, Prevost M C, Xie Z, Matsuyama S, Reed J C, Kroemer G. Science. 1998;281:2027–2031. doi: 10.1126/science.281.5385.2027. [DOI] [PubMed] [Google Scholar]